Introduction
Preimplantation development is a dynamic process characterized by a series of developmental events that cover the transition time from gametes to a pluripotent embryo (Lonergan et al., Reference Lonergan, O'Kearney-Flynn and Boland1999). This orchestrated process is regulated by differential expression of many genes, therefore the acquisition of knowledge on the expression patterns of these genes provides new insights into the complex molecular pathways that control early embryonic development in mammals (Steuerwald et al., Reference Steuerwald, Cohen, Herrera and Brenner1999; Khurana & Niemann, Reference Khurana and Niemann2000).
Reverse transcription quantitative real-time PCR (RT-qPCR) is a powerful technique for quantitative analysis, capable of combining accuracy, sensitivity, specificity and reproducibility, properties that make it currently the method most widely used to detect and quantify differences in gene expression. However, RT-qPCR assays are prone to errors and experimental variations, making necessary to minimize these variables for a well conducted study (Bustin, Reference Bustin2002; Huggett et al., Reference Huggett, Dheda, Bustin and Zumla2005; Wong & Medrano, Reference Wong and Medrano2005; Van Guilder et al., Reference Van Guilder, Vrana and Freeman2008). These differences can be controlled by normalizing data using reference genes, which should be expressed consistently in the samples regardless of tissue, experimental condition and/or treatment (Thellin et al., Reference Thellin, Zorzi, Lakaye, De Borman, Coumans, Hennen, Grisar, Igout and Heinen1999; Dheda et al., Reference Dheda, Huggett, Bustin, Johnson, Rook and Zumla2004; Ohl et al., Reference Ohl, Jung, Xu, Stephan, Rabien, Burkhardt, Nitsche, Kristiansen, Loening, Radonic and Jung2005; Bar et al., Reference Bar, Bar and Lehmann2009). However, the stability of reference genes can also vary depending on the state of development and experimental conditions, hence its experimental validation is essential for each model, as inappropriate use of these reference genes can lead to erroneous normalization of RT-qPCR data and therefore to a misinterpretation of the biological significance of the generated results (Haller et al., Reference Haller, Kulle, Schwager, Gunawan, Heydebreck, Sültmann and Füzesi2004; Zhang et al., Reference Zhang, Ding and Sandford2005; McCurley & Callard, Reference McCurley and Callard2008).
In the present study, we used RT-qPCR to measure the transcript levels of 10 genes commonly used as reference genes mostly in studies of gene expression in bovine embryos produced in vitro (Goossens et al., Reference Goossens, Van Poucke, Van Soom, Vandesompele, Van Zeveren and Peelman2005; Perez et al., Reference Perez, Tupac-Yupanqui and Dunner2008; Vireque et al., Reference Vireque, Camargo, Serapião, Rosa e Silva, Watanabe, Ferreira, Navarro, Martins and Ferriani2009; Walker et al., Reference Walker, Meier, Mitchell, Roche and Littlejohn2009a). We analyzed the relative gene expression stability of these genes in bovine blastocysts produced by in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI) and somatic cell nuclear transfer (SCNT), in order to identify the most stable genes and their optimum number to normalize RT-qPCR data for gene expression studies in bovine blastocysts produced by these methods.
Materials and methods
Biological material
Gene expression analysis was carried out on day 7 expanded bovine blastocysts produced in our laboratory by IVF, ICSI and SCNT, according to the methods already described (Felmer & Arias, Reference Felmer and Arias2011; Felmer et al., Reference Felmer, Arias, Muñoz and Rio2011; Arias et al., Reference Arias, Sáchez, Risopatrón and Felmer2012). Embryos generated by these methods were cultured under the same culture medium and culture conditions. Briefly, embryo culture was carried out in 50 μl drops (25 embryos per drop) under mineral oil at 38.5°C and 5% CO2, 5% O2, and 90% N2, in a humidified atmosphere. Culture medium consisted of KSOM (EmbryoMax, Millipore Corp, Billerica, MA, USA) 0.4% FAF-BSA for 3 days and then KSOM 5% FBS to day 7.
Selection of reference genes and primer design
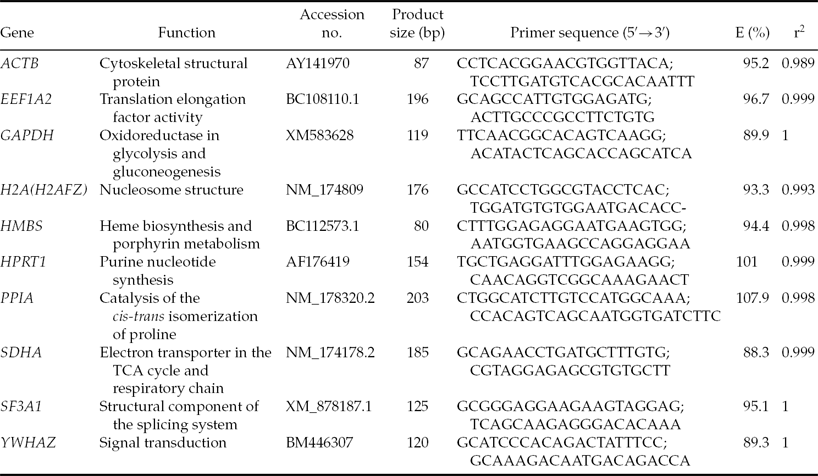
Ten candidate genes previously used as reference genes for normalization of gene expression data were selected for evaluation (Table 1). Primer sequences for ACTB and HPRT1 were taken from Goossens et al. (Reference Goossens, Van Poucke, Van Soom, Vandesompele, Van Zeveren and Peelman2005), EEF1A2, HMBS and SF3A1 from Perez et al. (Reference Perez, Tupac-Yupanqui and Dunner2008) and H2A from Vireque et al. (Reference Vireque, Camargo, Serapião, Rosa e Silva, Watanabe, Ferreira, Navarro, Martins and Ferriani2009). Primer sequences for remaining genes (SDHA, YWHAZ, GAPDH, and PPIA) were re-designed based on RNA or DNA bovine sequences found in the GenBank database using the FAST PCR software tool, as sequences described by Goossens et al. (Reference Goossens, Van Poucke, Van Soom, Vandesompele, Van Zeveren and Peelman2005) and Walker et al. (Reference Walker, Meier, Mitchell, Roche and Littlejohn2009a) did not allow us to set up the same annealing temperature for all genes under study.
Table 1 Information on the primers used for RT-qPCR

E, PCR efficiency; r2, correlation coefficient.
RNA extraction and cDNA synthesis
Three pools of embryos for each treatment (n = 5 blastocysts/pool) were lysed in 20 μl of extraction buffer (XB; Arcturus, Carlsbad, CA, USA) by incubation at 42°C for 30 min followed by centrifugation at 3000 g for 2 min. RNA was kept frozen at –80°C in the kit's extraction buffer until all samples were collected for analysis. Total RNA was extracted from each pool of embryos using the PicoPure RNA Isolation Kit (Arcturus, Carlsbad, CA, USA) according to the manufacturer's instructions; residual genomic DNA was removed by DNase I digestion, using 0.125 units final concentration of RNase-free DNase Set (Qiagen, Valencia, CA, USA). Final RNA was eluted from the purification column using 11 μl of the kit's elution buffer. Due to the low cell number used for RNA extraction, RNA quantity could not be measured by a NanoDrop 2000C (ThermoScientific) spectrophotometer.
Reverse transcription assay was carried out using the RevertAid™ H Minus First Strand Kit (Fermentas Inc., MD, USA), according to the manufacturer's instructions. Briefly, the following reagents were added to each 0.5 ml of RNase-free tube: 10 μl total RNA and 200 ng of random hexamers. The reaction tubes were incubated in a preheated PCR machine at 70°C for 5 min and transferred to ice. After denaturation, the following reagents were added to each reaction tube: 4 μl of 5× first-strand reaction buffer, 2 μl of 10 mM dNTPs, and 1 μl of Riboblock. After gentle mixing, reaction tubes were incubated at 25°C for 5 min. Then, 1 μl of RevertAid™ MuLV RT was added and the mixture incubated at 42°C for 60 min in a dry bath. The reaction was terminated by heating at 70°C for 10 min and chilled on ice. This first-strand cDNA was diluted five times and used for real-time experiments.
Quantitative real-time RT-PCR
Polymerase chain reactions (PCR) were performed using Brilliant II SYBR® Green QPCR Master Mix (Stratagene) in a thermocycler MX3000P (Agilent Technologies, CA, USA). All PCR reactions were performed in duplicate wells in a final volume of 20 μl containing 4 μl of diluted cDNA, 10 μl of Master mix, 4 μl of primer mix (300 nM final), and 2 μl of PCR-Grade water. PCR program consisted of an initial incubation step at 95°C for 5 min to activate Taq DNA polymerase, followed by 40 cycles of template denaturation step at 95°C for 20 s, a primer annealing step at 58°C for 20 s (same annealing for all primers), and an extension step at 72°C for 20 s. A control for removal of genomic DNA after DNase treatment (–RT) was performed with primers for ACTB and negative control tubes without cDNA template were included in each assay. At the end of the PCR reaction, melting curve analyses were performed for all genes, and the specificity as well as integrity of the PCR products was confirmed by the presence of a single peak (data not shown). PCR efficiencies (E) were estimated using a relative standard curve derived from a pooled cDNA mixture from in vitro-produced embryos (a 10-fold dilution series with five measuring points). These values were determined by the slopes of the curves according to the equation E = 10(–1/slope) established by Pfaffl (Reference Pfaffl2001; Table 1).
Gene expression stability analysis
RT-qPCR data (Ct values) were transformed into relative quantification data using the formula Q = (E)∆Ct described by Livak & Schmittgen (Reference Livak and Schmittgen2001) and then exported into an Excel datasheet (Microsoft® Excel 2003). To determine the most stable reference genes, the geNorm Visual Basic Application Program v3.4 described by Vandesompele et al. (Reference Vandesompele, De Preter, Pattyn, Poppe, Van Roy, De Paepe and Speleman2002) was used.
Results and Discussion
Given the high sensitivity of RT-qPCR to detect small changes in transcript abundance in bovine blastocysts produced in vitro, it is necessary to normalize the data by endogenous control genes commonly known as reference or housekeeping genes. Normalization by these genes is essential to control initial differences in embryo cell number, variations in RNA extraction yield, RNA abundance, reverse transcription efficiency, and the presence of inhibitors, thus enabling comparison of mRNA levels across different samples (Bustin et al., Reference Bustin, Benes, Garson, Hellemans, Huggett, Kubista, Mueller, Nolan, Pfaffl, Shipley, Vandesompele and Wittwer2009). However, many studies have made use of these genes without a proper validation of their stability (Vandesompele et al., Reference Vandesompele, De Preter, Pattyn, Poppe, Van Roy, De Paepe and Speleman2002). Furthermore, much evidence suggests that stability of reference genes can vary depending on the state of development and experimental conditions (Bustin, Reference Bustin2000; Warrington et al., Reference Warrington, Nair, Mahadevappa and Tsyganskaya2000); therefore its usefulness should be validated experimentally in each model (Zhang et al., Reference Zhang, Ding and Sandford2005; McCurley & Callard, Reference McCurley and Callard2008).
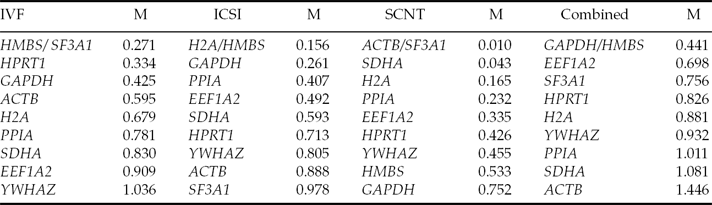
In the present study, in order to analyze the suitability of candidate reference genes for bovine blastocysts produced by different in vitro embryo production methods, the expression stability of 10 previously used reference genes was assessed by the geNorm algorithm (Vandesompele et al., Reference Vandesompele, De Preter, Pattyn, Poppe, Van Roy, De Paepe and Speleman2002). This program calculates the gene stability measure (M) by determining the average pairwise variation of a gene with all other control genes (Vandesompele et al., Reference Vandesompele, De Preter, Pattyn, Poppe, Van Roy, De Paepe and Speleman2002). In this way, genes with a low M value have a low variation, which represents a more stable expression (M values <1.5). Using this program we ranked the 10 candidate reference genes in bovine blastocysts produced by the different methods according to their expression stability (Table 2 and Fig. 1). Thus, a gradual stepwise exclusion of the least stable genes allowed us to identify HMBS and SF3A1 as the most stable genes for embryos produced by IVF (M values 0.271), H2A and HMBS for embryos produced by ICSI (M values 0.156), and ACTB and SF3A1 for embryos produced by SCNT (M values 0.010) (Table 2 and Fig. 1). The same analysis considering the gene expression data of all combined embryo production methods (IVF, ICSI, and SCNT) positioned GAPDH and HMBS as the most stable reference genes with an M value of 0.441, whereas ACTB showed the least stability in this analysis (M value 1.446; Table 2).
Table 2 Ranking of candidate reference genes according to their expression stability values (M) in each of the in vitro embryo production methods

Candidate genes are listed according to their expression stability with the highest on top and the lowest at the bottom.
It is necessary to note from these data that each embryo production method showed a different ranking for these genes, with a slightly higher difference for embryos produced by SCNT (Table 2), which could be explained by inefficiencies in the process of nuclear transfer attributable to incomplete or abnormal epigenetic reprogramming (Somers et al., Reference Somers, Smith, Donnison, Wells, Henderson, McLeay and Pfeffer2006; Beyhan et al., Reference Beyhan, Forsberg, Eilertsen, Kent-First and First2007; Suzuki et al., Reference Suzuki, Kondo, Wakayama, Cizdziel and Hayashizaki2008). These differences in the stability of the genes confirm that not only in vitro culture conditions may affect the gene expression patterns during embryogenesis in mammals (Niemann & Wrenzycki, Reference Niemann and Wrenzycki2000; Lazzari et al., Reference Lazzari, Wrenzycki, Herrmann, Duchi, Kruip, Niemann and Galli2002; Rizos et al., Reference Rizos, Lonergan, Boland, Arroyo-Garcia, Pintado, de la Fuente and Gutierrez-Adan2002; Rinaudo & Schultz, Reference Rinaudo and Schultz2004; Felmer et al., Reference Felmer, Arias, Muñoz and Rio2011), but also the methodological procedure used to generate these embryos, which agrees with previous studies between IVF and SCNT embryos (Zhou et al., Reference Zhou, Xiang, Walker, Farrar, Hwang, Findeisen, Sadeghieh, Arenivas, Abruzzese and Polejaeva2008; Ross et al., Reference Ross, Wang, Kocabas and Cibelli2010). A large variation in M value range between the three different embryo production groups was also observed; particularly the M values of SCNT embryos are lower (0.01 for the most stable gene to 0.752 for the least stable gene), a finding that suggested that these embryos have little variation in their expression patterns compared to IVF and ICSI embryos, respectively. This effect could be attributed to the differences in gene expression observed in male and female embryos as it would be the case for IVF and ICSI embryos, respectively (Bermejo-Alvarez et al., Reference Bermejo-Alvarez, Rizos, Rath, Lonergan and Gutierrez-Adan2008; Walker et al., Reference Walker, Kimura and Roberts2009b), an effect that is not observed in SCNT embryos as all embryos are of the same gender. It is also interesting to note that despite all candidate reference genes showed a high expression stability, as evidenced by their low M values (<1.4), somehow supporting the previous selection of these genes as reference genes (Goossens et al., Reference Goossens, Van Poucke, Van Soom, Vandesompele, Van Zeveren and Peelman2005; Perez et al., Reference Perez, Tupac-Yupanqui and Dunner2008; Vireque et al., Reference Vireque, Camargo, Serapião, Rosa e Silva, Watanabe, Ferreira, Navarro, Martins and Ferriani2009), differences in gene expression stability were still observed between these genes and the different embryo production methods, confirming that careful selection of the best candidate genes is strongly recommended for each experimental condition. This point is better exemplified by a gene expression analysis carried out with all combined data using the REST program (http://www.gene-quantification.de/rest-2009.html), where selecting GAPDH (the most stable) or ACTB (the least stable) as reference genes gave different gene expression results (data not shown).

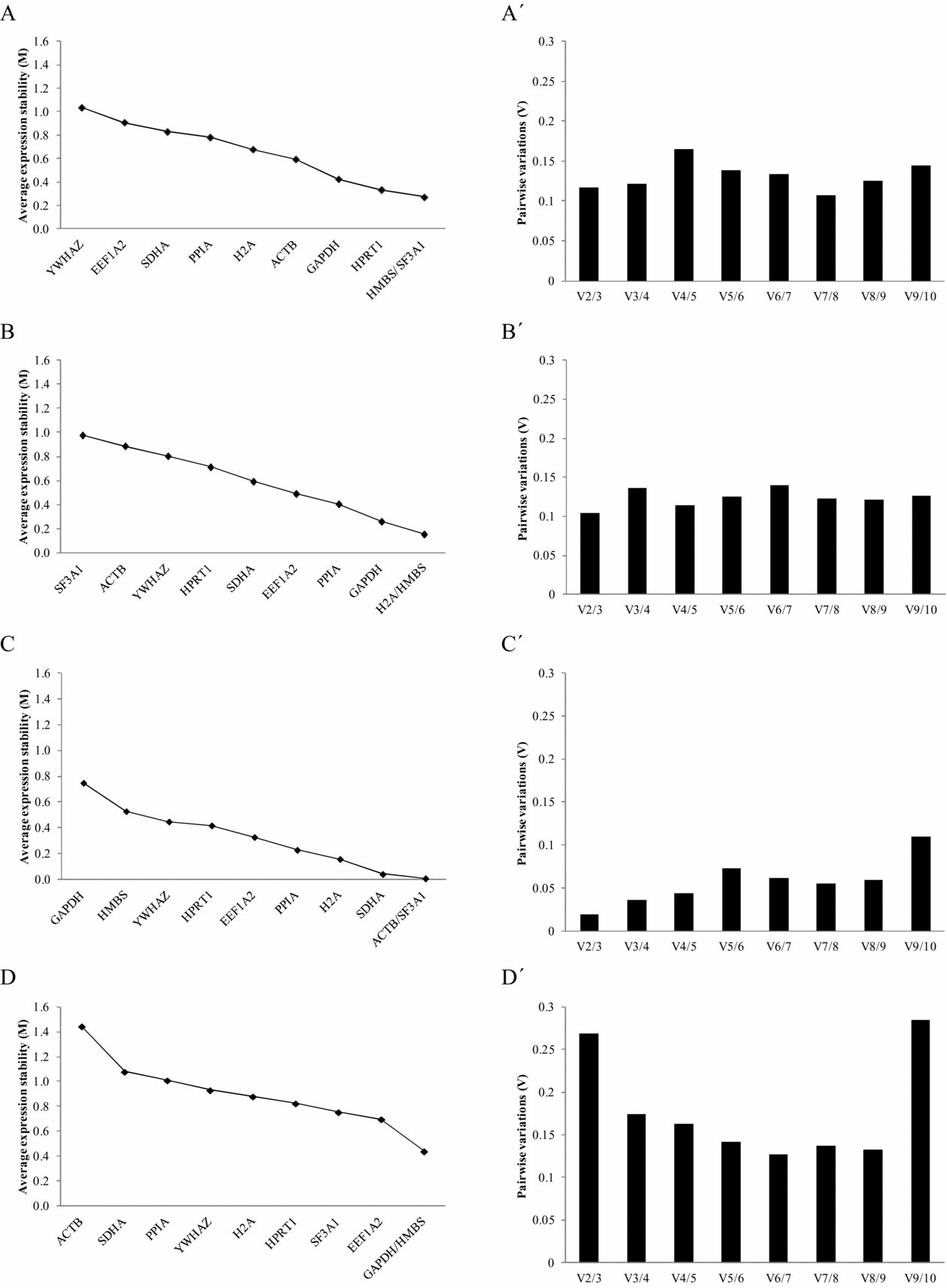
Figure 1 Gene expression stability of candidate reference genes analyzed by the geNorm program. Left panel: Average expression stability values (M) of candidate reference genes plotted from the least stable (left) to the most stable (right). (A) In vitro fertilization (IVF). (B) Intracytoplasmic sperm injection (ICSI). (C) Somatic cell nuclear transfer (SCNT). (D) All assays combined. (A′–D′). Right panel: Pairwise variation analysis (Vn/Vn+1) between the normalization factors NFn and NFn+1 to determine the optimal number of reference genes for normalization: (A′) IVF; (B′) ICSI; (C′) SCNT; and (D′) combined assay.
Considering that variations in gene expression stability may always exist for any reference gene subjected to a determined experimental condition (Bustin, Reference Bustin2000; Warrington et al., Reference Warrington, Nair, Mahadevappa and Tsyganskaya2000; Remans et al., Reference Remans, Smeets, Opdenakker, Mathijsen, Vangronsveld and Cuypers2008), normalization of gene expression data against a single reference gene can bias the generated results and further compromise its validity (Dheda et al., Reference Dheda, Huggett, Bustin, Johnson, Rook and Zumla2004; Jemiolo & Trappe, Reference Jemiolo and Trappe2004). Accordingly, Vandesompele et al. (Reference Vandesompele, De Preter, Pattyn, Poppe, Van Roy, De Paepe and Speleman2002) demonstrated that using a single reference gene leads to a moderate error and validated the geometric mean of multiple reference genes as an accurate normalization factor. Therefore, in addition, we calculated the optimal number of reference genes needed for an accurate normalization of gene expression data in bovine blastocysts generated by these in vitro embryo production methods. This optimum was determined using the normalization factor (NFn), which is based on the geometric mean of the expression values of the n best reference gene, calculated by the stepwise inclusion of an additional less stable reference gene (Vandesompele et al., Reference Vandesompele, De Preter, Pattyn, Poppe, Van Roy, De Paepe and Speleman2002). An arbitrary cut-off value of 0.15 indicates acceptable stability of the control gene combination, a value below which the inclusion of an additional reference gene is not required; therefore, in our case, the inclusion of a third gene had no significant effect on the NF value (Fig. 1). However, Vandesompele et al. (Reference Vandesompele, De Preter, Pattyn, Poppe, Van Roy, De Paepe and Speleman2002) recommended the minimal use of three reference genes and, therefore, we considered three genes to be sufficient for accurate normalization when analyzing gene expression data in bovine blastocysts produced either by IVF (HMBS, SF3A1, and HPRT1), ICSI (H2A, HMBS, and GAPDH), and SCNT (ACTB, SF3A1, and SDHA) (Fig. 1A′, B′ and C′, respectively). Conversely, the comparison of gene expression data between blastocysts produced by these in vitro embryo production methods would require the inclusion of a fifth reference gene (Fig. 1D′). The use of five reference genes leads to a waste of resources, particularly in this case when most of the genes showed a relatively stable expression (M values <1.5). Therefore, we suggest the use of the geometric average of the three most stable genes, in this case GAPDH, HMBS, and EEFIA2, as the best combination for normalization of gene expression data in bovine blastocysts.
Previous studies on the stability of reference genes determined that GAPDH, YWHAZ, and SDHA were the best endogenous control genes in preimplantation embryo samples and that ACTB was the least stable reference gene (Goossens et al., Reference Goossens, Van Poucke, Van Soom, Vandesompele, Van Zeveren and Peelman2005). Our results, which compared all combined gene expression data for bovine blastocysts produced by the different embryo production methods, are in agreement with this study as GAPDH and ACTB showed the highest and the lowest stability values, respectively. Although some differences could be observed in the ranking for YWHAZ, and SDHA genes, this finding could be attributed either to the different set of genes used in both studies, the different set of primers (see Materials and methods section), or the different developmental stage of embryos and culture conditions. In a separate study that compared IVF and SCNT embryos, ACTB was also found to change significantly at the blastocyst stage, a situation that could be the result of abnormal nuclear reprogramming in SCNT embryos (Ross et al., Reference Ross, Wang, Kocabas and Cibelli2010). A similar result was also observed by Bower et al. (Reference Bower, Moser, Hill and Lehnert2007), who reported unstable expression of ACTB in a microarray experiment that compared the transcriptome of SCNT to IVF blastocysts. These data are of particular relevance for ACTB, as this gene has been used previously as a single reference gene in different studies of gene expression in embryos; this approach highlights the caution that must be exercised for an appropriate selection of internal control genes. Our data also show that although some reference genes maintained a certain level of similarity in the gene expression stability in each of the in vitro embryo production methods, differences were still observed. In addition, the fact that expression of reference genes may also vary under other experimental conditions must be taken into consideration, a situation that is particularly relevant when nuclear transfer embryos are in evaluation.
In conclusion, the results of the present study showed that experimental validation of reference genes is essential for each experimental model and that not only the culture conditions may affect the expression patterns of bovine blastocysts produced in vitro, but also the embryo production method may have an important effect. We determined the best combination and the optimal number of reference genes for gene expression studies in bovine blastocysts produced by the different in vitro embryo production methods.
Acknowledgements
Provision of ovaries by our local Slaughterhouse (Frigorifico Temuco) and funding support from FONDECYT 1080216 and 1100449 CONICYT, Chile are gratefully acknowledged.
Conflict of interest
The authors declare that they have no conflicts of interest.