Introduction
The mammalian ovary contains thousands of follicles at birth and preantral (i.e., primordial, primary and secondary) follicles represent 90% of this follicular population. However, the vast majority (99.9%) of these follicles become atretic during their growth and maturation (Markström et al., Reference Markström, Svensson, Shao, Svanberg and Billig2002). Although many preantral follicles degenerate (Van den Hurk et al., Reference Van den Hurk, Spek, Hage, Fair, Ralph and Schotanus1998), numerous healthy ones still can be used in assisted reproductive programmes after in vitro growth and maturation (Silva et al., Reference Silva, Ferreira, Costa, Santos, Carvalho, Rodrigues, Lucci, Báo and Figueiredo2002). Because of this, different isolation techniques and culture systems for preantral follicles have been developed in the past years. In various species (bovine: Gutierrez et al., Reference Gutierrez, Ralph, Telfer, Wilmut and Webb2000; ovine: Cecconi et al., Reference Cecconi, Barboni, Coccia and Mattioli1999; murine: Cortvrindt et al., Reference Cortvrindt, Smitz and Van Steirteghem1996; caprine: Zhou & Zang, 2005; Huanmin & Young, Reference Huanmin and Yong2000), preantral follicles have been cultured up to antral stages of development. Systemically delivered pituitary hormones and locally produced growth factors have an important role in maintaining follicular viability and to promote follicle growth and differentiation (for reviews, see Fortune, Reference Fortune2003; Van den Hurk & Zhao, Reference Van den Hurk and Zhao2005). These compounds act on follicles through their receptors. Such binding sites for FSH (FSH-R) have been demonstrated in granulosa cells of preantral follicles in hamsters (Roy & Treacy, Reference Roy and Treacy1993), cows (Wandji et al., Reference Wandji, Fortier and Sirard1992), sheep (Eckery et al., Reference Eckery, Moeller, Nett and Sawyer1997) and rats (Monniaux & Renviers, Reference Monniaux and Reviers1989), suggesting that FSH is involved in early follicle growth. Indeed, FSH has been demonstrated to stimulate in vitro growth of primary and secondary follicles from various mammalian species (e.g., human: Roy & Treacy, Reference Roy and Treacy1993; Abir et al., Reference Abir, Franks, Mobberley, Moore, Margara and Winston1997; Wright et al., Reference Wright, Hovatta, Margara, Trowe, Winston, Franks and Hardy1999; cow: Hulshof et al., Reference Hulshof, Figueiredo, Beckers, Bevers, Van der Donk and Van den Hurk1995; Wandji et al., Reference Wandji, Eppig and Fortune1996; sheep: Newton et al., Reference Newton, Picton and Gosden1999; and mouse: Spears et al., Reference Spears, Murray, Allison, Boland and Gosden1998; Cortvrindt et al., Reference Cortvrindt, Smitz and Van, Steirteghem1997). In rat secondary follicles, LH receptors (LH-R) have been demonstrated in the theca layer (Kishi & Greenwald, Reference Kishi and Greenwald1999a), which was stimulated to produce progesterone and androstendione after addition of equine chorionic gonadotrophin to the follicular culture medium (Kishi & Greenwald, Reference Kishi and Greenwald1999b). As in rodents, bovine secondary follicles also expressed LH receptors in theca cells (Braw-Tal & Roth, Reference Braw-Tal and Roth2005). In rat ovaries, growth hormone receptor (GH-R) mRNA was abundantly expressed in granulosa cells (Carlsson et al., Reference Carlsson, Nilsson, Isaksson and Billig1993; Zhao et al., Reference Zhao, Taverne, van der Weijden, Bevers and van den Hurk2002), while positive immunostaining for GHR was detected in both oocytes and granulosa cells (Zhao et al., Reference Zhao, Taverne, van der Weijden, Bevers and van den Hurk2002). In vivo experiments showed that GH promotes ovarian follicle development (Gong et al., Reference Gong, Bramley and Webb1991, Reference Gong, Baxter, Bramley and Webb1997) and reduces regression of mouse follicles (Danilovich et al., Reference Danilovich, Bartke and Winters2000). In ovine (Eckery et al., Reference Eckery, Moeller, Nett and Sawyer1997) and human (Abir et al., Reference Abir, Garor, Felz, Nitke, Krissi and Fisch2008) preantral follicles, expression of GH-R mRNA was demonstrated in both oocytes and granulosa cells. Thus far, however, there is little information on the relative expression of hypophyseal hormone receptors and growth factors in goat preantral follicles.
Pituitary hormones may influence ovarian folliculogenesis through the control of follicular growth factor production, and locally produced growth factors on their turn may regulate hormone actions on follicles through up-regulation or down-regulation of the receptors of these endocrine compounds (Van den Hurk & Zhao, Reference Van den Hurk and Zhao2005). Among the ovarian growth factors, epidermal growth factor (EGF) is expressed in human (Reeka et al., Reference Reeka, Berg and Brucker1998; Qu et al., Reference Qu, Godin, Nisolle and Donnez2000) and hamster (Garnett et al., Reference Garnett, Wang and Roy2002) primordial and primary follicles, and in caprine primordial, primary and secondary follicles (Silva et al., Reference Silva, van den Hurk and Figueiredo2006a). In goats, EGF promotes primordial follicle growth (Silva et al., Reference Silva, van den Hurk, Matos, Santos, Pessoa, Moraes and Figueiredo2004a), while in rats, EGF locally controls the action of FSH and LH by inhibiting LH-R synthesis (Hattori et al., Reference Hattori, Yoshino, Shinohara, Horiuchi and Kojima1995) and increases that of FSH-R (Luciano et al., Reference Luciano, Pappalardo, Ray and Peluso1994).
Fibroblastic growth factor-2 (FGF-2) mRNA is localized in granulosa cells and theca cells of developing rat follicles (Guthridge et al., Reference Guthridge, Bertolini, Cowling and Hearn1992; Ortega et al., Reference Ortega, Salvetti, Amable, Dallard, Baravalle, Barbeito and Gimeno2007) and in preantral human follicles (Quennel et al., Reference Quennell, Stanton and Hurst2004; Ben-Haroush et al., Reference Ben-Haroush, Abir, Ao, Jin, Kesler-Icekson, Feldberg and Fisch2005). FGF-2 has been found to activate mouse (Nilsson et al., Reference Nilsson, Parrot and Skinner2001) and caprine (Matos et al., Reference Matos, van den Hurk, Lima-Verde, Luque, Santos, Martins, Báo, Lucci and Figueiredo2006) primordial follicles and to stimulate subsequent growth to primary stages and beyond. Transcripts for IGF-1 mRNA were detected in rat granulosa cells of developing secondary, early antral and preovulatory follicles (Oliver et al., Reference Oliver, Aitman, Powell, Wilson and Clayton1989). In goat follicles IGF-1 mRNA is expressed in all stages of development (Silva et al., Reference Silva, Brito, Leitão, Silva, Passos, Fernandes, Vasconcelos and Figueiredo2007) and the IGF-1 protein stimulates preantral follicle growth in goats (Zhou & Zang, 2005) and rats (Zhao et al., Reference Zhao, Taverne, Van Der Weijden, Bevers and Van den Hurk2001). IGF-1 binds with higher affinity for IGFR-I and low affinity for IGFR-II (Silva et al., Reference Silva, Figueiredo and van den Hurk2009), which are present in granulosa cells of primary follicles, secondary and antral (Monget et al., Reference Monget, Monniaux and Durand1989). Like IGF-1 mRNA, Kit ligand (KL) expression was detected at all stages of caprine follicular development (Silva et al., Reference Silva, van den Hurk, Van Tol, Roelen and Figueiredo2006b). In rabbit and murine follicles, Kl is produced by granulosa cells and has an important role in primordial follicle activation, recruitment of theca cells, antrum formation and oocyte maturation (Hutt et al., Reference Hutt, Mclaughlin and Holland2006). The receptor for Kl (c-kit) is expressed by oocytes and thecal-interstitial cells, enabling them to respond to this growth factor (Knight & Glister, Reference Knight and Glister2006).
During human folliculogenesis, vascular endothelial growth factor (VEGF) is produced by theca cells (Yamamoto et al., Reference Yamamoto, Konishi, Tsuruta, Nanbu, Kuroda, Matsushita, Hamid, Yura and Mori1997) and, late in follicle development, also in granulosa cells (Kamat et al., Reference Kamat, Brown, Manseau, Senger and Dvorak1995). In response to gonadotrophins, the ovarian VEGF level is increased in rats (Koos, Reference Koos1995), whereby it promotes growth of preantral follicles (Danforth et al., Reference Danforth, Arbogast, Ghosh, Dickerman, Rofagha and Friedman2001), the number of healthy follicles and follicular angiogenesis (Tajima et al., Reference Tajima, Yoshii, Fukuda, Orisaka, Miyamoto, Amsterdam and Kotsuji2005). In the goat, VEGF receptor-2 is expressed in oocytes and granulosa cells of all follicular stages, while VEGF maintains follicular ultrastructural integrity and promotes primordial follicle growth (Bruno et al., Reference Bruno, Celestino, Lima-Verde, Lima, Matos, Araújo, Saraiva, Martins, Name, Campello, Báo, Silva and Figueiredo2009).
TGF-β family members, like the bone morphogenetic proteins 6 (BMP-6) and 15 (BMP-15) and growth differentiation factor-9 (GDF-9), are also implicated in the growth of preantral follicles. BMP-6 is expressed in goat preantral follicles (Silva et al., Reference Silva, Brito, Leitão, Silva, Passos, Fernandes, Vasconcelos and Figueiredo2007) and promotes granulosa cell viability in cows (Glister et al., Reference Glister, Kemp and Knight2004). This factor plays its biological activity by binding with the receptors bone morphogenetic protein receptor II (BMPRII) and BMPRIB (also termed ALK6) (Shimasaki et al., Reference Shimasaki, Kelly Moore, Otsuka and Erickson2004). BMP-15 is expressed in the oocytes of all types of goat follicles (Silva et al., Reference Silva, van den Hurk, Van Tol, Roelen and Figueiredo2004b), and stimulates both granulosa cell proliferation and development of primordial and primary follicles (Juengel & McNatty, Reference Juengel and McNatty2005). GDF-9 is secreted by oocytes (mouse: Chang et al., Reference Chang, Brown and Matzuk2002) and granulosa cells (goat: Silva et al., Reference Silva, van den Hurk, Van Tol, Roelen and Figueiredo2004b) of preantral follicles. In humans, it promotes follicular viability and growth of preantral follicles by granulosa cell proliferation (Hreinsson et al., Reference Hreinsson, Scott, Rasmussen, Swahn, Hsueh and Hovatta2002). It has recently been established that GDF-9 signalling involves interaction with TGF-βRI (also known as ALK5) and BMPRII on the target cell surface, while BMP-15 signalling involves BMPRIB and BMPRII (Juengel & McNatty, Reference Juengel and McNatty2005). Expression of each of these receptor types has been detected in granulosa cells from the primordial/primary follicle stage onwards (Juengel & McNatty, Reference Juengel and McNatty2005).
To optimize preantral follicle development in vitro, it is important to study follicular mRNA levels of hormone receptors and growth factors. Hereby, the choice of a correct reference gene to normalize gene expression in quantitative real-time PCR is essential to truly reflect biological processes (Garcia-Vallejo et al., Reference Garcia-Vallejo, Van het Hof, Robben, Van Wijk, Van Die, Joziasse and Van Dijk2004). Housekeeping genes are used as endogenous controls for normalizing expression levels evaluated with RT-PCR. Ideal housekeeping genes are constitutively expressed, do not respond to external stimuli and exhibit little or no sample-to-sample or run-to-run variation (Banda et al., Reference Banda, Bommineni, Thomas, Luckinbill and Tucker2008). Most of the housekeeping genes are involved in basic cell metabolism [for example: glyceraldehyde-3-phosphate dehydrogenase (GAPDH), phosphoglycerokinase (PGK), 18S rRNA, ubiquitin (UBQ) and ribosomal protein 19 (RPL-19)] or are cytoskeletal structural proteins (β-tubulin and β-actin). However, after several reports of variation in putative housekeeping genes (Schmittgen & Zakrajsek, Reference Schmittgen and Zakrajsek2000; Schmid et al., Reference Schmid, Cohen, Henger, Irrgang, Schlondorff and Kretzler2003; Radonic et al., Reference Radonic, Thulke, Mackay, Landt, Siegert and Nitsche2004; Huggett et al., Reference Huggett, Dheda, Bustin and Zumla2005) it has become clear that no reference gene can be assumed to be suitable for any given set of condition. Vandesompele et al. (Reference Vandesompele, De Preter, Pattyn, Poppe, Van Roy and De Paepe2002) demonstrated that errors in expression data up to 20-fold can be generated by use of only a single reference gene. Various studies showed the stability of housekeeping genes in tissues from mice (Kouadjo et al., Reference Kouadjo, Nishida, Cadrm-Girard, Yoshioka and St Amand2007), pig (Nygard et al., Reference Nygard, Jorgensen, Cirera and Fredholm2007) and in embryos of bovine (Goossens et al., Reference Goossens, Van Poucke, Van Soom, Vandesompele, Van Zeveren and Peelman2005), swine (Kuyk et al., Reference Kuyk, du Puy, van Tol, Haagsman, Colenbrander and Roelen2007) and murine (Mamo et al., Reference Mamo, Gal, Bodo and Dmnyes2007) species. Currently, however, there are no data about both the stability of housekeeping genes and the levels of mRNA for hormone receptors, like FSH, LH and GH, and growth factors, like EGF, GDF-9, BMP-15, VEGF, FGF-2, BMP-6, IGF-1 and KL, in caprine preantral follicles.
The aim of the present study is to investigate the stability of GAPDH, β-tubulin, β-actin, PGK, UBQ, RNA 18S, RPL19 expression in fresh and in-vitro cultured goat preantral follicles, and to evaluate the relative expression of growth factors (EGF, GDF-9, BMP-15, VEGF, FGF-2, BMP-6, IGF-1 and KL) and hormone receptors (FSH, LH and GH) in fresh follicles.
Material and methods
Ovaries, follicle isolation and in vitro culture
Ovaries of adult goats (n = 10) were collected in local abattoir immediately after slaughter. After collection, the ovaries were washed once in 70% alcohol for about 10 s, and then twice in 0.9% saline solution and transported to the laboratory at 4°C for up to 1 h. Then, the ovaries were carefully dissected and placed immediately in warmed culture medium, consisting of α-MEM. Briefly, ovarian cortical slices (1–2 mm thick) were cut from the ovarian surface and large preantral follicles were visualized under the stereomicroscope, manually isolated using 26-gauge needles attached to a syringe and washed two times in α-MEM. After isolation, follicles were transferred to 100 μl drops containing fresh medium at room temperature for evaluation. For this study, only follicles (150–200 μm in diameter) with a centrally located oocyte surrounded by compact layers of granulosa cells, an intact basal membrane, and no antral cavity were selected. Isolated follicles from five ovaries were randomly distributed into three groups of 10 preantral follicles that were stored at –80°C until RNA extraction.
For in vitro culture, another group of isolated follicles (n = 30) from five ovaries were randomly transferred to 100-μl drops containing fresh medium under mineral oil to further evaluate the follicular quality. Then, health preantral follicles were individually cultured in 25-μl drops of culture medium in petri-dishes (60 × 15 mm, Corning, USA). The culture medium was called α-MEM+ and consisted of α-MEM (pH 7.2–7.4 supplemented with 1.25 mg/ml bovine serum albumin (BSA), ITS (insulin 6.25 ng/ml, transferrin 6.25 ng/ml and selenium 6.25 ng/ml), 2 mM glutamine, 2 mM hypoxanthine and 50 μg/ml of ascorbic acid under mineral oil. Incubation of follicles was conducted at 39°C, for 12 days. Fresh media were prepared and immediately incubated for 1 h at 39°C prior to use. Preantral follicles were randomly distributed in α-MEM+ that was supplemented with 100 ng/ml FSH (from day 0 to day 6) and 500 ng/ml FSH (from day 6 to day 12). Every other day, 5 μl of the culture media was added because of evaporation and at least 30 follicles were cultured. After culture, three groups of 10 cultured follicles were collected and stored at –80°C until RNA extraction.
RNA extraction and cDNA synthesis
Total RNA was extracted using the Trizol reagent (Invitrogen). The RNA concentration was estimated by reading the absorbance at 260 nm and was checked for purity at 280 nm in a spectrophotometer (Amersham Biosciences). For each sample, the RNA concentrations were adjusted and 44 ng/ml was used to synthesize cDNA. Before the reverse transcription reaction, samples of RNA are incubated for 5 min at 70°C and then cooled in ice. The reverse transcription was performed in a total volume of 20 μl composed of 10 μl of sample RNA, 4 μl reverse transcriptase buffer (Invitrogen), 8 units RNasin, 150 units of reverse transcriptase Superscript III, 0036 U random primers, 10 mM DTT and 0.5 mM of each dNTP (Invitrogen). The mixture was incubated at 42°C for 1 h, subsequently at 80°C for 5 min, and finally stored at –20°C. The negative control is prepared under the same conditions, but without addition of reverse transcriptase.
PCR amplification and determination of gene stability
To identify the most stable housekeeping gene for its use in studies with fresh and cultured preantral follicles, quantification of mRNA for glyceraldehyde-2-phosphate dehydrogenase (GAPDH), β-tubulin, β-actin, phosphoglycerokinase (PGK), 18S rRNA, ubiquitin (UBQ) and ribosomal protein 19 (RPL-19) was performed with the use of SYBR Green. Each reaction in real time (20 μl) containing 10 μl of SYBR Green Master Mix (Applied Biosystems), 7.3 μl of ultra pure water, 1 μl of cDNA and 0.85 M of each primer real-time PCR is performed in a thermocycler (Master Cycler). The primers, chosen to carry out amplification of different housekeeping genes, are shown in Table 1. The reactions of the cDNA by PCR amplification consist of initial denaturation and polymerase activation for 10 min at 95 °C, followed by 40 cycles of 15 s at 95°C, 30 s at 58°C and 30 s at 60°C. The extension will be held for 20 min at 72°C.
Table 1 Primer pairs used in real-time PCR for quantification of growth factors mRNAs in fresh and 12-day cultured caprine preantral follicles

Gene stability was evaluated using the geNorm software program (Vandersompelle et al., Reference Vandesompele, De Preter, Pattyn, Poppe, Van Roy and De Paepe2002). Briefly, this approach relies on the principle that the expression ratio of two perfect reference genes would be identical in all samples in all experimental conditions or cell types. Variation in the expression ratios between different samples reflects expression instability of one or both of the genes. Therefore, increasing variation in this ratio corresponds to decreasing expression stability. The geNorm software can be used to calculate the gene expression stability measure (M), which is the mean pair-wise variation for a gene compared with all other tested control genes. Genes with higher M-values have greater variation in expression. The stepwise exclusion of the gene with the highest M-value allows the ranking of the tested genes according to their expression stability.
Quantification of mRNA for hormone receptors and growth factors
Quantification of mRNA for different growth factors and hormone receptors were performed to compare their expression in fresh preantral follicles. The primers chosen to carry out amplification of different growth factors (EGF, FGF-2, BMP-6, BMP-15, KL, VEGF, GDF-9 and IGF-1) as well as the receptor for LH, FSH and GH are shown in Tables 2 and 3, respectively.
Table 2 Primer pairs used in real-time PCR for quantification of housekeeping genes in fresh and 12-day cultured caprine preantral follicles

Table 3 Primer pairs used for mRNA quantification of RNA for FSH-R, GH-R and FSH-R in fresh and 12-day cultured caprine preantal follicles

Statistical analysis
Data of mRNA expression of different growth factors (EGF, FGF-2, BMP-6, BMP-15, KL, VEGF, GDF-9 and IGF-1) in large preantral follicles were analysed by the paired t-test (p < 0.05). Comparison among R-LH, R-FSH and R-GH were performed using the non-parametric Kruskal–Wallis test (p < 0.05).
Results
Stability of housekeeping genes in caprine preantral follicles
Analysis of starting cDNA determined gene expression stability in fresh and 12-day cultured goat preantral follicles and resulted in gene expression stability values M for each gene. Therefore, stepwise exclusion of unstable genes and subsequent recalculation of the average M-values resulted in a ranking of the genes based on their M-values with the two most stable genes leading the ranking. After stepwise elimination of the least stable gene (18S RNA) it was revealed that the genes with the highest expression stability in goat preantral follicles before and after in vitro culture were β-actin and ubiquitin (Fig. 1a, b).

Figure 1 Stability of housekeeping genes in goat preantral follicles before (a) and after (b) elimination of the least stable housekeeping gene (18S RNA).
Expression of FSH-R, LH-R, GH-R and growth factors in caprine preantral follicles
Real-time PCR demonstrated that the steady-state levels of FSH-R mRNA in fresh preantral follicles are significantly higher than those of LH-R mRNA. However, neither significant difference was observed between FSH-R and GH-R no between LH-R mRNA and GH-R mRNA in caprine preantral follicles (Fig. 2). Expression profiles for eight genes (EGF, GDF-9, BMP-15, VEGF, FGF-2, BMP-6, IGF-1 and KL) were determined relative to the least expressed gene. As shown in Table 4, EGF had the lowest levels of mRNA (1.0) in goat preantral follicles and was used as calibrator. When compared with EGF, all growth factors studied had significantly higher relative mRNA expression rates, i.e., in sequence of increasing expression, GDF-9, BMP-15, BMP-6, FGF-2, VEGF, Kl and IGF-1.
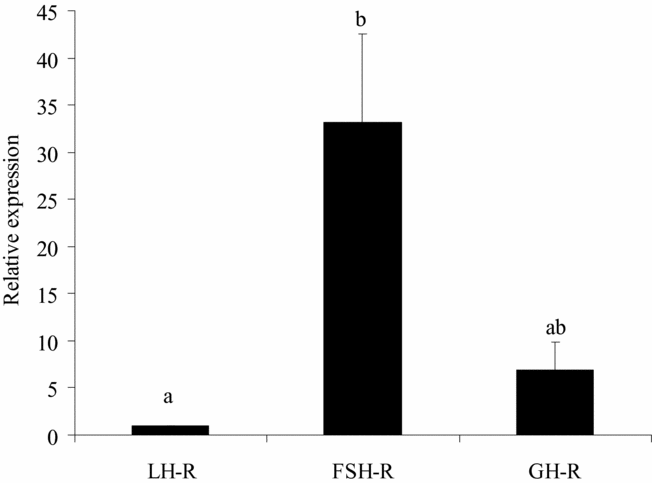
Figure 2 Steady-state levels of FSH-R, LH-R, and GH-R mRNA in caprine preantral follicles. a,bValues with different superscripts are significantly different (p < 0.05).
Table 4 Steady-state levels of mRNA for various growth factors compared with that of EGF in caprine preantral follicles

*Significantly different (p < 0.05) from growth factor.
Relative to GDF-9, the steady-state levels of BMP-6 and IGF-1 mRNA were significantly higher (Fig. 3a), whereas, compared to BMP-15 mRNA, IGF-1 and Kl mRNA had higher levels (Fig. 3b). No significant differences were observed between the mRNA levels of VEGF, BMP-6, FGF-2, Kl and IGF-1 (Fig. 3c), but the mRNA levels of Kl and IGF-1 were significantly higher than that of FGF-2 (Fig. 3b). Furthermore, the mRNA level of IGF-1 was significantly higher than that of FGF-2 (Fig. 3e), but not different from that of Kl (Fig. 3f).

Figure 3 Steady-state level (mean ± SEM) of mRNA for the growth factors BMP-15, VEGF, FGF-2, BMP-6, IGF-1, and Kl compared with that of GDF-9 (a); BMP-15 (b); VEGF (c); FGF-2 (d); IGF-1 (e); and Kl (f) in goat preantral follicles.
*Significantly different (p < 0.05) from growth factor.
Discussion
The current study demonstrated that, among the tested housekeeping genes, β-actin and ubiquitin are the most stable genes in goat preantral follicles, while 18sRNA is the least stable gene. In comparison, ubiquitin, β-actin and GAPDH were the three most stable housekeeping genes in bovine oocytes, while ubiquitin and PGK were the most stable genes in fresh and cultured bovine cumulus cells (Van Tol et al., Reference Van Tol, van Eerdenburg, Colenbrander and Roelen2007). Different from bovine oocytes, GAPDH was considered the least stable gene in zebrafish embryos (Lin et al., Reference Lin, Spikings, Zhang and Rawson2009). The data confirm the opinion of Garcia-Vallejo et al. (Reference Garcia-Vallejo, Van het Hof, Robben, Van Wijk, Van Die, Joziasse and Van Dijk2004) that assessment of the most suitable housekeeping gene(s) for different types of animal tissues and cells is inevitably, and that no reference gene can at forehand be assumed to be suitable for them.
In caprine preantral follicles, derived from the same animals that have been used for the housekeeping gene studies, the level of FSH-R mRNA was higher compared with that of LH-R. Thus far, little is known about the relative expression of hypophyseal hormone receptors in ovarian preantral follicles. Previous studies showed binding sites/receptors for FSH in granulosa cells of preantral and antral follicles and that FSH may act on the development of (Wandji et al., Reference Wandji, Fortier and Sirard1992; Hulshof et al., Reference Hulshof, Figueiredo, Beckers, Bevers, Van der Donk and Van den Hurk1995; Gutierrez et al., Reference Gutierrez, Ralph, Telfer, Wilmut and Webb2000) and growth factor expression in (Joyce et al., Reference Joyce, Pendola, Wigglesworth and Eppig1999; Wang & Roy, Reference Wang and Roy2004; Thomas et al., Reference Thomas, Ethier, Shimasaki and Vanderhyden2005) early and more advanced follicles of various mammalian species. Braw-Tal & Roth (Reference Braw-Tal and Roth2005) demonstrated the presence of LH-R in theca cells of bovine preantral follicles and that this is accompanied by reduced follicular atresia. FSH and LH appeared both able to support murine preantral follicle development in vitro (Cortvrindt et al., Reference Cortvrindt, Hu, Liu and Smitz1998; Wu et al., Reference Wu, Nayudu, Kiesel and Michelmann2000).
In caprine preantral follicles, the level of GH-R mRNA was not significantly aberrant from those of FSH-R and LH-R. To our knowledge there is nothing known in literature about the relative expression levels of these hormones in preantral follicles of other animals. Eckery et al. (Reference Eckery, Moeller, Nett and Sawyer1997) previously demonstrated abundant expression of GH-R mRNA in oocytes and granulosa cells of ovine preantral and antral follicles. In the bovine ovary, GH-R mRNA was detected in oocytes of primordial and primary follicles and in follicular cells from the primary follicle stage onward (cow: Kolle et al., Reference Kolle, Sinowatz, Boie and Lincoln1998; rat: Zhao et al., Reference Zhao, Taverne, van der Weijden, Bevers and van den Hurk2002). In cows, GH was found to promote granulosa cell proliferation and to increase progesterone synthesis (Langhout et al., Reference Langhout, Spicer and Geisert1991), and in rodents, to promote preantral follicle development by positively affecting the proliferation and ultrastructure of both granulosa and theca cells (Liu et al., Reference Liu, Andoh, Yokota, Kobayashi, Abe, Yamada, Mizunuma and Ibuki1998; Kobayashi et al., Reference Kobayashi, Mizunuma, Kikuchi, Liu, Andoh, Abe, Yokota, Yamada, Ibuki and Hagiwara2000; Zhao et al., Reference Zhao, Dorland, Taverne, Van Der Weijden, Bevers and Van Den Hurk2000; Kikuchi et al., Reference Kikuchi, Andoh, Abe, Yamada, Mizunuma and Ibuki2001).
Among the studied growth factor mRNAs in caprine preantral follicles, IGF-1 appeared most highly expressed, followed by KL, VEGF, FGF-2, BMP-6, BMP-15, GDF-9 and EGF, in decreasing extent of expression successively. Involvement of IGF-1 in early stages of folliculogenesis was shown in knock-out experiments with mice, since the development of preantral and antral follicles was impaired in animals lacking the IGF-1 gene (Elvin & Matzuk, Reference Elvin and Matzuk1998). In vitro studies demonstrated that IGF-1 stimulates the development of bovine (Gutierrez et al., Reference Gutierrez, Ralph, Telfer, Wilmut and Webb2000; Fortune et al., Reference Fortune, Rivera and Yang2004) and rat (Zhao et al., Reference Zhao, Taverne, Van Der Weijden, Bevers and Van den Hurk2001) preantral follicles. Kl did increase the number of developing follicles during culture of mouse ovaries, suggesting a role in primordial follicle activation (Parrot & Skinner, Reference Parrot and Skinner1999). The expression of Kl was stimulated by BPM-15, while Kl inhibited BMP-15 expression. In contrast, GDF-9 inhibited Kl expression in cultured granulosa cells (Joyce et al., Reference Joyce, Clark, Pendola and Eppig2000).
VEGF production was brought about by theca cells of human developing follicles (Yamamoto et al., Reference Yamamoto, Konishi, Tsuruta, Nanbu, Kuroda, Matsushita, Hamid, Yura and Mori1997) and in granulosa cells of human maturing follicles (Kamat et al., Reference Kamat, Brown, Manseau, Senger and Dvorak1995), and was found to promote follicular angiogenesis in ruminants and primates (Redmer & Reynolds, Reference Redmer and Reynolds1996). Injection of VEGF in mouse ovaries not only increased vascularization, but also promoted follicle development and reduced follicular apoptosis (Quintana et al., Reference Quintana, Kopcow, Sueldo, Marconi, Rueda and Barañao2004). Alterations in the levels of mRNA for VEGF were observed during primordial to primary follicle transition in rat ovaries (Kezele et al., Reference Kezele, Ague, Nilsson and Skinner2005). In preantral follicles, FGF-2 expression was previously shown in bovine oocytes and granulosa cells (Van Wezel et al., Reference Van Wezel, Umapathysivam, Tilley and Rodgers1995) and in human oocytes (Ben-Haroush et al., Reference Ben-Haroush, Abir, Ao, Jin, Kesler-Icekson, Feldberg and Fisch2005) and granulosa cells (Quennell et al., Reference Quennell, Stanton and Hurst2004). FGF-2 appeared an important regulator of early folliculogenesis, promoting primordial follicle activation (mouse: Nilsson et al., Reference Nilsson, Parrot and Skinner2001; goat: Matos et al., Reference Matos, van den Hurk, Lima-Verde, Luque, Santos, Martins, Báo, Lucci and Figueiredo2006), growth of activated follicles (goat: Matos et al., Reference Matos, van den Hurk, Lima-Verde, Luque, Santos, Martins, Báo, Lucci and Figueiredo2006), and granulosa cell proliferation (chicken: Roberts & Ellis, Reference Roberts and Ellis1999). FGF-2 also plays a major role in ovarian angiogenesis (Reynolds & Redmer, Reference Reynolds and Redmer1998; Berisha et al., Reference Berisha, Sinowatz and Schams2004).
In ovine ovaries, BMP-6 mRNA was previously detected in oocytes of all follicular stages, but not in granulosa and theca cells (Juengel et al., Reference Juengel, Heath, Quirke and McNatty2006). However, in rat (Erickson & Shimasaki, Reference Erickson and Shimasaki2003) ovaries BMP-6 was expressed in both oocytes and granulosa cells. In mouse granulosa cells, BMP-6 was found to inhibit FSH-R expression and to increase FSH-stimulated progesterone production (Otsuka et al., Reference Otsuka, Yamamoto, Erickson and Shimasaki2001). Expression of BMP-15 mRNA was previously detected in all preantral follicle stages (Silva et al., Reference Silva, van den Hurk, Van Tol, Roelen and Figueiredo2004b). In-vitro studies furthermore showed that BMP-15 decreases expression of FSH-R (Otsuka et al., Reference Otsuka, Yamamoto, Erickson and Shimasaki2001) and increases that of Kl in rat granulosa cells (Otsuka & Shimasaki, Reference Otsuka and Shimasaki2002). Thomas et al. (Reference Thomas, Ethier, Shimasaki and Vanderhyden2005) also demonstrated that BMP-15 increases expression of Kl in cultured oocytes and granulosa cells, and that the level of BMP-15 transcripts was reduced in the presence of FSH. BMP15 induced granulosa cell proliferation was inhibited after blocking Kl and c-kit signalling during co-culture of rat granulosa cells and oocytes, which suggests that BMP-15 acts via Kl (Otsuka & Shimasaki, Reference Otsuka and Shimasaki2002; Shimasaki et al., Reference Shimasaki, Moore, Erickson and Otsuka2003). Shimasaki et al. (Reference Shimasaki, Moore, Erickson and Otsuka2003) hypothesized that an interaction between BMP-15 and Kl promotes the development of primordial follicles, while GDF-9 was secreted by oocytes from growing follicles to stimulate granulosa cell proliferation and to modulate Kl action. In the current experiments, expression of BMP-15 may have caused the relative high level of Kl in caprine preantral follicles.
Despite being one of the least expressed growth factor in caprine preantral follicles, GDF-9 was 91 times more expressed than the most weakly expressed EGF gene. Hreinsson et al. (Reference Hreinsson, Scott, Rasmussen, Swahn, Hsueh and Hovatta2002) showed that GDF-9 promotes survival and growth of cultured human preantral follicles, while it inhibits Kl expression in granulosa cells (mouse: Joyce et al., Reference Joyce, Clark, Pendola and Eppig2000; hamster: Wang & Roy, Reference Wang and Roy2004) and preantral follicle development (hamster: Wang & Roy, Reference Wang and Roy2004). Consequently, relatively low expression of GDF-9 may have contributed for high levels of Kl in caprine preantral follicles. In addition, GDF-9 synergizes with FSH to promote growth and differentiation of murine preantral follicles (Hayashi et al., Reference Hayashi, Mcgee, Min, Klein, Rose, Van Duin and Hsueh1999). In bovine, GDF-9 reduced the production of progesterone and oestradiol by granulosa cells (Spicer et al., Reference Spicer, Aad, Allen, Mazerbourg and Hsueh2006) and that of progesterone and androstenedione by theca cells (Spicer et al., Reference Spicer, Aad, Allen, Mazerbourg, Payne and Hsueh2008), respectively.
In caprine preantral follicles, EGF came up as the least expressed gene among the growth factor mRNAs tested. In mice, the follicular EGF mRNA level is regulated by LH (Hsieh et al., Reference Hsieh, Zamah and Conti2009), while EGF, amphiregulin, epiregulin, and betacellulin are potent stimulators of oocyte maturation and cumulus expansion. Several papers reported that EGF inhibits the expression of LH-R (e.g., Hattori et al., Reference Hattori, Yoshino, Shinohara, Horiuchi and Kojima1995) and increases the expression of FSH-R (e.g., Luciano et al., Reference Luciano, Pappalardo, Ray and Peluso1994). In vitro studies furthermore showed that EGF stimulates growth of bovine preantral follicles (Gutierrez et al., Reference Gutierrez, Ralph, Telfer, Wilmut and Webb2000), and promotes granulosa cell proliferation in porcine preantral follicles (Morbeck et al., Reference Morbeck, Flowers and Britt1993), as well as growth of these follicles up to the antral stage, while reducing degeneration of the granulosa cells (Mao et al., Reference Mao, Smith, Rucker, Wu, McCauley, Cantley, Prather, Didion and Day2004).
In conclusion, ubiquitin and β-actin are the most stable genes among several tested putative housekeeping genes in fresh and cultured caprine preantral follicles, and thus are most useful in normalizing starting quantities of cDNA during RT-PCR analysis. Among eight tested growth factors, IGF-1 was most abundantly expressed in goat follicles, whereas EGF showed the weakest expression. Knowing the levels of mRNA for hormone receptors and growth factors in fresh preantral follicles is very important for in vitro studies, since will provide helpful information to choose which growth factor or hormone will be used as a supplement for culture medium.
Acknowledgements
This study was supported by CNPq and FUNCAP.









