Introduction
Embryological analyses of fish species are important to achieve a detailed knowledge about their biology and systematics, particularly related to ontogenetic variation in morphology, growth, feeding, behaviour and mortality. Besides serving to identify new stocks and for fisheries management, such studies might be useful for the identification of aquatic habitats in which species are recruited (Nakatani et al., Reference Nakatani, Agostinho, Baumgartner, Bialetzki, Sanches, Makrakis and Pavanelli2001).
An understanding of the ontogeny of species is also favourable to the development of biotechnological processes for environmental monitoring by evaluating the effects of toxic substances over the ontogenetic pattern in aquatic fauna. Moreover, embryonic studies can assist fish culture practices and provide insights on evolution, heredity and the development of structural traits in organisms (Lagler, Reference Lagler1959; Flores et al., Reference Flores, Araiza and Valle2002; Botero et al., Reference Botero, Fresneda, Montoya and Ángel2004; Ninhaus-Silveira et al., Reference Ninhaus-Silveira, Foresti and Azevedo2006; Marques et al., Reference Marques, Nakaghi, Faustino, Ganeco and Senhorini2008).
Compared with the high number of native fish in Brazil, little information is known about their reproductive biology and most studies have focussed on commercially important species for fisheries and aquaculture. Reports on embryogenesis in Characiformes species can cite Brycon cephalus (Alexandre et al., Reference Alexandre, Ninhaus-Silveira, Verissimo-Silveira, Buzollo, Senhorini and Chaguri2010), B. insignis (Andrade-Talmelli et al., Reference Andrade-Talmelli, Kavamoto, Romagosa and Fenerich-Verani2001), B. orbignyanus (Ganeco et al., Reference Ganeco, Franceschini-Vicentini and Nakaghi2008), Leporinus piau (Borçato et al., Reference Borçato, Bazzoli and Sato2004), Prochilodus lineatus (Ninhaus-Silveira et al., Reference Ninhaus-Silveira, Foresti and Azevedo2006) and B. amazonicus (Nakaghi et al., Reference Nakaghi, Neumann, Faustino, Mendes and Braga2013). Amongst Siluriformes, embryonic traits are known for Rhamdia hilarii (Godinho et al., Reference Godinho, Fenerich and Narahara1978), R. sapo (Cussac et al., Reference Cussac, Matkovic and Maggese1985), Pseudoplatystoma corruscans (Cardoso et al., Reference Cardoso, Alves, Ferreira and Godinho1995), Parauchenipterus galeatus (Sanches et al., Reference Sanches, Nakatani and Bialetzki1999), Pimelodus maculatus (Luz et al., Reference Luz, Reynalte-Tataje, Ferreira and Zaniboni-Filho2001; Buzzolo et al., Reference Buzzolo, Veríssimo-Silveira, Oliveira-Almeida, Alexandre, Okuda and Ninhaus-Silveira2011), Rhinelepis aspera (Perini et al., Reference Perini, Sato, Rizzo and Bazzoli2009), and R. quelen (Amorim et al., Reference Amorim, Gomes, Martins, Sato, Rizzo and Bazzoli2009; Rodrigues-Galdino et al., Reference Rodrigues-Galdino, Maiolino, Forgati, Donatti, Mikos, Carneiro and Rios2009).
The species Leiarius marmoratus is widespread in South American basins (Amazonas, Essequibo & Orinoco). This catfish may reach about 100 cm in length, and is characterized by 9–10 rays in its dorsal fins and dark patches over a yellowish-brown background along the body and fins (http://www.fishbase.org; Reis et al., Reference Reis, Kullander and Ferraris-Jr2003). According to Ramírez-Gil & Ajiaco-Martinez (Reference Ramírez-Gil and Ajiaco-Martinez1997), this fish reaches a mean weight of 12 kg, being found in deep freshwater environments with a pH range of 5.8–7.2 and a mean temperature of 24–26°C. In its natural habitat, this fish presents a piscivorous diet (Layman et al., Reference Layman, Winemiller, Arrington and Jepsen2005).
Since 1986, this catfish has been bred artificially via hormonal induction (Kossowski, Reference Kossowski1996). According to Cruz-Casallas et al. (Reference Cruz-Casallas, Díaz-Olarte, Marciales-Caro, Pabón-Penã, Medina-Robles and Curz-Casalhas2008), L. marmoratus is easily adapted to captivity and artificial feeding using dry food, yielding high growth rates during early life stages in preliminary studies. Usually, this species has been selected to produce hybrids in crosses with P. reticulatum and Pimelodus blochii (Kossowski & Madrid, Reference Kossowski and Madrid1985, Reference Kossowski and Madrid1991; Kossowski, Reference Kossowski1991, Reference Kossowski1992, Reference Kossowski1996a,Reference Kossowskib).
Even though this species has been routinely raised in captivity, information about its reproductive biology in specialized literature is scarce. Therefore, the goal of the present study was to characterize the morphological events that take place during embryogenesis of L. marmoratus using light and electron microscopy.
Materials and methods
The work was carried out during the spawning season of L. marmoratus (Fig. 1A) from January to February 2010. To obtain the embryos, fish specimens were selected from the broodstock available in National Centre for Research and Conservation of Continental Fish, Chico Mendes Institute for Biodiversity Conservation–CEPTA/ICMBio, Pirassununga, São Paulo, Brazil and Muriti Fishculture Farm, Nova Mutum, Mato Grosso, Brazil.

Figure 1 (A) Leiarius marmoratus; (B) hydrated egg. Asterisk – perivitelline space. Bar – 100 μm.
Analysis of embryonic development
To analyze the temporal–morphological changes in embryos of L. marmoratus, random samples of nearly 200 eggs were collected at different times of embryonary development, considering the moment of fecundation as time zero. The eggs were incubated at 28.3 ± 0.07°C, and the collection of samples occurred at 5 min during the first h of development and each 10 min up to the first 2 h of embryogenesis. Subsequent samplings were performed in 1-h intervals up to larval hatching. The collected embryos were divided into two fractions: one was fixed in a solution of 2% glutaraldehyde and 4% paraformaldehyde in sodium phosphate buffer 0.1 M, pH 7.3 for 24 h. Afterwards, the material was transferred into 70% ethanol (light microscopy). The second parcel was fixed and stored in 2.5% glutaraldehyde solution diluted in sodium phosphate buffer 0.1 M, pH 7.3 (scanning electron microscopy).
The prefixed material was then transported to the Neotropical Ichthyology Laboratory (LINEO) – Departament of the Biology and Zootecny, UNESP/FE, Ilha Solteira, São Paulo. Brazil.
Analysis using light microscopy
Fifty embryos of each sample were selected for in toto analyses. The chorion was removed using watchmaker's forceps and a needle, stained with Harris haematoxylin–eosin (HE), analyzed and photographed in a stereomicroscope Motic SMZ 168, equipped with a digital camera Moticam 2500/5.0 Mega Pixels USB 2.0.
For histological studies, the selected embryos were embedded in glycol methacrylate (Technovit 7199/historesin) and microtomed into serial transversal and sagittal cuts of 3 and 2 μm. The cuts were placed onto histological slides and then stained with Harris haematoxylin–eosin. Then the histological slides were analyzed using a light microscope (Olympus–CX41) and microphotographed (Moticam 2500–5.0 MPixel USB 2.0).
Analysis by scanning electron microscopy (SEM)
For SEM, the prefixed embryos in 2.5% glutaraldehyde were post-fixed in osmium tetroxide, dehydrated in critical-point Balzers, metalized in a Balzers Metalizer (MED-010 Balzers Union), analysed and electromicrographed using a scanning electron microscope (SEM Quanta 200 - FEI).
Ethics statement
The methodological procedures used for the development of this work is in accordance with the Ethical Principles of Animal Experimentation adopted by the Brazilian College of Animal Experimentation (COBEA) and was approved by the Ethics Committee on Animal Use (CEUA), Faculty of Engineering, UNESP/Ilha Solteira, São Paulo, Brazil (Protocol no. 007/2011/CEUA).
Results
The eggs of L. marmoratus are spherical, yellowish and demersal with a narrow perivitelline space and a well defined transparent chorion after hydration. Fat droplets in the yolk sac were absent during the entire embryonic development. No gelatinous layer was detected surrounding the eggs of L. marmoratus after fertilization (Fig. 1B). After hydration the perivitelline space and the diameter of the eggs showed about 74.51 ± 20.40 μm and 930.88 ± 27.01 μm, respectively.
Embryogenesis
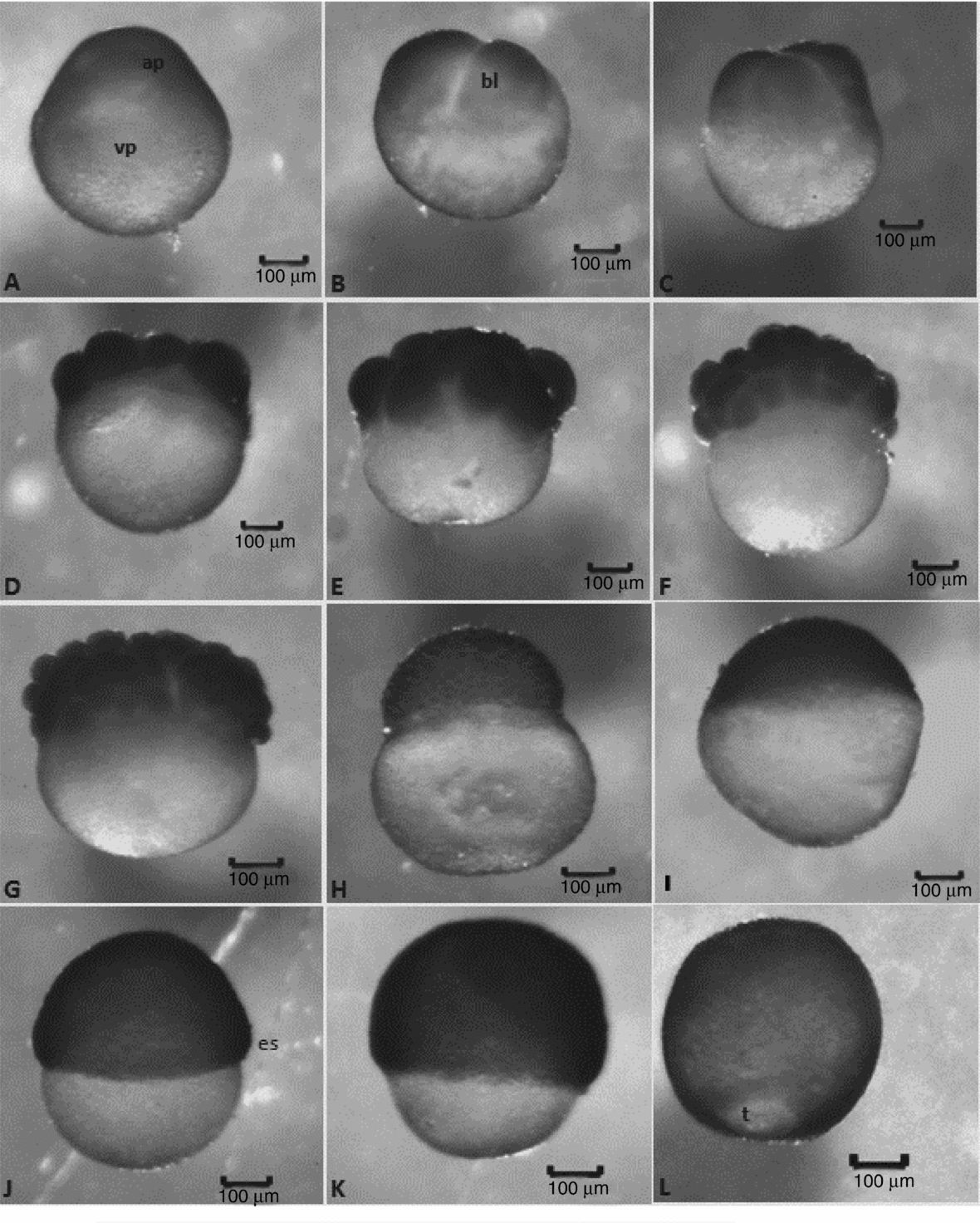
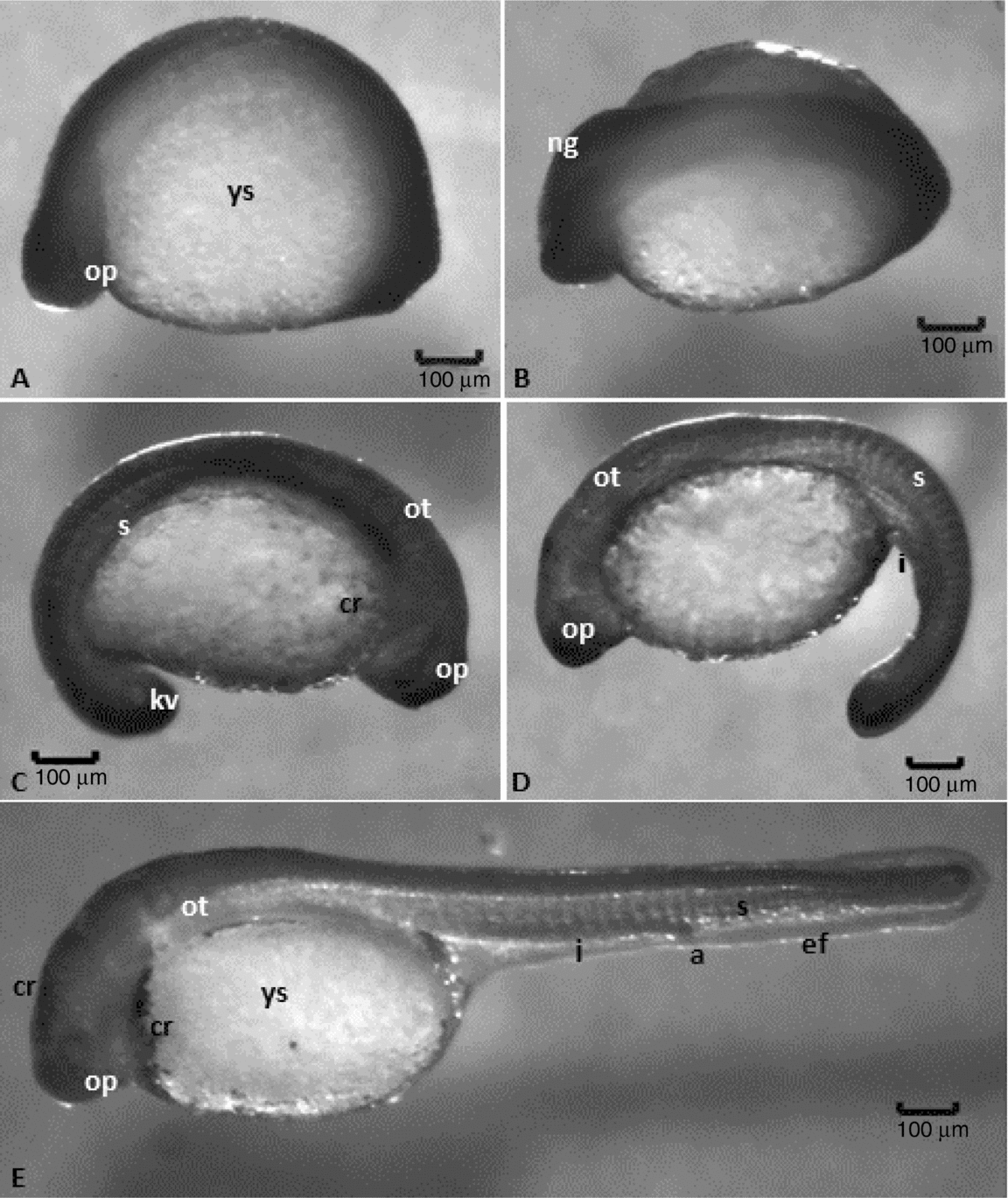
The incubation period of L. marmoratus comprised 14 h at a mean temperature of 28.3°C. The embryonic stages observed were: (i) zygote; (ii) cleavage: 2-cell, 4-cell, 8-cell, 16-cell, 32-cell, 64-cell and morula; (iii) gastrula: epiboly (morphogenetic movement in which the blastoderm covers the yolk vesicle) at 25, 50, 75 or 90% and blastopore closure; (iv) organogenesis: neurula, segmentation and pre-larval phases; and (v) hatching (Figs. 2 and 3).

Figure 2 Embryonic development stages of Leiarius marmoratus. Zygote stage: (A) 1-cell embryo (post-fertilization without chorion). Cleavage stage: (B) 2-cell embryos; (C) 4-cell embryos; (D) 8-cell embryos; (E) 16-cell embryos; (F) 32-cell embryos; (G) 64-cell embryos; (H) morula. Gastrula stage: (I) 25% of epiboly; (J) 50% of epiboly; (K) 75% of epiboly; (L) 90% of epiboly. Staining: haematoxylin–eosin (HE). ap, animal pole; bl, blastomere; t, blastopore closure; vp, vegetal pole.

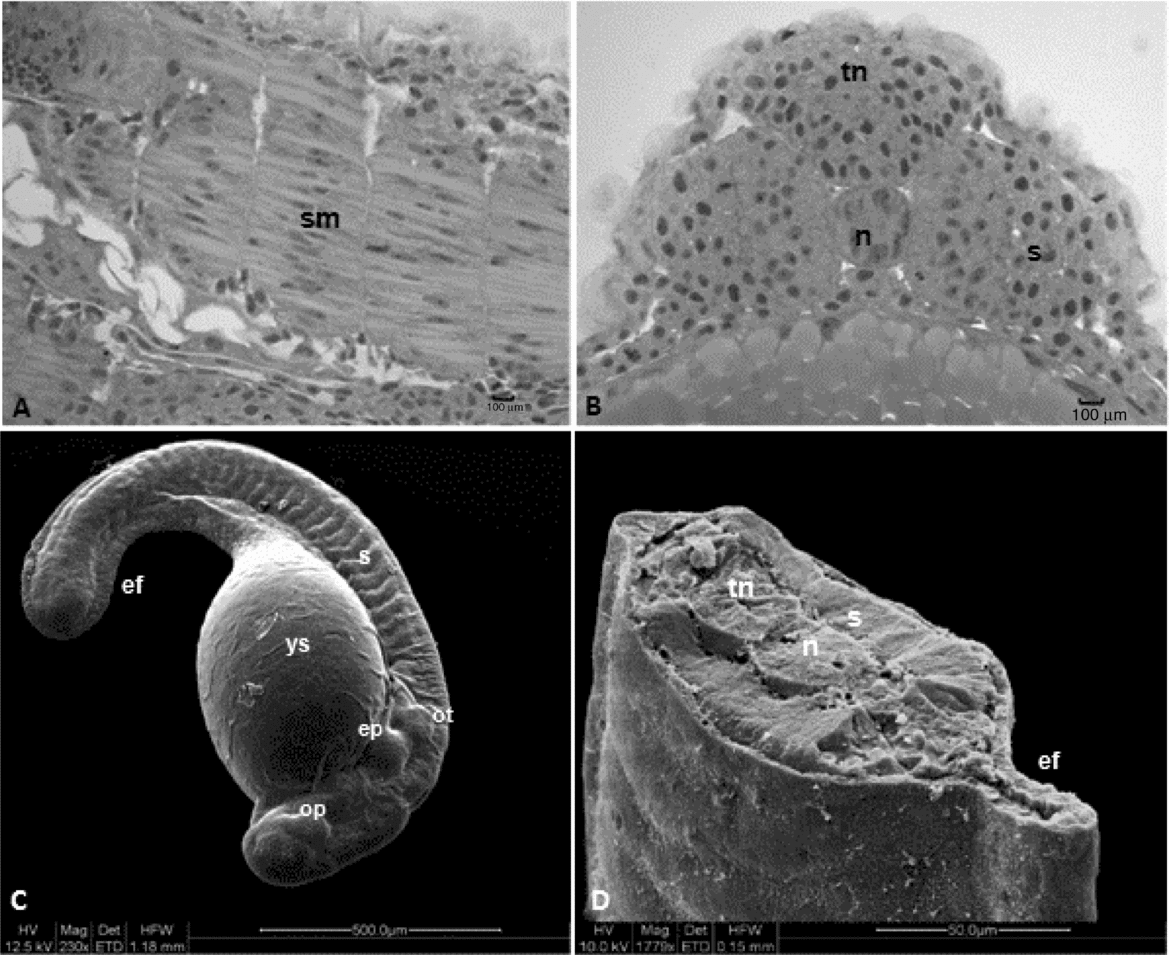
Figure 3 Embryonic development stages of Leiarius marmoratus. Organogenesis stage: (A, B) neurula; (C) embryo with about 15 somites, optic vesicle, attached tail and pigmentation of body; (D) 24 somites, free tail, rudiments of the posterior intestine. Hatching stage: (E) 30 somites, posterior intestine well developed, anal pore delimited. Staining: haematoxylin–eosin (HE). a, anal pore; cr, chromatophore; ef, embryonary fin; I, intestine; kv, Kupffer's vesicle; ng, neural groove; op, optic vesicle; ot, otic vesicle; s, somites; ys, yolk sac.
Some degree of heterogeneity was observed in embryo development, i.e., at the same moment, embryos in different stages or phases of embryogenesis were detected.
Zygote stage
After fertilization and egg hydration, the perivitelline space increased and animal and vegetal poles were defined. The animal pole is composed of active cytoplasm and a nucleus, being identified in vivo as a more transparent area, while the vegetal pole is denser in vivo and encompasses the yolk vesicles. As animal and vegetal poles were clearly distinguished and a large amount of yolk was present, the eggs were classified as telolecithal. For the first 30 min of incubation, all embryos observed were in the zygote stage (Fig. 2A).
Cleavage stage
After 40 min incubation the first cell divisions were observed. The cleavage type was meroblastic or incomplete, according to the following pattern: the first cleavage was vertical, giving rise to two blastomeres of similar size; the second one was vertical and perpendicular to the first division, forming four blastomeres, the third cleavage was vertical and parallel to the first one, giving rise to eight blastomeres in a 4 × 2 arrangement; the forth cleavage was vertical and parallel to the second one, producing 16 blastomeres in a 4 × 4 formation; the fifth cleavage was vertical and parallel to the first one, giving rise to 32 blastomeres in a 4 × 8 formation; the sixth cleavage was horizontal, producing two cell layers with 64 blastomeres in total. During the morula phase (>100 cells), the cells were arranged in several layers, composing a ‘half-berry’-shaped cell mass (Fig. 2B–H). Although most embryos followed the above mentioned cleavage pattern, odd cleavage patterns giving rise to 6, 13 or 15 blastomeres were sometimes observed. Up to fifth cleavage the blastomeres exhibited incomplete division, once the cleavage groove was unable to cope with the large amount and high density of yolk.
At this stage, the cells (blastomeres) increased in number but their size decreased, displaying a more homogeneous morphology up to the fifth cleavage (Fig. 2A–E). From the sixth cleavage onwards, the division was horizontal, giving rise to blastomeres of different sizes and complete cleavage (Fig. 2G). In this period, embryos with detached blastomeres and deformities in the yolk vesicle were observed as well as a high number of deformed and unviable eggs.
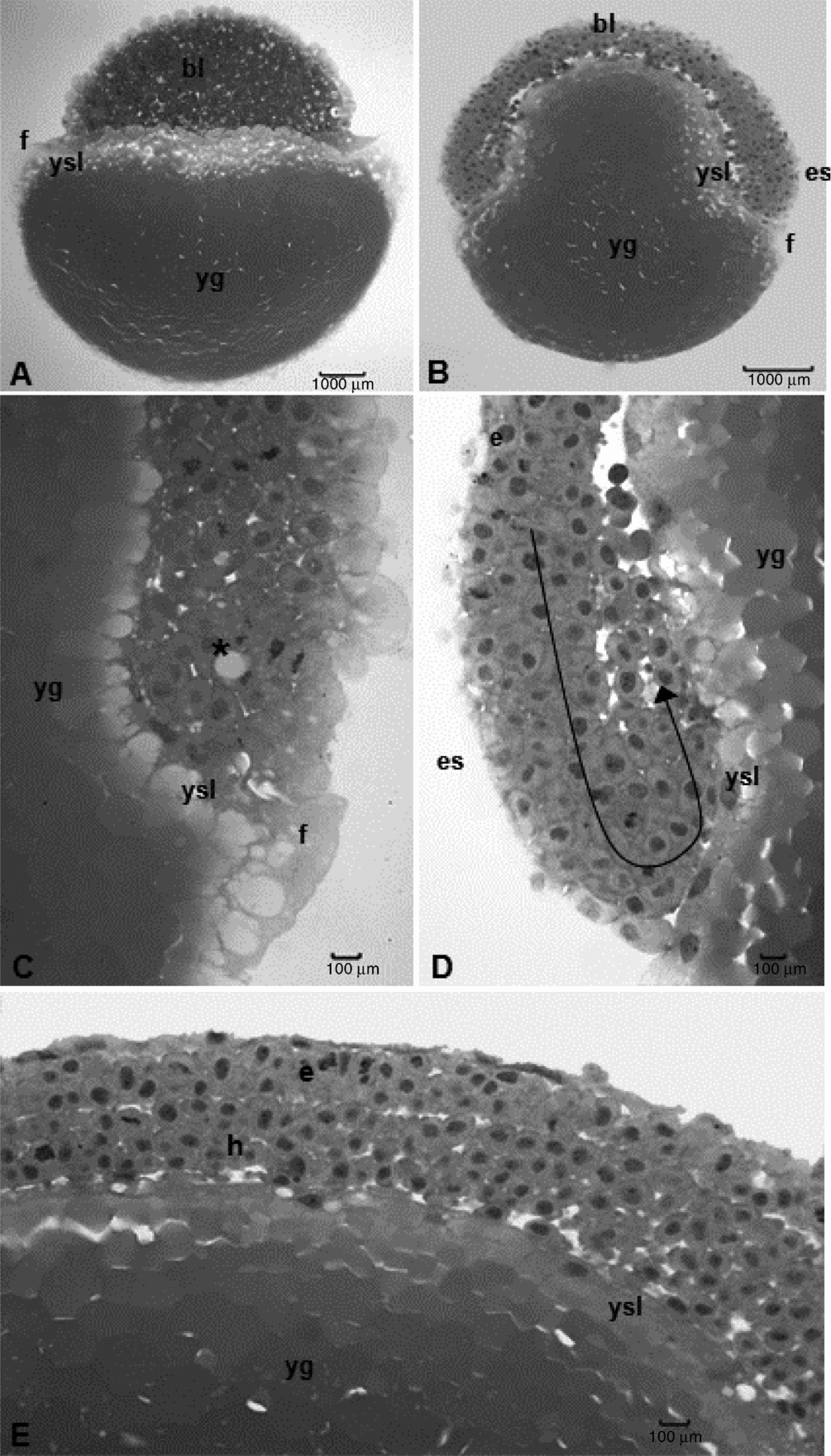
Up to the 64 cell phase, histological analyses showed no distinguishable layer between blastoderm and yolk as the yolk globules penetrated into blastomeres in a scattered way. In addition, individualized nuclei were absent in blastomeres. The first nuclei and nuclear divisions could be visualized from the morula phase onwards, as well as the beginning of the formation of the yolk syncytial layer or periblast (Fig. 4A). There was no formation of a blastocele but of a more compact blastoderm that, at the end of cleavage stage, assumed a flattened half-berry shape that covered one pole of the yolk sac (Figs. 2H and 4A). This stage ended after 2–3 h of incubation.

Figure 4 Histological sections of Leiarius marmoratus embryos. (A) Cleavage stage, morula phase. (B–E) Gastrula stage, 50% epiboly phase. Staining: haematoxylin–eosin (HE). bl: blastomeres; e, epiblast; es, embryonic shield; f, fringe; h, hypoblast; yg: yolk globules; ysl, yolk syncytial layer. Arrow – morphogenetic movement of involution. Asterisk: penetration of yolk globules in the embryo.
Gastrula stage
This stage was characterized by the beginning of morphogenetic movements (epiboly) in which the blastoderm cells undergo rapid mitotic divisions. As a result, the surface cells became flattened while the intern cells intercalated with external cells (intercalation) thereby extending blastoderm up to the full covering of the yolk vesicle (Fig. 2I–L).
Morphogenetic movements were observed from the third hour of embryogenesis; in the fourth hour of incubation the L. marmoratus embryos reached the 50% epiboly stage with half of the yolk sac covered (Fig. 2J). Simultaneously, cell involution started inasmuch as the expanding layer folded to form a second layer, which extended towards an opposite direction in relation to the first one, determining the thickening of blastoderm border, called germ ring. Cells accumulated perpendicularly to the germ ring, forming the embryonic shield. These morphogenetic movements then determined the formation of epiblast and hypoblast as well as convergence, directing the cell layers in the embryonic shield to establish the head–tail and dorsal–ventral axes (Fig. 4B–E).
At 5 h, all embryos presented 75% of yolk covered by blastoderm (Fig. 2K). At the 6 h, epiboly in embryos reached 90%, with just a small yolk portion still exposed: the yolk plug or blastopore (Fig. 2L). Light microscope analyses revealed the periblast had formed a fringe along the blastoderm border since its formation up to the blastopore closure (Fig. 4C). Periblast was characterized by a cytoplasmatic layer with several nuclei with patched yolk globules in cytoplasm and no membrane among them (Fig. 4C–E).
Even though most embryo cells presented a single nucleolus, several cells with two nucleoli were observed. The gastrula stage ended between the 6–7 h of embryonic development, when the whole yolk vesicle became covered by embryonic cells and the blastopore was closed by the periblast.
Organogenesis stage
This stage started after 7–8 h of incubation and was typified by the origin of rudimentary organs and systems from embryonic layers. Somites, notochord, neural tube, optic and otic vesicles, encephalon and early delimitation of intestines were observed as the embryos grew and elongated, mainly through the head–tail axis (Figs. 5 and 6). After 7 h of embryogenesis, all embryos reached the neurula phase with differentiation between cephalic and caudal regions and neural keel (Fig. 3B). At this point, the thickening of dorsal epiblast determined the formation of neural keel that later fused to give rise to the neural plate. The notochord was composed of elongated and aligned cells (Fig. 5D, E), placed underneath the neural plate. The mesendoderm that will produce the somites (segmentation phase) after segmentation was visualized to be lateral to the notochord (Figs. 3A–E and 6B–D).

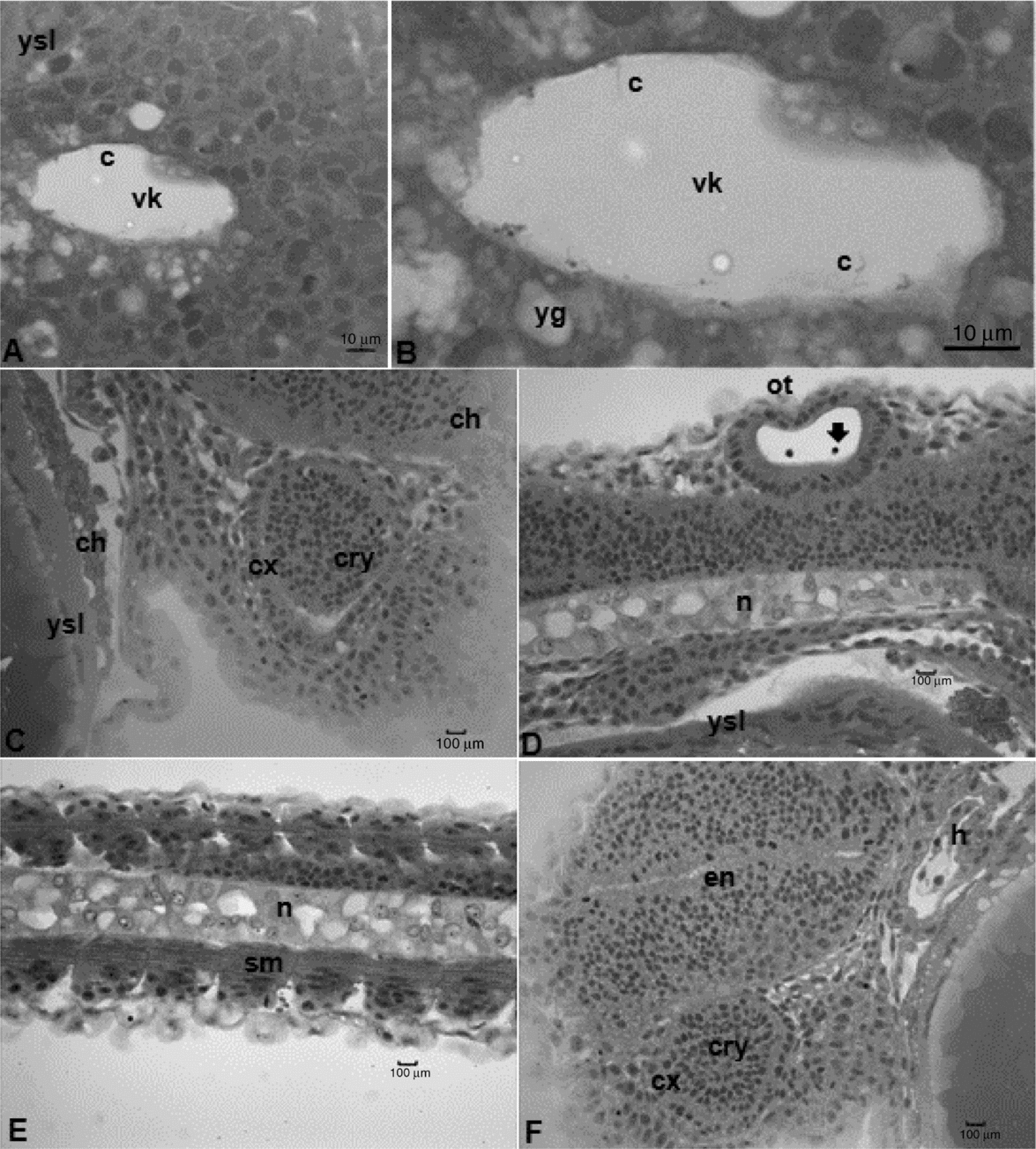
Figure 5 Histological sections of Leiarius marmoratus embryos in organogenesis stage. (A) Neurula phase. (B, C) Segmentation phase. (D, E) Hatching stage. (F) Staining: haematoxylin–eosin (HE). c, cilium; ch, chromatophore; cry, crystalline; cx, optical calyx; en, cephalic vesicle; h, heart; n, notochord; op, optic vesicle; ot, otic vesicle; sm, somites in myogenesis; vk, Kupffer's vesicles; yg, yolk globules; ys, yolk sac; ysl, yolk syncytial layer. Arrow – otolithes.

Figure 6 Structural and ultrastructural analysis of Leiarius marmoratus embryos in organogenesis stage, segmentation phase. (A, B) Histological sections staining with Harris haematoxylin–eosin. (C, D) Scanning electron microscopy. ef, embryonary fin; n, notochord; op, optic vesicle; ot, otic vesicle; s, somites; sm, somites in myogenesis; tn, neural tube; ys, yolk sac; ysl, yolk syncytial layer.
The differential growth of neural tube portions gave rise to the encephalic region, divided into three regions: prosencephalon, mesencephalon and rhombencephalon. After 8 h of development, the embryos present the optic vesicle and segmentation of the first somites took place. At 9 h, it was possible to visualize the Kupffer's vesicle, an egg-shaped structure at the tail region, composed of a ciliated cubic cell layer and a central lumen (Figs. 3C and 5A, B). The otic vesicle could also be defined at this period, being located between the optic vesicle and the first somite and characterized by a layer of elongated cells organized in parallel with nuclei in the basal region. Two otolithes were observed within the otic vesicle (Figs. 3C–E and 5D). Kupffer's vesicle could be no longer visualized after 10 h of embryogenesis as the first pigmented cells (chromatophores) appeared throughout the yolk sac membrane, near the cardiac region (Figs. 3C, E and 5C).
The pre-larval phase was detected at 11 h of incubation once embryos presented a shape similar to recently hatched larvae, which include: evident tail detached from the yolk sac, absence of Kupffer's vesicle, notochord visible from head to tail (Figs. 5D and 6B) and a well defined rudimentary intestine. Somites in this phase undergo myogenesis, leading to differentiation of myoblasts with spherical and large nuclei and myomeres with flattened nuclei (Figs. 5E and 6A).
Based on SEM analysis of L. marmoratus embryos this structure was located under the otic vesicle and comprised two pairs of solid elevations with a depression observed between them. This structure is probably the beginning of the formation of the operculum (Fig. 6C).
Spasmodic movements were another feature of this stage, which increased as long as embryos developed. At the end of this stage, the embryos presented vigorous swimming movements that play a major role in chorion rupture.
Hatching stage
This stage started after 13–14 h of incubation and was characterized by chorion softening, followed by its rupture and presence of free-swimming larvae. The larval heads were attached to the anterior region of the yolk sac. They lacked individualized fins, but had an embryonic fin along the ventral and dorsal portions of caudal region (Figs. 3E and 7B). Light microscopy allowed the observation of a rudimentary heart, located in front of the yolk sac (Fig. 5F), and the development of an optic calyx, formed by cells with elongated nuclei and involving a cell mass from which the crystalline arises (Fig. 5C, F). At this stage, the chromatophores were concentrated in the membrane that surrounds the yolk sac, mostly located at anterior and posterior regions, but also in the head of L. marmoratus (Fig. 5C).

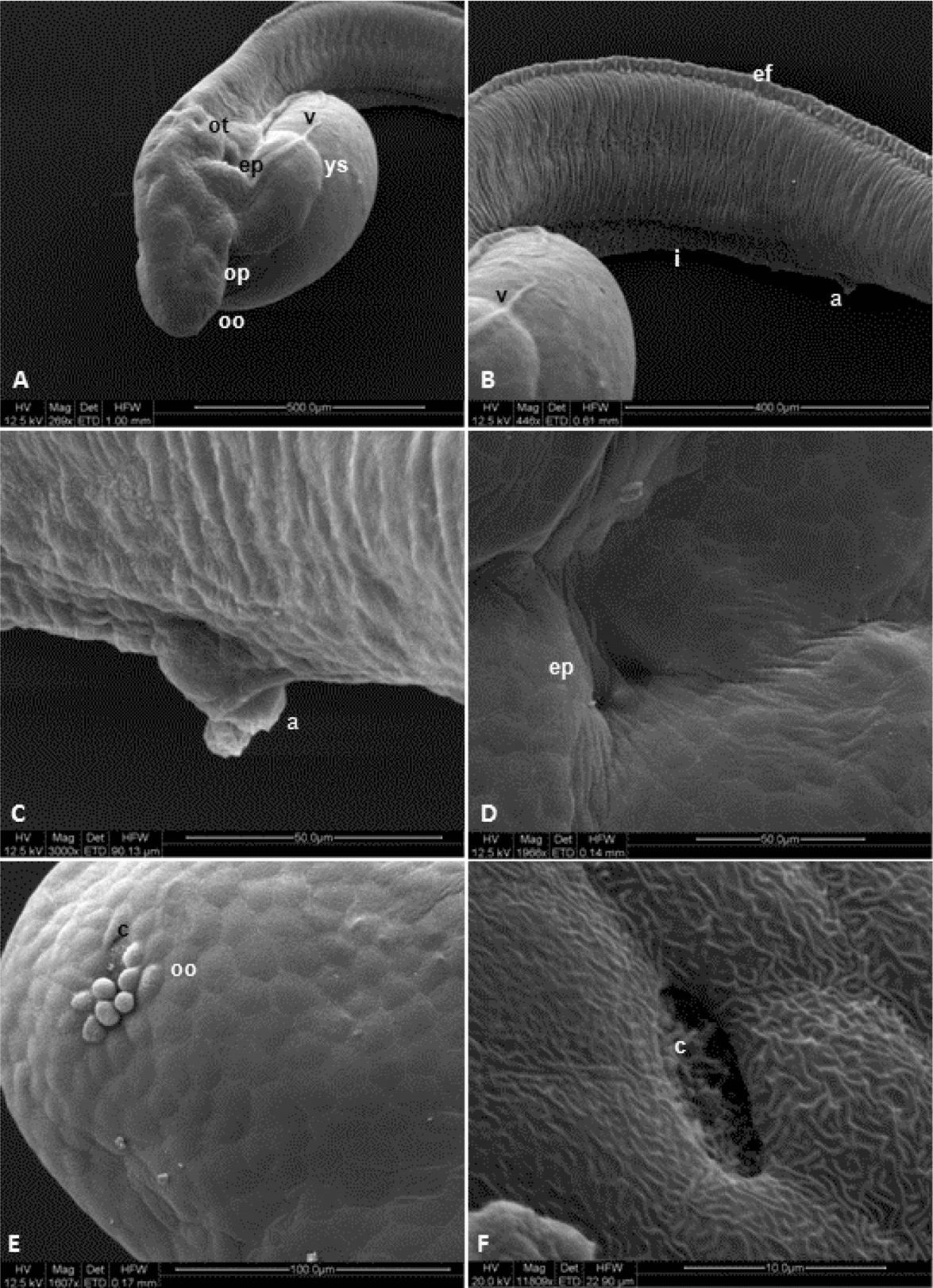
Figure 7 Ultrastructural (SEM) analysis of Leiarius marmoratus embryos in hatching stage. a, anal pore; c, olfactory cilium; ep, non-identified structure; ef, embryonary fin; i, intestine; oo, olfactory organ; op, optic vesicle; ot, otic vesicle; v, yolk veins; ys, yolk sac.
Differentiation of notochord cells were also observed, changing from elongated and aligned cells into egg-shaped cells with several clear areas among them. Analysis by SEM revealed the development of olfactory organ in the form of a small circular depression with cilium-like structures in the inner portion (Fig. 7E, F).
Based on SEM analysis of L. marmoratus embryos, yolk veins were observed at the dorsal region of the yolk sac (Fig. 7A, B). Furthermore, the structures identified as possibly forming the operculum were more prominent, between both the cellular clumps as a depression (Fig. 7A, D). Hatching of L. marmoratus larvae occurred after 14 h of development, thus completing the embryonic cycle.
Discussion
Fish eggs are classified as macrolecithal due to their large amount of yolk, and telolecithal as the yolk is located in the vegetative pole, and the cytoplasm and organelles are at the animal pole (Ganeco et al., Reference Ganeco, Franceschini-Vicentini and Nakaghi2008; Marques et al., Reference Marques, Nakaghi, Faustino, Ganeco and Senhorini2008). These characteristics have been reported for all species of neotropical fishes studied, including Prochilodus lineatus (Ninhaus-Silveira et al., Reference Ninhaus-Silveira, Foresti and Azevedo2006), P. corruscans × P. reticulatum hybrids (Faustino et al., Reference Faustino, Nakaghi, Marques, Makino and Senhorini2007), P. corruscans (Marques et al., Reference Marques, Nakaghi, Faustino, Ganeco and Senhorini2008), Zungaro jahu (Nogueira et al., Reference Nogueira, Azevedo, Canelhas, Bedore, Lopes and Godinho2012), Rhinelepis aspera (Perini et al., Reference Perini, Sato, Rizzo and Bazzoli2009), B. cephalus (Alexandre et al., Reference Alexandre, Ninhaus-Silveira, Verissimo-Silveira, Buzollo, Senhorini and Chaguri2010) and the present study with L. marmoratus.
Rheophilic species such as Prochilodus lineatus (Ninhaus-Silveira et al., Reference Ninhaus-Silveira, Foresti and Azevedo2006), B. orbignyanus (Ganeco et al., Reference Ganeco, Franceschini-Vicentini and Nakaghi2008), Piaractus mesopotamicus and Colossoma macropomum (Ribeiro et al., Reference Ribeiro, Leme dos Santos and Bolzan1995) produce eggs with a large perivitelline space after hydration that protects embryos against injury during embryogenesis, favouring their survival in fast-flowing waters. Conversely, the perivitelline space in eggs of R. hilarii is reduced (Godinho et al., Reference Godinho, Fenerich and Narahara1978), in a similar manner to the pattern observed in hydrated eggs of L. marmoratus. Such differences in the perivitelline space are possibly related to the reproductive strategies of these species and the habitat in which the eggs develop, such as the bottom of rivers in the case of L. marmoratus.
The presence of a gelatinous hyaline layer surrounding the eggs has been observed in Steindachneridion parahybae (Honji et al., Reference Honji, Tolussi, Mello, Caneppele and Moreira2012), hybrids between P. corruscans and P. reticulatum (Faustino et al., Reference Faustino, Nakaghi, Marques, Makino and Senhorini2007), Rhinelepis aspera (Perini et al., Reference Perini, Sato, Rizzo and Bazzoli2009) and Pimelodus maculatus (Buzzolo et al., Reference Buzzolo, Veríssimo-Silveira, Oliveira-Almeida, Alexandre, Okuda and Ninhaus-Silveira2011). According to Rizzo et al. (Reference Rizzo, Sato, Barreto and Godinho2002), this gelatinous layer is typical of Siluriformes, being composed of coiled delicate fibrils. Nonetheless, this structure was absent on the chorion of the presently studied species.
In bony fishes, fat droplets may be present in the yolk mass in distinct number and size according to each fish family, as reported in the freshwater B. cephalus (Lopes et al., Reference Lopes, Senhorini and Soares1995) and marine Abudefduf sexfasciatus (Shadrim & Emel’yanova, Reference Shadrim and Emel’yanova2007). However, no fat droplets were detectable in the yolk of L. marmoratus, following the pattern observed in Prochilodus lineatus (Ninhaus-Silveira et al., Reference Ninhaus-Silveira, Foresti and Azevedo2006), B. orbignyanus (Ganeco et al., Reference Ganeco, Franceschini-Vicentini and Nakaghi2008) and B. insignis (Andrade-Talmelli et al., Reference Andrade-Talmelli, Kavamoto, Romagosa and Fenerich-Verani2001).
Brummett & Dumont (Reference Brummett and Dumont1981) observed that, right after the rupture of cortical alveoli, a protoplasmatic cover accumulates at the animal pole, thus forming the blastodisc, in which the zygote is cleaved into blastomeres. According to Faustino et al. (Reference Faustino, Nakaghi, Marques, Ganeco and Makino2010a), a cytoplasm movement has taken place after the cortical reaction in eggs of hybrids between P. corruscans and P. reticulatum, determining the differentiation of both animal and vegetative poles and the beginning of cleavage – changes that are also observed in L. marmoratus embryos.
The stages observed through the embryonic development of the species analysed in this study (zygote, cleavage, gastrula, organogenesis and hatching) are similar to those reported in P. corruscans × P. reticulatum hybrids (Faustino et al., Reference Faustino, Nakaghi, Marques, Makino and Senhorini2007; Shadrim & Emel’yanova, Reference Shadrim and Emel’yanova2007), P. corruscans (Marques et al., Reference Marques, Nakaghi, Faustino, Ganeco and Senhorini2008) and Zungaro jahu (Nogueira et al., Reference Nogueira, Azevedo, Canelhas, Bedore, Lopes and Godinho2012). The only exception is the blastula stage, described by these authors but not found in the present study, because blastocele formation was not observed in L. marmoratus embryogenesis.
According to Zhang et al. (Reference Zhang, Bhattacharya, Li and Jamieson2009), holoblastic cleavage results in a ball-shaped cell layer, while meroblastic cleavage forms the blastodisc. However, the features that typify the blastula stage are the formation of peripheral cells in morula and the formation of a cavity filled with liquid named the blastocele. In species with oligolecithal eggs and holoblastic cleavage, the blastocele gives rise to the blastocyst (Moore & Persaud, Reference Moore and Persaud2003), whilst in individuals with telolecithal eggs and meroblastic cleavage it originates a cavity below the blastoderm or in irregular spaces among blastoderm cells as often reported (Kimmel & Law, Reference Kimmel and Law1985; Trinkaus, Reference Trinkaus1992; Kimmel et al., Reference Kimmel and Law1995; Ninhaus-Silveira et al., Reference Ninhaus-Silveira, Foresti and Azevedo2006; Marques et al., Reference Marques, Nakaghi, Faustino, Ganeco and Senhorini2008; Ganeco et al., Reference Ganeco, Franceschini-Vicentini and Nakaghi2008). In Brachidanio rerio, irregular spaces interspersed with blastoderm cells were observed that suggested that this stage should be defined as stereoblastula as it lacked a typical blastocele (Kimmel et al., Reference Kimmel, Ballard, Kimmel, Ullmann and Schilling1995). In other species such trout (Lagler et al., Reference Lagler, Bardach, Miller and Passino1977) a typical blastocele has been reported between the blastoderm and the periblast. However, in fish such as Oreochromis niloticus (Morrison et al., Reference Morrison, Miyake and Wright2001), the hybrids between P. corruscans and P. reticulatum (Faustino et al., Reference Faustino, Nakaghi, Marques, Ganeco and Makino2010a) and the species in the present study, neither a characteristic cavity nor irregular spaces, both indicators of stereoblastula, could be identified.
The type of cleavage, meroblastic or partial, observed in L. marmoratus has been reported in most teleosteans (Lagler et al., Reference Lagler, Bardach, Miller and Passino1977; Ninhaus-Silveira et al., Reference Ninhaus-Silveira, Foresti and Azevedo2006; Ganeco et al., Reference Ganeco, Franceschini-Vicentini and Nakaghi2008; Marques et al., Reference Marques, Nakaghi, Faustino, Ganeco and Senhorini2008; Amorim et al., Reference Amorim, Gomes, Martins, Sato, Rizzo and Bazzoli2009; Perini et al., Reference Perini, Sato, Rizzo and Bazzoli2009; Alexandre et al., Reference Alexandre, Ninhaus-Silveira, Verissimo-Silveira, Buzollo, Senhorini and Chaguri2010; Faustino et al., Reference Faustino, Nakaghi and Neumann2010b; Buzzolo et al., Reference Buzzolo, Veríssimo-Silveira, Oliveira-Almeida, Alexandre, Okuda and Ninhaus-Silveira2011).
The first cleavage divided the blastodisc in two cells of similar size, as described in Xiphister atropurpureus (Wourms & Evans, Reference Wourms and Evans1974), Cynolebias (Carter & Wourms, Reference Carter and Wourms1991), Oryzias layipes (Iwamatsu, Reference Iwamatsu1994) and Brachidanio rerio (Kimmel et al., Reference Kimmel, Ballard, Kimmel, Ullmann and Schilling1995). Cleavage usually begin from the centre to the edges of the blastodisc (Matkovik et al., Reference Matkovik, Cussac, Cukier, Guerrero and Maggese1995; Shardo, Reference Shardo1995), as corroborated by the results in L. marmoratus.
In O. latipes (Iwamatsu, Reference Iwamatsu1994), blastomeres were arranged in a single layers composed of four central and 13 peripheral blastomeres when eggs reached the 16-cell stage; at the 32-cell stage, the blastomeres formed four series of eight cells each. The same result has been reported for Alosa sapidíssima (Shardo, Reference Shardo1995) and B. orbignyanus (Ganeco et al., Reference Ganeco, Franceschini-Vicentini and Nakaghi2008). Yet, most teleosteans bear four series with four cells each in this phase, forming a single cell layer (Trinkaus, Reference Trinkaus1992; Shardo, Reference Shardo1995). This 4 × 4 arrangement has been also observed in Catostomus commersoni (Long & Ballard, Reference Long and Ballard1976), Danio rerio (Kimmel et al., Reference Kimmel, Ballard, Kimmel, Ullmann and Schilling1995), Oreochromis niloticus (Morrison et al., Reference Morrison, Miyake and Wright2001) and in the present species.
Morrison et al. (Reference Morrison, Miyake and Wright2001) suggested that variation in both embryogenesis and embryo development (asynchrony and malformations) is related to temperature at incubation and age of broodstock. In Prochilodus lineatus, Ninhaus-Silveira et al. (Reference Ninhaus-Silveira, Foresti and Azevedo2006) reported that, although using young breeders (1–2 years old) and a constant water temperature, a higher asynchrony in embryonic development and an accentuated variation in blastomere division occurred during incubation at 24°C rather than at 28ºC. Kimmel et al. (Reference Kimmel, Ballard, Kimmel, Ullmann and Schilling1995) and Morrison et al. (Reference Morrison, Miyake and Wright2001) also observed that asynchrony in embryonic development might be present even in a single spawn incubated under optimal conditions. Hisaoka & Firlit (Reference Hisaoka and Firlit1960) verified that mitotic divisions in zebrafish are synchronic up to the 64-cell phase, but they become asynchronic when the embryos reach 64 cells. The results in the experiment with L. marmoratus supported these findings, indicating that genetic and environmental traits are essential to the embryonic development of bony fishes.
In Z. jahu, Nogueira et al. (Reference Nogueira, Azevedo, Canelhas, Bedore, Lopes and Godinho2012) reported that the yolk globules penetrated the blastomeres after fragmentation, in a similar manner to the observations in the presently studied species. According to Ninhaus-Silveira et al. (Reference Ninhaus-Silveira, Foresti and Azevedo2006), such fragmentation might facilitate the absorption of yolk globules by cells.
The periblast or yolk syncytial layer is an important structure to the embryonic development of teleosteans (Ninhaus-Silveira et al., Reference Ninhaus-Silveira, Foresti, de Azevedo, Agostinho and Veríssimo-Silveira2007). Kimmel et al. (Reference Kimmel, Ballard, Kimmel, Ullmann and Schilling1995) stated that this layer is an organ found only in teleosteans as an extra-embryonic structure that contributes in the formation of an embryo body (Balinsky, Reference Balinsky1970). This cell layer, also called the periblast, can be differentiated from other blastoderm cells as it is more basophilic than the latter (Hisaoka & Firlit, Reference Hisaoka and Firlit1960). It also plays a key role in yolk breakage, making it available to the embryos, which allows their development (Balinsky, Reference Balinsky1970).
The formation of the periblast during the morula phase has been reported for Catostomus commersoni (Long & Ballard, Reference Long and Ballard1976), P. lineatus (Ninhaus-Silveira et al., Reference Ninhaus-Silveira, Foresti and Azevedo2006), Z. jahu (Nogueira et al., Reference Nogueira, Azevedo, Canelhas, Bedore, Lopes and Godinho2012), and in the species studied here. Conversely, the periblast is firstly observed at the start of the blastula stage in other fish species (Wourms & Evans, Reference Wourms and Evans1974; Iwamatsu, Reference Iwamatsu1994; Kimmel et al., Reference Kimmel, Ballard, Kimmel, Ullmann and Schilling1995; Ganeco et al., Reference Ganeco, Franceschini-Vicentini and Nakaghi2008; Faustino et al., Reference Faustino, Nakaghi, Marques, Ganeco and Makino2010a; Faustino et al., Reference Faustino, Nakaghi and Neumann2010b).
As the yolk syncytial layer increases, it involves the yolk, independently of the blastoderm, serving as a primary cause of epiboly movement (Devillers, Reference Devillers1961; Betchaku & Trinkaus, Reference Betchaku and Trinkaus1986, Reference Trinkaus1993; Ninhaus-Silveira et al., Reference Ninhaus-Silveira, Foresti, de Azevedo, Agostinho and Veríssimo-Silveira2007). According to these authors, the peripheral yolk syncytial layer contracts to facilitate the migration of nuclei to the inner portion, thereby completing its formation as the syncytial layer grows towards the vegetative pole along with the blastoderm, which is firmly attached, thus triggering epiboly. The present results corroborate this suggestion.
The gastrula stage initiates when the first epiboly movements begins and ends when the blastopore is closed by the blastoderm and the tail bud is formed. These findings agree with the reports for B. orbignyanus (Ganeco et al., Reference Ganeco, Franceschini-Vicentini and Nakaghi2008), P. lineatus (Ninhaus-Silveira et al., Reference Ninhaus-Silveira, Foresti and Azevedo2006), R. aspera (Perini et al., Reference Perini, Sato, Rizzo and Bazzoli2009), B. gouldingi (Faustino et al., Reference Faustino, Nakaghi and Neumann2010b), Pimelodus maculatus (Buzzolo et al., Reference Buzzolo, Veríssimo-Silveira, Oliveira-Almeida, Alexandre, Okuda and Ninhaus-Silveira2011) and in the species studied here.
According to Trinkaus (Reference Trinkaus1992), the cells presenting epiboly around the yolk also undergo involution on the edges of the germ ring while the embryonic shield is converted, directing these cells to anterior and dorsal positions. These movements form the chordomesoderm that precedes the formation of the notochord. The cells adjacent to the paraxial mesoderm cells form the mesoderm somites (Kimmel et al., Reference Kimmel, Ballard, Kimmel, Ullmann and Schilling1995). This affirmation is supported by results in L. marmoratus and other species such Prochilodus lineatus (Ninhaus-Silveira et al., Reference Ninhaus-Silveira, Foresti and Azevedo2006), B. orbignyanus (Ganeco et al., Reference Ganeco, Franceschini-Vicentini and Nakaghi2008), Zungaro jahu (Nogueira et al., Reference Nogueira, Azevedo, Canelhas, Bedore, Lopes and Godinho2012), R. quelen (Amorim et al., Reference Amorim, Gomes, Martins, Sato, Rizzo and Bazzoli2009) and hybrids between P. corruscans and P. reticulatum (Faustino et al., Reference Faustino, Nakaghi, Marques, Makino and Senhorini2007). Conversely, Oreochromis niloticus embryos cannot extend over the entire vegetative pole because of the size of the yolk sac and then present a rudimentary organogenesis (segmentation of somites) prior the cessation of epiboly movements (Morrison et al., Reference Morrison, Miyake and Wright2001).
By the end of the gastrula stage, the first mesoderm tissues were observed along both sides of notochord, arranged in segments called somites, as described in Brachidanio rerio (Hisaoka & Firlit, Reference Hisaoka and Firlit1960; Kimmel et al., Reference Kimmel, Ballard, Kimmel, Ullmann and Schilling1995), Oreochromis niloticus (Galman & Avtalion, Reference Galman and Avtalion1989), Oryzias latipes (Iwamatsu, Reference Iwamatsu1994) and B. orbignyanus (Ganeco et al., Reference Ganeco, Franceschini-Vicentini and Nakaghi2008).
Kimmel et al. (Reference Kimmel, Ballard, Kimmel, Ullmann and Schilling1995) regarded the neurula as a phase within the organogenesis stage. Brummett & Dumont (Reference Brummett and Dumont1978) and Morrison et al. (Reference Morrison, Miyake and Wright2001) observed Kupffer's vesicle in the early steps of the organogenesis stage. In L. marmoratus, this vesicle appeared at the neurula phase. According to Hisaoka & Firlit (Reference Hisaoka and Firlit1960), Kupffer's vesicle represents a remnant structure from the archenteron located above the periblast and below the notochord. In Oncorhynchus keta (Mahon & Hoar, Reference Mahon and Hoar1956 cited by Hisaoka & Firlit, Reference Hisaoka and Firlit1960), Kupffer's vesicle is described as an oblique and elongated cavity with columnar epithelial walls, which is separated from the periblast by a layer of endoderm cells, similar to the pattern observed by Ninhaus-Silveira et al. (Reference Ninhaus-Silveira, Foresti and Azevedo2006) in P. lineatus and in L. marmoratus from this study.
Brummett & Dumont (Reference Brummett and Dumont1978) hypothesized that this vesicle might have a digestive activity bring helpful to yolk absorption, once cilium-like cells were present in Kupffer's vesicle and the intestine of Fundulus heterocliltus. Essner et al. (Reference Essner, Amack, Nyholm, Harris and Yost2005) showed that, in Danio rerio, Kupffer's vesicle contained fluids and cilia and suggested that this transitory embryonic structure could be responsible for the organ asymmetry during development. In the species analysed in this study, cilia were detected on the epithelium of this vesicle. In contrast, these features were not observed in neotropical species, such as B. cephalus (Alexandre et al., Reference Alexandre, Ninhaus-Silveira, Verissimo-Silveira, Buzollo, Senhorini and Chaguri2010) and Pimelodus maculatus (Buzzolo et al., Reference Buzzolo, Veríssimo-Silveira, Oliveira-Almeida, Alexandre, Okuda and Ninhaus-Silveira2011). By non-visualization these cilia could be linked to the monociliar feature to the cells lining Kupffer's vesicle, as demonstrated in zebrafish (Okabe et al., Reference Okabe, Xu and Burdine2008), and the histological technique used by the authors cited.
According to Gilbert (Reference Gilbert2010), the neural tube arises from a solid cell cord at neural plates, forming a string-like structure that migrates into the embryo to form the tube. The uppermost portion of neural tube undergoes drastic changes by expanding into three primary vesicles: anterior brain (prosencephalon), middle brain (mesencephalon) and posterior brain (rhombencephalon). In L. marmoratus, the anterior region of neural tube expanded to form these three regions, as reported for Prochilodus lineatus (Ninhaus-Silveira et al., Reference Ninhaus-Silveira, Foresti and Azevedo2006), B. orbignyanus (Ganeco et al., Reference Ganeco, Franceschini-Vicentini and Nakaghi2008), Brachidanio rerio (Kimmel et al., Reference Kimmel, Ballard, Kimmel, Ullmann and Schilling1995), R. sapo (Cussac et al., Reference Cussac, Matkovic and Maggese1985) and P. corruscans (Marques et al., Reference Marques, Nakaghi, Faustino, Ganeco and Senhorini2008).
In R. sapo (Cussac et al., Reference Cussac, Matkovic and Maggese1985), the notochord cells form vacuoles that move the nuclei towards the peripheral region. Falk-Petersen (Reference Falk-Petersen2005) reported that the notochord is composed of vacuolated cells separated by thin cell membranes. The histological analyses of this structure in the species analyzed in this work corroborate these previous reports.
The development of circulatory system begins with the formation of the heart and it continues through the larval development (Langeland & Kimmel, Reference Langeland, Kimmel, Gilbert and Raunio1997; Yelon & Stainier, Reference Yelon and Stainier1999; Hu et al., Reference Hu, Sedmera, Post and Clark2000). According to Hu et al. (Reference Hu, Sedmera, Post and Clark2000), the heart is the first definitive organ to develop and it is functional during embryogenesis. Other authors reports that the heart initially appears as a single tube with a multilayer wall (Morrison et al., Reference Morrison, Miyake and Wright2001; Hall et al., Reference Hall, Smith and Johnston2004). Rodrigues-Galdino et al. (Reference Rodrigues-Galdino, Maiolino, Forgati, Donatti, Mikos, Carneiro and Rios2009) also describes yolk veins with circulating blood in R. quelen. The present data in L. marmoratus support the above-mentioned reports.
The V-like embryonic muscles formed by myotomes are established in early somitogenesis (Patterson et al., Reference Patterson, Mook and Devoto2008). Muscular contractions occur initially in individual myotomes and then spread to coordinated series of myotomes. These contractions in late embryos arise in bursts as the circuits of motor and sensory reflexes develop functionally (Zhang et al., Reference Zhang, Bhattacharya, Li and Jamieson2009). According to Rodrigues-Galdino et al. (Reference Rodrigues-Galdino, Maiolino, Forgati, Donatti, Mikos, Carneiro and Rios2009), when the somites are arranged in a V-shape, the embryos display lateral body flexion. These movements were firstly detected in pre-larval embryos of L. marmoratus.
The otic vesicle was established in early organogenesis and otolithes were detected at the end of this stage, following the pattern described by Zhang et al. (Reference Zhang, Bhattacharya, Li and Jamieson2009). Rodrigues-Galdino et al. (Reference Rodrigues-Galdino, Maiolino, Forgati, Donatti, Mikos, Carneiro and Rios2009) observed a pair of otolithes similar in form to small granules attached to the inner surface of each otocyst.
The olfactory plates are formed when the embryo are near the hatching stage (Blaxter, Reference Blaxter, Hoar and Randall1988) as observed in the studied species. In Z. jahu (Nogueira et al., Reference Nogueira, Azevedo, Canelhas, Bedore, Lopes and Godinho2012) and B. gouldingi (Faustino et al., Reference Faustino, Nakaghi and Neumann2010b), the olfactory organ was observed at the hatching stage with a small amount of rudimentary cilia. The olfactory plates with cilia are present in the borders before the eyes (Zhang et al., Reference Zhang, Bhattacharya, Li and Jamieson2009).
The chorion rupture in bony fishes is favoured by hatching glands. Prior to hatching, these glands secrete proteolytic enzymes, chorionases, which degrade the inner layers of the chorion, facilitating the exit of embryos (Zhang et al., Reference Zhang, Bhattacharya, Li and Jamieson2009). According to these authors, both tail and body movements are also helpful to the hatching process. Chorion deterioration and embryo movements were also observed in L. marmoratus in the pre-larva phase near to the hatching stage.
The analysis of chromatophores and pigments on eyes and body are important traits in taxonomy and species identification (Meijide & Guerrero, Reference Meijide and Guerrero2000). The cells from the reticular epithelium and skin melanophores are the first to show pigments during embryogenesis (Kimmel et al., Reference Kimmel, Ballard, Kimmel, Ullmann and Schilling1995), as observed in L. marmoratus, as well as other species such as Z. jahu (Nogueira et al., Reference Nogueira, Azevedo, Canelhas, Bedore, Lopes and Godinho2012) and R. aspera (Perini et al., Reference Perini, Sato, Rizzo and Bazzoli2009).
Cussac et al. (Reference Cussac, Matkovic and Maggese1985) also detected three pairs of a similar structure to that observed in L. marmoratus, so-called anterior mesoderm bundles, located at the dorsal region in embryos of R. sapo. These authors showed that the first anterior mesoderm bundle remains undifferentiated at hatching, while the second one differentiates into the anterior and outer wall of opercular cavity. In the third bundle, the cells were arranged in parallel to form the branchial fissures. A similar structure was observed using SEM by Faustino et al. (Reference Faustino, Nakaghi, Marques, Ganeco and Makino2010a) in P. corruscans and P. corruscans × P. reticulatum hybrids, but identified as primordial barbels.
The embryogenesis period in L. marmoratus was similar to that reported by Faustino et al. (Reference Faustino, Nakaghi, Marques, Ganeco and Makino2010a) in hybrids of P. corruscans and P. reticulatum. Variation in embryonic periods is related to sensitivity of teleostean embryogenesis to environmental changes, mainly in temperature.
A fast embryonic development is typical of teleosts species with seasonal reproductive strategies, high fecundity and no parental care (Vandevalle et al., Reference Vandevalle, Germeau, Besancenet, Parmentier and Baras2005) such as L. marmoratus. Blaxter (Reference Blaxter, Hoar and Randall1988) reports that most recently hatched fish larvae lack mouth, anus, gills, swimming bladder, fins, pigmentation and visual accuracy. Overall, these features were also observed in L. marmoratus larvae, differing in the presence of pigmentation in the region of the yolk sac and head, an anal pore and a single embryonic fin.
Acknowledgements
This work was financially supported by FAPESP (Foundation of Research of the São Paulo State) and the facilities and fish used in this study were provided by the National Centre for Research and Conservation of Continental Fish of the Chico Mendes Institute for Biodiversity Conservation (CEPTA/ICMBio), Pirassununga, São Paulo and the fish farming centre at Buriti, Nova Mutum, Mato Grosso, Brazil.