Introduction
Inhibitor of apoptosis (IAP) proteins are structurally characterized by the existence of one to three Zn2+-finger motifs termed baculoviral IAP repeats (BIRs) (Crook et al., Reference Crook, Clem and Miller1993; Uren et al., Reference Uren, Coulson and Vaux1998; Hinds et al., Reference Hinds, Norton, Vaux and Day1999). By structural and functional analysis, two classes of BIR-bearing proteins are identified: type I and type II. Members of type I, including survivin, primarily play roles during cell division. In contrast, members of type II are primarily involved in the blocking of apoptosis by directly regulating caspase activation (Suzuki et al., Reference Suzuki, Hayashida, Ito, Kawano, Nakano, Miura, Akahane and Shiraki2000; Uren et al., Reference Uren, Wong, Pakusch, Fowler, Burrows, Vaux and Choo2000). Antisense RNA-survivin expression leads to failure in cell cleavage (Chen et al., Reference Chen, Wu, Tahir, Kroeger, Rosenberg, Cowsert, Bennett, Krajewski, Krajewska, Welsh, Reed and Ng2000). Survivin may be crucial in regulating chromosome segregation and cytokinesis (Fraser et al., Reference Fraser, James, Evan and Hengartner1999; Yoon & Carbon, Reference Yoon and Carbon1999; Speliotes et al., Reference Speliotes, Uren, Vaux and Horvitz2000). Previous results indicate that survivin plays an important role in cell division; therefore, here we investigated the influence of survivin on the progression of meiotic cell cycle in rat oocytes.
Chromosomal passenger complex (CPC) is comprised of aurora-B kinase and three other non-enzymatic members: inner centromere protein (INCENP), survivin and borealin (Ruchaud et al., Reference Ruchaud, Carmena and Earnshaw2007). Survivin and aurora-B kinase bind different domains of INCENP. The N-terminus of aurora-B kinase binds to the C-terminus of INCENP, while survivin and borealin bind the N-terminus of INCENP. Previous studies imply that different domains of survivin target the CPC complex to its different location during cell division (Lens et al., Reference Lens, Vader and Medema2006; Vader et al., Reference Vader, Kauw, Medema and Lens2006). It is now accepted that, in the period of chromosome alignment at the metaphase plate, accurate kinetochore-microtubule arrangements and equal physical tension generation are the prerequisite for the initiation of metaphase–anaphase transition. Whereas aberrant kinetochore-microtubule attachments or misalignment of chromosomes create abnormal tension, CPC, a key kinetochore protein complex, is activated in order to detect and eliminate these errors (Kotwaliwale & Biggins, Reference Kotwaliwale and Biggins2006; Ruchaud et al., Reference Ruchaud, Carmena and Earnshaw2007). This role of the CPC appears to destabilize microtubules from kinetochores by aurora-B phosphorylating Ndc80, which is known as the core microtubule attachment site at the kinetochore (DeLuca et al., Reference DeLuca, Dong, Hergert, Strauss, Hickey, Salmon and McEwen2005; Ruchaud et al., Reference Ruchaud, Carmena and Earnshaw2007). However, the function of survivin, as a member of CPC, has remained elusive in the progress of tension translation into signals. Therefore, we aim to show that survivin may be involved in major cellular functions in meiotic maturation of mammalian oocytes.
The meiosis of rat oocytes is different from mouse oocytes in some aspects. For example, released from the follicles, rat oocytes develop to the metaphase I (MI) stage earlier than mouse oocytes (rat 6 h, mouse 8 h). In addition, ovulated rat oocytes were observed to activate spontaneously, but not arrest at the MII stage during in vitro culture (Keefer & Schuetz, Reference Keefer and Schuetz1982). After extruding their second polar bodies, the spontaneously activated oocytes enter into a next metaphase-like stage, termed the third meiotic metaphase (MIII), instead of proceeding to interphase with the formation of pronuclei (Fan et al., Reference Fan, Tong, Teng, Lian, Li, Yang, Chen, Schatten and Sun2003). Compared with mouse oocytes, rat oocytes have became a unique model for studying the role of survivin in mammalian meiotic maturation.
In the present study, we report the subcellular localization and possible functional roles of survivin in meiotic maturation for the first time in rat oocytes. Our results prove that the survivin-dependent signal is essential for the normal progression of meiotic cell cycle in rat oocytes.
Materials and methods
Antibodies
Rabbit polyclonal anti-survivin antibody (catalogue number: #2808) was purchased from Cell Signaling Technology and mouse monoclonal anti-α-tubulin antibody (catalogue number: F2168) was purchased from Sigma Chemical Company. Fluorescein-conjugated affiniPure goat anti-rabbit IgG (H+L) (catalogue number: ZF-0311) and rabbit IgG (catalogue number: ZDR-5002) were purchased from Zhongshan Golden Bridge Biotechnology Company, Ltd.
Oocytes collection and culture
Animal care and use were conducted according to the guidelines of the Animal Research Committee of the Institute of Zoology, Chinese Academy of Sciences. Female SD rats used in this study were purchased from Vital River Laboratory Animal Technology Co. Ltd. Oocytes displaying a germinal vesicle (GV) were collected from ovaries of 4–5-week-old SD rats after being injected with 20 IU of pregnant mare's serum gonadotrophin (PMSG) for 48 h. They were cultured in M2 medium (Sigma Chemical Company) under paraffin oil at 37 °C in a humidified 5% CO2 incubator. Oocytes were collected for immunostaining at different times of culture. For MII oocytes collection, after injection with 20 IU PMSG for 48 h, rats were injected with 20 IU human chorionic gonadotrophin (hCG), and then sacrificed after 14–16 h.
Immunofluorescence and confocal microscopy
Oocytes at the desired stages were collected and fixed in 4% paraformaldehyde in PBS for 30 min at room temperature. They were then transferred to membrane permeabilization solution (0.5% Triton X-100) for 20 min at room temperature. After a 1 h block in blocking buffer (PBS containing 1% BSA), oocytes were incubated overnight at 4°C with 1:800 rabbit anti-survivin or 1:200 anti-α-tubulin-FITC antibody. After three washes in washing buffer (0.1% Tween 20 and 0.01% Triton X-100 in PBS), they were labelled with FITC-conjugated goat anti-rabbit IgG for 2 h at room temperature. After three additional washes in washing buffer, oocytes were co-stained with PI (propidium iodide, 10 μg/ml in PBS) for 5 min.
Finally, following extensive washing, oocytes were mounted on glass slides and examined with a confocal laser-scanning microscope (Zeiss LSM 510 META).
Each experiment was repeated three times and at least 50 oocytes were examined each time. The same instrument settings were used for each replicate.
Antibody microinjection
Microinjection was performed using a Nikon Diaphot ECLIPSE TE 300 (Nikon UK Ltd) inverted microscope equipped with Narishige MM0–202N hydraulic three-dimensional micromanipulators (Narishige Inc.). A microinjection volume of 7 pl of anti-survivin antibody was injected into the cytoplasm of a fully grown GV oocyte in all experiment. The oocytes were kept in M2 medium supplemented with 0.2 mM IBMX to prevent GV breakdown (GVBD) during the injection period. After microinjection, oocytes were washed thoroughly with M2 medium and cultured in the same medium. IgG injection oocytes were microinjected with the same amount of rabbit IgG. Each experiment was repeated three times, and at least 30 oocytes were examined each time.
Statistical analysis
All percentages from three repeated experiments were expressed as mean ± SEM and the number of oocytes observed was labelled in brackets as (n = ). Data were analyzed by chi-squared test. Differences at p < 0.05 were considered to be significant.
Results
Subcellular localization of survivin in rat oocytes during meiotic maturation
We used immunofluorescence staining to examine the localization of survivin at different stages of meiotic maturation. The oocytes were cultured for 0, 3, 5, 6 and 9 h in vitro, corresponding to GV, GVBD, pro-MI, MI, AI/TI stages, respectively, and MII oocytes were obtained in vivo.
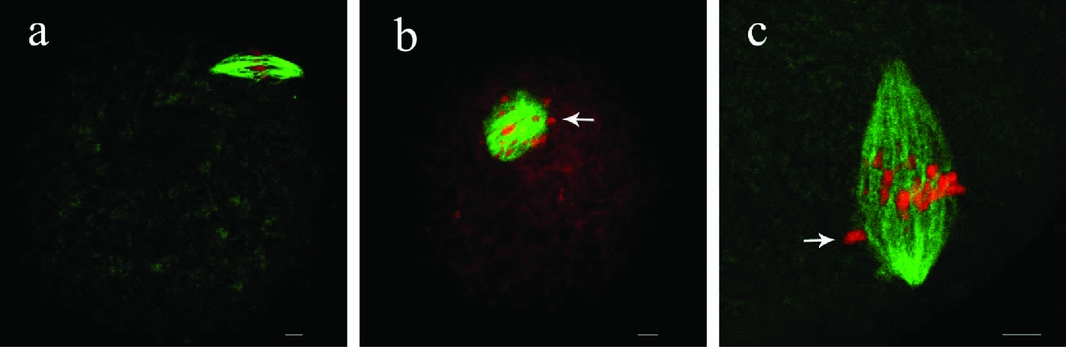
As shown in Fig. 1, at the GV stage, survivin was mainly concentrated in the GV. After GVBD, survivin began to be concentrated at the condensed chromosomes. At pro-MI and MI, survivin was mainly localized at the kinetochores, with a minor proportion detected on the chromosome arms. It was worth to mention that we observed the dynamic signal change of survivin from kinetochores and chromosome arms to midzone of spindle at anaphase I stage (Fig. 1AI ①,AI ② ). As the oocytes progressed through anaphase I and telophase I stages, survivin migrated to the midbody, and signals on the kinetochores and chromosomes disappeared. At MII, survivin became mainly localized at the kinetochores, with a minor proportion on the chromosomes again, similar to the MI stage.

Figure 1 Subcellular localization of survivin during rat oocyte meiotic maturation. Rat oocytes were processed for immunofluorescent staining at different stages of maturation. GV, oocytes at germinal vesicle; pro-MI, oocytes at first prometaphase; MI, oocytes at first metaphase; AI, oocytes at first anaphase; TI, oocytes at first telophase; MII, oocytes at second metaphase. At the GV stage, survivin mainly distributed in the germinal vesicle. From GVBD to MI, survivin accumulated at the kinetochores (MI, arrow). Survivin localized to the midzone at anaphase, midbody (TI, arrow) at telophase and localized to centromeres at MII. Bar = 10 μm. This figure is available in colour online.
Effect of anti-survivin antibody microinjection on spindle assembly and chromosome alignment
In this part, we used the microinjection technology to block the protein function. For evaluating that whether the injection procedure itself affected the spindle assembly and chromosome alignment, we conducted two groups including control group without any injection procedure and IgG injection group. The results showed that the oocytes with injection procedure can grow as normally as the oocytes of control group (Fig. 2a,b). The ratio of the abnormal spindles and chromosome misalignments in the injection group were not significantly different from the control (Fig. 2c,d). Thus, the data showed that injection procedure itself did not induce the influence on the spindle assembly and chromosome alignment.

Figure 2 The effect of microinjection procedure itself on oocyte spindle assembly and chromosome alignment. In the control group (a) and injection group (b) most oocytes showed normal growth. Bar = 10 μm. Percentage of abnormal growth was shown in the control group and the injection group (c, d). The same superscripts indicate that there was no statistical difference (p > 0.05). This figure is available in colour online.
To explore the role of the survivin protein, we depleted the protein function by microinjecting anti-survivin specific antibody into the cytoplasm. As shown in Fig. 3, the microinjection of anti-survivin antibody caused various morphologically defective spindles, including spindles without well organized poles, spindles with numerous microtubules emanating from the multipoles and compressed spindle microtubules. The rate of abnormal spindles generated in the anti-survivin-injected group was 80.06 ± 0.94% (n = 176), which was significantly higher than that in the IgG injection group (30.19 ± 2.38%, n = 164) p < 0.05.

Figure 3 Disruption of cytoplasmic survivin leads to abnormal spindles at metaphase I (MI) stage. MI spindle morphology after microinjection of rabbit IgG and survivin antibody in rat oocytes. In the rabbit IgG injection group, most oocytes showed normal spindle morphology (a); while in the antibody injection group, most oocytes showed spindles with numerous microtubules emanating from the multipoles (b, arrow) or spindles without well-organized poles and compressed spindle microtubules (c, arrows). Bar = 10 μm. This figure is available in colour online.
Repression of survivin not only induced abnormal spindles but also affected chromosome alignment. Confocal images revealed that chromosomes did not align normally on the equatorial plate at MI stage in the survivin-repressed oocytes (Fig. 4). The oocytes often displayed lagging or scattered chromosomes. We chose 6 h after GV when most oocytes reached MI stage to examine the status of chromosome alignment in the antibody-injected oocytes. After injection at GV stage, the oocytes were cultured to the time point corresponding to 6 h after GV. Misaligned chromosomes occurred with a higher percentage (81.24 ± 1.12%, n = 164) in the anti-survivin group compared to the IgG injection group (23.18 ± 1.63%, n = 176). Thus, the repression of survivin significantly increased the incidence of misaligned chromosomes.
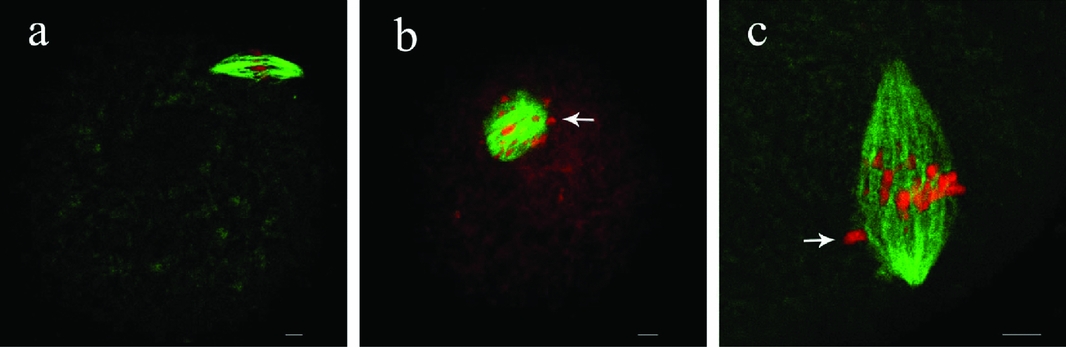
Figure 4 Chromosome alignments induced by the inhibition of survivin at metaphase I (MI) stage. MI homologous chromosomes alignment after microinjection of rabbit IgG and survivin antibody in rat oocytes. In the rabbit IgG injection group, most oocytes displayed normal chromosome alignment (a); while in the antibody injection group, most oocytes showed misaligned chromosomes scattering along the entire spindle (b, arrow) or chromosome alignment disruption with one or more lagging chromosomes (c, arrow). Bar = 10 μm. This figure is available in colour online.
Anti-survivin antibody microinjection induces earlier first polar body emission
In order to investigate the effects of survivin function during the whole meiotic process, here we microinjected specific anti-survivin antibody (Sigma) at GV stage to block its function in rat oocytes. We continuously examined the ratio of the first polar body extrusion (PBE) at every 1 h after antibody microinjection (Fig. 5). The ratio of the PBE in the IgG injection group was not significantly different from the control. Thus, the data showed that injection procedure itself did not induce the influence on the process of oocyte maturation. When the oocytes were cultured to 9 h after GV stage (GV9h), we found that 60 ± 18.4% of oocytes extruded the first polar body in the survivin-antibody-injection group, whereas in the IgG injection group, the ratio was only 5.7 ± 2.8% (p < 0.05). This result suggests that inhibiting survivin during meiosis leads to earlier emission of the first polar body.

Figure 5 Effect of survivin antibody injection on polar body extrusion (PBE). Oocytes were injected with anti-survivin antibody at the GV stage and then cultured for different times. Percentages of oocytes with PBE are shown by mean ± SEM in the anti-survivin antibody injection group, the IgG injection group and the control group. Different superscripts indicate statistical difference (p < 0.05). The same superscripts indicate that there was no statistical difference (p > 0.05). This figure is available in colour online.
Discussion
Oocytes undergo structural and physiological changes during their meiotic maturation. There are different activities being adjusted and controlled punctually at precise space throughout the meiotic maturation. The maturation of mammalian oocytes is a complex and significant process before fertilization. Healthy oocytes regain the capability of meiosis just when liberated from ovarian follicles. Then, the oocytes undergo GV, GVBD, pro-MI, MI, AI, TI stages, and sequentially first polar body (PB1) emission. Finally, oocytes are arrested at MII stage until fertilization.
It is essential for oocytes to preserve the genetic integrity and fidelity for the survival of species before forming a zygote. During oocyte maturation, the accuracy of chromosome segregation prevents aneuploidy, which largely results in tumourigenesis and congenital defects. The attachment of microtubules to kinetochores adjusts the chromosome segregation, which is monitored by a supervision mechanism known as chromosomal passenger complex (CPC) (Ruchaud et al., Reference Ruchaud, Carmena and Earnshaw2007). In mitosis, the activation of CPC plays important roles in these events: (1) it destabilizes improper chromosome-spindle attachments thereby promoting biorientation; (2) regulates the chromosome condensation, sister chromatid cohesion, spindle assembly; and (3) controls the completion of cytokinesis (Adams et al., Reference Adams, Carmena and Earnshaw2001; Lens et al., Reference Lens, Vader and Medema2006). To properly execute the function of CPC, the CPC complex needs the wholeness of its components, which include the survivin protein.
The survivin expression is limited to the embryonic and fetal tissues, even adult tissues, for example, testis, haematopoietic cells and a variety of tumours. All of these are characterized by high levels of cellular proliferation (Ambrosini et al., Reference Ambrosini, Adida and Altieri1997; Kobayashi et al., Reference Kobayashi, Hatano, Otaki, Ogasawara and Tokuhisa1999; Fukuda & Pelus, Reference Fukuda and Pelus2001; Tanaka et al., Reference Tanaka, Iwamoto, Gon, Nohara, Iwamoto and Tanigawa2000), which prompted us to investigate the temporospatial distribution and regulation of survivin during the oocyte maturation.
We observed the distribution of survivin in rat oocytes at various stages by confocal microscopy. The cytoplasmic distribution profile during the meiotic division in rat oocytes clearly indicates a major role of survivin during oocyte maturation. In our study, we blocked the function of survivin by microinjecting anti-survivin specific antibody into rat oocytes at GV stage, and found that the knockdown of survivin, strikingly, did lead to defective MI chromosome alignment, spindle assembly and a higher percentage of oocytes with precocious first polar body emission compared with the control group. Thus we speculate that survivin may be involved in regulating several significant events and controls multiple steps of meiotic maturation of rat oocytes.
The specific cellular roles of survivin are less clear. Aurora-B, the same as survivin, is a subunit of CPC (Sun et al., Reference Sun, Wei, Li, Lin, Xu, Liang, Kim, Schatten, Lu and Sun2009). The inhibition of aurora-B in rat spermatocytes causes defects in chromosome alignment and spindle assembly (Wang et al., Reference Wang, Toppari, Parvinen and Kallio2006). In the present study, we blocked the function of survivin and got the same phenotypes. Chromosome dynamics during meiosis are complicated. At anaphase I, the resolution of cohesion at chiasmata allows the separation of homologous chromosomes. This release requires the CPC (Kaitna et al., Reference Kaitna, Pasierbek, Jantsch, Loidl and Glotzer2002; Rogers et al., Reference Rogers, Bishop, Waddle, Schumacher and Lin2002). However, the CPC has a dual role in the protection of meiotic centromeric cohesion. That is to say it preserves centromere cohesion through the onset of anaphase of meiosis, while allowing its release at MII (Ruchaud et al., Reference Ruchaud, Carmena and Earnshaw2007). Remarkably, survivin simultaneously localized to the kinetochore and chromosome arms from pro-MI to anaphase I. Until telophase I, survivin migrated to the midbody. Furthermore, in our study, depletion of survivin by microinjecting antibody causes defective homologue segregation. By analysis of connection of localization-function, survivin is most likely to participate in the CPC's function towards both chiasmata cohesion and centromere cohesion, but this needs further verification.
The CPC plays a role in detecting the kinetochore-microtubule interactions. Studies of several distinct groups prompted us to speculate that survivin may be the first supervisor to a great extent. Initially, survivin, as a sensor, perceives the tension generated from kinetochore–microtubule interactions. Ultimately, aurora-B, as an effector, executes the function of CPC (Lens & Medema, Reference Lens and Medema2003; Kotwaliwale & Biggins, Reference Kotwaliwale and Biggins2006). That is to say, when kinetochores are not under proper tension, survivin would activate aurora-B, resulting in destabilization of microtubules. On the contrary, once biorientation is correctly achieved again, survivin would no longer activate aurora-B, thereby stabilizing the kinetochore–microtubule attachments. Although the pattern of chromosome segregation is different between mitosis and meiosis I, the function of CPC in detecting attachment is conservative (Ruchaud et al., Reference Ruchaud, Carmena and Earnshaw2007). We demonstrate that oocytes lacking survivin protein enter anaphase I prematurely and cause the first polar body emission to occur earlier, in a fashion that does not allow sufficient time for CPC to play a part in rectifying the abnormal chromosome segregation. In the future, it will be critical to understand how survivin, as a subunit of CPC, plays regulatory roles in multiple steps of meiotic maturation of rat oocytes, such as the initiation of cohesion release and the detection of kinetochore-microtubule attachments.
Acknowledgements
This work was supported by the National Natural Science Foundation of People's Republic of China (No. 30770247) and the Hebei Provine Natural Science Foundation of China (No. 2007000894). We thank Yi Hou, Ying-Chun OuYang, Shi-Wen Li for their technical assistance. We also thank Sheng-Li Lin, Mo Li, Jv Yuan, Sen Li, Qing-Hua Zhang, Jing-Shan Tong, Xin Huang for their helpful discussions.