Introduction
Preimplantation embryo-derived stem cells are a good tool for studying early developmental biology and for biomedical research and genetic engineering in mammalian species (Blomberg & Telugu, Reference Blomberg and Telugu2012). Trophoblast stem cells are the first cells to differentiate from the embryo, have oligopotent stem cell characteristics, contribute to the formation of the fetal parts of the placenta and fetal membranes, and have the capacity to self-renew indefinitely (Latos & Hemberger, Reference Latos and Hemberger2016; Peter et al., Reference Peter, Beg, Ahmad and Bergfelt2017). Camel (Camelus dromedarius) is an important mammalian species in the Arabian Peninsula and some other African and Asian countries because it can tolerate harsh arid conditions with maintaining meat and milk production. Moreover, the differentiation process of trophoblasts remains poorly understood in the camel because only few studies have described the early embryonic development in this species. There have been few reports regarding the embryonic and adult stem cells from camel (C. dromedarius) (Mohammadi-Sangcheshmeh et al., Reference Mohammadi-Sangcheshmeh, Shafiee, Seyedjafari, Dinarvand, Toghdory, Bagherizadeh, Schellander, Cinar and Soleimani2013; Saadeldin et al., Reference Saadeldin, Swelum, Elsafadi, Moumen, Alzahrani, Mahmood, Alfayez and Alowaimer2017a; Saadeldin et al., Reference Saadeldin, Abdel-Aziz Swelum, Alzahrani and Alowaimer2018a, Reference Saadeldin, Swelum, Elsafadi, Mahmood, Alfayez and Alowaimerc). Trials have been performed to isolate in vitro-produced trophoblast cells from several mammalian species such as cow, buffalo, mouse, rabbit, and pig, either through the use of feeder cells (Talbot et al., Reference Talbot, Powell, Camp and Ealy2007) or feeder-free culture conditions (Shimada et al., Reference Shimada, Nakano, Takahashi, Imai and Hashizume2001; Wutz et al., Reference Wutz, Tan, Tang, Zhang, Niu, Chen, Li, Wei and Ji2011; Kubaczka et al., Reference Kubaczka, Senner, Araúzo-Bravo, Sharma, Kuckenberg, Becker, Zimmer, Brüstle, Peitz, Hemberger and Schorle2014; Dean et al., Reference Dean, Mohapatra, Sandhu, Singh, Singla, Chauhan, Manik and Palta2015; Saadeldin et al., Reference Saadeldin, Kim and Lee2015). While, derivation of camel trophoblast and embryonic stem cells has been reported using in vivo-derived camel embryos (Saadeldin et al., Reference Saadeldin, Swelum, Elsafadi, Moumen, Alzahrani, Mahmood, Alfayez and Alowaimer2017a); no reports have been declared regarding the isolation of trophoblast stem cells from in vitro-derived embryos.
Several trials have been reported on the beneficial effects of various growth factor supplementation on the development of preimplantation embryos (Lee & Fukui, Reference Lee and Fukui1995; Ahumada et al., Reference Ahumada, Salvador, Cebrian-Serrano, Lopera and Silvestre2012; Pan et al., Reference Pan, Cui, Baloch, He, Fan, He, Li, Yang, Zhang and Yu2015). A few studies have reported the effect of growth factors on embryo-derived outgrowths in mice (Haimovici & Anderson, Reference Haimovici and Anderson1993), while no reports have been declared regarding the effects of growth factors on trophoblast derivation from camels.
Therefore, the current study aimed to optimize the conditions for the successful culture of trophoblast cells from in vitro-derived camel embryos through supplementation of culture medium with basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF).
Material and methods
Unless otherwise stated, all the chemicals used were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA).
Camel ovaries were collected from a slaughterhouse in Riyadh and transported in 0.9% (v/v) saline solution (NaCl) at 30 to 33°C to the laboratory within 4 to 6 h. Antral follicles (2–8 mm in diameter) were aspirated and cumulus–oocyte complexes (COCs) with evenly granulated cytoplasm and enclosed in more than three layers of compact cumulus cells were selected. COCs were washed three times with HEPES-buffered tissue culture medium-199 (TCM-199) supplemented with 2 mM sodium bicarbonate, bovine serum albumin 0.1%, and 5 ng/ml gentamycin sulfate. COCs were in vitro matured in 100 μl drops of bicarbonate-buffered TCM-199 supplemented with 10% camel follicular fluid, 10 μg/ml follicle stimulating hormone (FSH), 10 μg/ml luteinizing hormone (LH), 10 ng/ml EGF, 0.3 μM cysteamine, 0.15 mg/ml l-glutamine, and 50 μg/ml gentamycin sulfate at 38.5°C in a humidified atmosphere of 5% CO2 in air for 30 h (Yaqoob et al., Reference Yaqoob, Saadeldin, Swelum and Alowaimer2017). The culture medium was overlaid with mineral oil. After maturation, cumulus cells were stripped from oocytes by pipetting in 1 mg/ml hyaluronidase in HEPES-buffered TCM-199 and washed three times in TCM-199 supplemented with 10% fetal bovine serum (FBS). Cumulus-free oocytes extruding the first polar body (around 65% of total COCs) were activated in TCM-199 supplemented with 10% FBS and 5 µM ionomycin for 5 min in a dark chamber, as previously described (Saadeldin et al., Reference Saadeldin, Swelum, Yaqoob and Alowaimer2017b). The parthenogenetically activated oocytes were cultured in microdrops of KSOMaa medium (one oocyte/5 µl) overlaid with oil in a humid atmosphere of 5% CO2, 5% O2, and 90% N2 at 38.5°C for 8 days until blastocyst formation and hatching. Blastocyst formation was about 20% and hatching was about 50% of the total blastocysts.
Under a stereomicroscope and by using a microblade, mechanical isolation of trophoblast and inner cell mass from the hatched embryos was performed as previously described (Strom et al., Reference Strom, Inzunza, Grinnemo, Holmberg, Matilainen, Stromberg, Blennow and Hovatta2007) with modifications. Briefly, a microblade was used to dislodge the inner cell mass cell dark clumps from the trophectoderm and then the dislodged trophoblast portions were cultured in culture medium for 24 h to form trophoblastic vesicles (TVs). TVs (n=48) were randomly divided in to four groups; 12 TVs from each group in four replicates (three TVs each). TVs were placed on 4-well dishes (Nunclon Surface, ThermoFisher Scientific, Waltham, MA, USA) freshly coated with basement membrane matrix (Matrigel, BD Biosciences) (Armant, Reference Armant2006; Saadeldin et al., Reference Saadeldin, Kim and Lee2015) to maintain feeder-free conditions. Culture medium of TVs was either supplemented with 10 ng/ml EGF (EGF group), 10 ng/ml FGF (bFGF, Miltenyi Biotec, GmbH, Bergisch Gladbach, Germany) (FGF group), or 10 ng/ml EGF+10 ng/ml FGF (EGF+FGF group). Plain culture medium was used for the control group.
TVs culture medium was comprised of Dulbecco’s modified Eagle’s medium supplemented with 1% non-essential amino acids, 2 mM l-glutamine, 1% insulin-transferrin-selenium, 0.1 mM β-mercaptoethanol, 10% FBS, and 1 mg/ml gentamycin. Culture was performed in a humid atmosphere of 5% CO2 at 38.5°C.
Trophoblast vesicle attachment to Matrigel and cuboidal cells outgrowths of trophoblasts were recorded for each treatment. Trophoblast passaging was performed through mechanical chopping and culturing over freshly prepared Matrigel, as previously mentioned (Saadeldin et al., Reference Saadeldin, Swelum, Elsafadi, Moumen, Alzahrani, Mahmood, Alfayez and Alowaimer2017a).
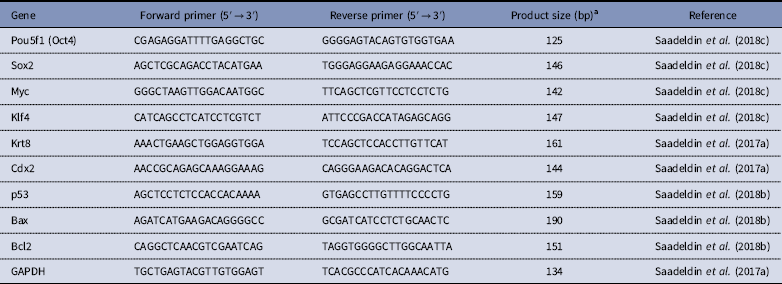
Total RNA was isolated from the control and FGF+EGF-supplemented TVs (three triplicates, five each) using a total RNA isolation kit (Intron Biotechnology, Daegu, Korea). Total RNA concentration and quality were determined using a NanoDrop 2000 spectrophotometer (ThermoFisher). Reverse transcription (RT) was performed to generate complementary DNA and relative quantification of mRNA transcripts was determined by relative quantitative polymerase chain reaction (qRT-PCR) according to the method described by (Saadeldin et al., Reference Saadeldin, Swelum, Elsafadi, Moumen, Alzahrani, Mahmood, Alfayez and Alowaimer2017a). Normalization to the reference gene GAPDH was performed and the fold change and relative quantification of Oct4, Sox2, Klf4, c-Myc, Cdx2, Krt8, p53, Bax, and Bcl2 transcripts were carried out using the 2−ΔΔCt method (Schmittgen & Livak, Reference Schmittgen and Livak2008). In all assays, non-template control (NTC) and reactions without RT resulted in negative amplification. Expression of each transcript in control trophoblast vesicles was set as an arbitrary unit to calculate the fold-change in the treated groups. Details on primers and approximate product size are listed in Table 1. Five biological replicates and three technical replicates were used.
Table 1 Primers used for relative quantitative PCR
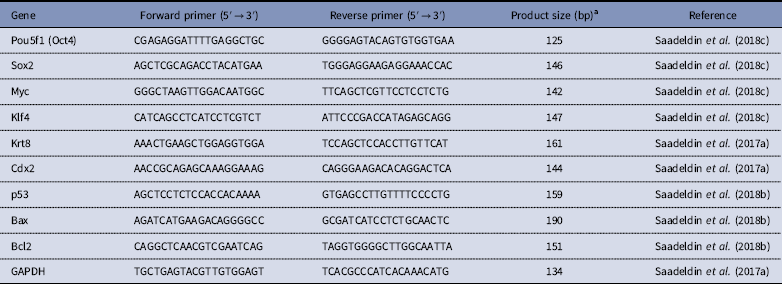
aThe melting curve for each primer was evaluated by ViiA™ 7 apparatus-associated software and the product size was confirmed by gel electrophoresis of PCR products on agarose 1.5% referred by a 1 kb DNA ladder.
Data of blastocyst attachment and trophoblast outgrowths were calculated as percentages and analyzed with the chi-squared test. Data of qRT-PCR were expressed as the mean ± SEM and compared by Student’s t-test using the SAS program (Cary, NC, USA). Statistical significance was considered when P<0.05.
Results and discussion
TVs co-supplemented with EGF and FGF showed significant increases in attachment to Matrigel and trophoblast outgrowths, passaged to the fifth passage, and maintained the morphological criteria of the trophoblasts (Table 2 and Fig. 1). Therefore, culture medium co-supplemented with 10 ng/ml EGF and 10 ng/ml bFGF showed better developmental characteristics of cultured TVs over Matrigel basement membrane matrix when compared with the control group and the groups supplemented with each alone.
Table 2 Effects of EGF and/or FGF on trophoblastic vesicle development

*The total number of four replicates, three TVs each.
TE. Trophoblast outgrowths.
a,b,cValues carrying different superscripts are statistically different at P<0.05.
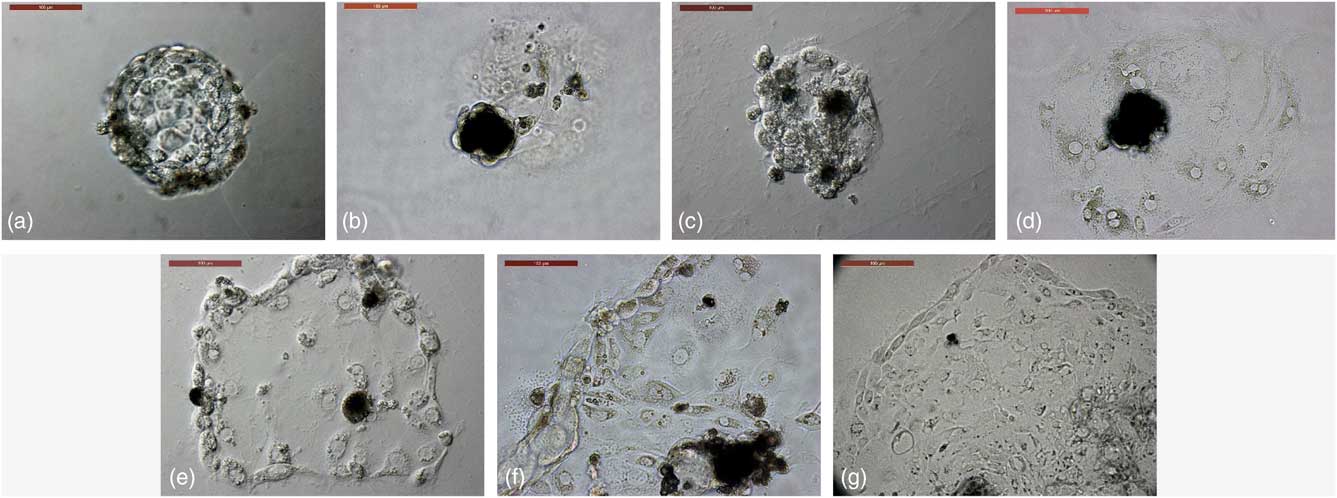
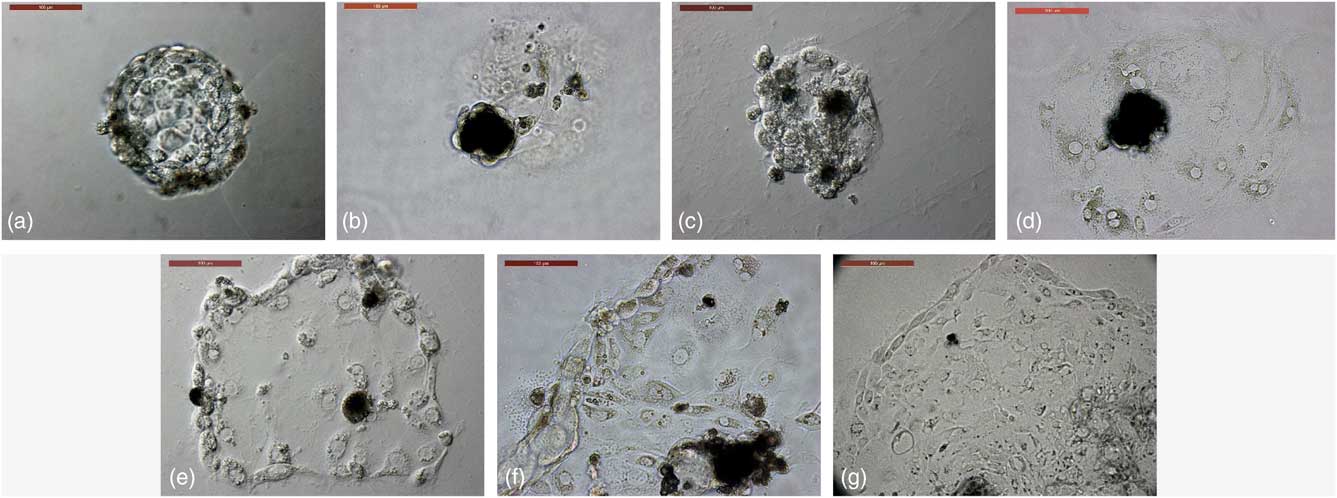
Figure 1 Trophoblast derivation from in vitro-produced camel embryos. (a) Trophoblastic vesicles (TVs) were cultured in 4-well dishes coated with freshly prepared Matrigel. (b) TVs growth in plain culture medium as a control group showing a lack of outgrowths. (c) TVs growth in EGF-supplemented culture medium, showing attachment with a lack of expanding outgrowths. (d) TVs growth in bFGF-supplemented culture medium, showing trophoblast outgrowths with a few cells that could not be passaged. (e–g) TVs growth in combined supplementation of culture medium with EGF and FGF, showing trophoblast outgrowths in primary culture – first passage and fifth passage, respectively. Scale bar represents 100 µm.
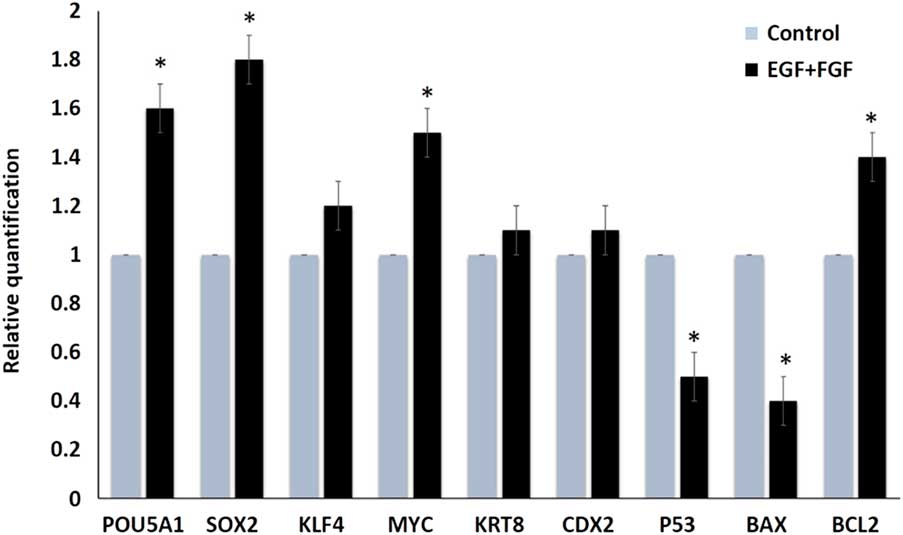
Additionally, combined supplementation of EGF and bFGF resulted in significant increases in pluripotency markers Pou5fa1 (Oct4), Sox2, and Myc when compared with the control group (1.6-, 1.8-, and 1.5-fold increases, respectively; Fig. 2). These increments might be required for the stemness criteria of the embryonic trophoblasts. Additionally, combined supplementation resulted in increased expression (1.45-fold) of the anti-apoptotic gene Bcl2 and decreased expression of apoptotic genes Bax and p53 (i.e. 2.5- and 2-fold increase in the control group, respectively). The Bax/Bcl2 ratio was significantly decreased in combined growth factor-supplemented TVs when compared with the control group (0.28 vs. 1; P<0.05). These changes might indicate the cellular death and replication defects that were observed in the control group (Fig. 2).
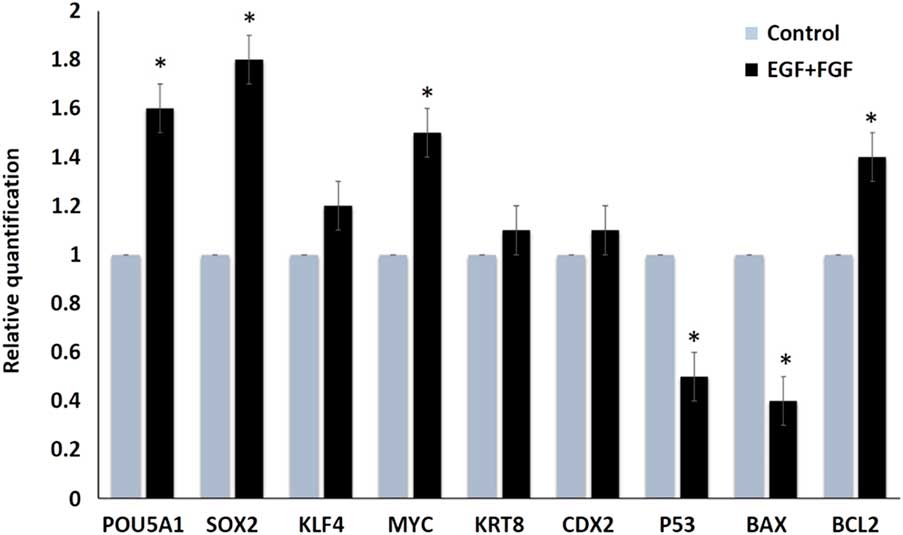
Figure 2 Relative quantification (fold change) of different transcripts after culturing TVs in plain culture medium (control) and medium supplemented with EGF and FGF. Asterisk (*) indicate significant difference at P<0.05.
Studies have demonstrated the supporting role of bFGF in the derivation and growth of trophoblast stem cells in mice (Tanaka et al., Reference Tanaka, Kunath, Hadjantonakis, Nagy and Rossant1998) and humans (Kunath et al., Reference Kunath, Yamanaka, Detmar, MacPhee, Caniggia, Rossant and Jurisicova2014). Recently, Okae et al. (Reference Okae, Toh, Sato, Hiura, Takahashi, Shirane, Kabayama, Suyama, Sasaki and Arima2018) showed that EGF was essential for derivation and long-term maintenance of proliferative human trophoblast stem cells.
The current results reflect the first trial to optimize trophoblast culture from in vitro-derived camel embryos in feeder-free culture conditions by a synergistic effect between EGF and bFGF. This study provides a paradigm to further understand the stemness properties of trophoblasts derived from in vitro-produced camel embryos.
Acknowledgement
The author would like to thank Dr Islam M. Saadeldin, for careful reading and commenting on the manuscript.
Financial support
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. G-640–662–1439. The authors, therefore, recognize and thank the DSR for providing technical and financial support.
Conflict of interest
The author declares that there is no conflict of interest that can be perceived as prejudicing the impartiality of the research reported.
Ethical standards
Not applicable.