Introduction
The matrinxã Brycon amazonicus belongs to the Characidae family and can be found in the Amazon River and in some Brazilian tributaries (Lima, Reference Lima2003). It is a rheophilic fish, with an annual reproductive cycle and total spawning. It presents with good zootechnical performance and acceptance in the consumer market (Ninhaus-Silveira et al., Reference Ninhaus-Silveira, Foresti, Veríssimo-Silveira and Senhorini2006). As it does not reproduce spontaneously in captivity, hormone induction techniques using pituitary fish extract and synthetic inducers have been used to aid artificial reproduction in these fish, and mainly in studies that have focused on oocyte quality (Ramos et al., Reference Ramos, Ramos and Medonça1997; Ramos, Reference Ramos2000; Romagosa et al., Reference Romagosa, Narahara, Borella and Fenerich-Verani2001; Pardo-Carrasco et al., Reference Pardo-Carrasco, Suárez-mahecha, Munõz-Lara, Arias-Castellanos and Gil2002, Reference Pardo-Carrasco, Zaniboni-Filho, Arias-Castellanos, Suárez-Mahecha, Atencio-García and Cruz-Casallas2006; Zaniboni-Filho & Weingartner, Reference Zaniboni-Filho and Weingartner2007; De Alexandre et al., Reference De Alexandre, Ninhaus-Silveira, Veríssimo-Silveira, Buzollo, Senhorini and Chaguri2010; Nakaghi et al., Reference Nakaghi, Neumann, Faustino, Mendes and De Braga2014; Bashiyo-Silva et al., Reference Bashiyo-Silva, Costa, Ribeiro, Senhorini, Veríssimo-Silveira and Ninhaus-Silveira2016).
The pituitary extracts of mature fish used for ovulation induction and spermiation of rheophilic species since the 1930s have more recently been replaced by synthetic analogues of gonadotrophin releasing hormone – GnRHa (Zohar & Mylonas, Reference Zohar and Mylonas2001), such as Ovaprim™, which is a synthetic product of salmon GnRHa with domperidone, a dopaminergic inhibitor, successfully employed in spermiation of salmonids, cyprinids (Mousavi et al., Reference Mousavi, Yousefian, Masoudnia and Fahimi2011; Cejko et al., Reference Cejko, Żarski, Krejszeff, Kucharczyk and Kowalski2013) and some Brazilian native species (Viveiros et al., Reference Viveiros, Gonçalves, Di Chiacchio, Nascimento, Romagosa and Leal2013).
Although female gametes have been afforded greater emphasis in breeding studies, it is also necessary to characterize semen samples to verify the potential for fertilizing breeding fish. This task includes the evaluation of any morphological abnormalities in spermatozoa. However, studies on sperm morphology in Brazilian native fish have so far been limited (Kavamoto et al., Reference Kavamoto, Barnabe, Campos and Andrade-Talmelli1999; Streit Jr et al., Reference Streit, Sirol, Ribeiro, Moraes, Galo and Digmayer2008; Murgas et al., Reference Murgas, Felizardo, Ferreira, Andrade and Veras2011; Maria et al., Reference Maria, Azevedo, Santos and Carneiro2012). The increase in sperm diseases has led to reduced sperm motility and vigour (Lahnsteiner et al., Reference Lahnsteiner, Berger, Weismann and Patzner1998). As a result, there has been a decrease in fertilization capacity (Rurangwa et al., Reference Rurangwa, Kime, Ollevier and Nash2004). Determination of the acceptable percentage change in these parameters is important to gauge semen quality. This information can aid in the selection of hormone therapies for fish artificial reproduction, due the importance of morphological alterations in spermatozoa after hormone treatment (Kavamoto et al., Reference Kavamoto, Barnabe, Campos and Andrade-Talmelli1999; Moraes et al., Reference Moraes, Streit, Ribeiro, Sakaguti, Souza and Povh2004; Streit Jr et al., Reference Streit, Sirol, Ribeiro, Digmayer, Galo, Moraes and Povh2012). Therefore, this study aimed to investigate if hormone treatment with CPE or Ovaprim™ affected the frequency of sperm abnormalities in B. amazonicus.
Materials and methods
Four-year-old males from a wild stock belonging to the Federal University of Amazonas (UFAM) Experimental Farm Aquaculture Station, Manaus – Amazonas – Brazil (02°38′56.1′′S; 060°03′14.7′′W) were used in the study. The fish were kept in earthen ponds (550 m2) and fed twice daily to their apparent satiation with commercial fish feed that contained 32% crude protein.
Twelve specimens were selected for hormone treatment in December 2014, transported to Aquaculture Station reproduction area and placed in two circular glass fibre tanks with a capacity of 3000 litres each. Water was constantly renewed and aerated, and kept at an average temperature of 27.7±1.0ºC and pH 7.9±0.2.
The specimens were identified with transponders (AnimallTAG) and coloured threads attached to the dorsal fin. They were randomized in two treatment groups (inducing hormones) and a control group, with four replicates each. Males received single dose injections intramuscularly in the base of the dorsal fin, according to treatment protocols: (1) Ovaprim™ – 0.5 ml/kg (Syndel Laboratories Ltd, Canada); (2) carp pituitary extract (CPE) – 2.0 mg/kg (Danúbio Aquaculture Ltd, Brazil); and (3) 0.9% NaCl solution (control) – 0.5 ml/kg. Body weight was 1.159±0.15 kg and dosages were calculated per kg body weight of the breeding fish. The animals had not been subjected previously to hormone therapy.
Semen collection occurred 12 h after injection. Each specimen was taken from the tank, placed in a container containing Eugenol™ 50 mg/l solution (Vidal et al., Reference Vidal, Furuya, Graciano, Schamber, Silva, Santos and Souza2007) to reduce stress during this procedure. Semen samples from each specimen were fixed with buffered saline formaldehyde at a ratio of 10:990 (semen:fixative). Two wet preparations were obtained from each animal with a 10-μl aliquot of the fixed semen, stained with 3% Rose Bengal (Streit Jr et al., Reference Streit, Moraes, Ribeiro, Povh, Souza and Oliveira2004), placed on a slide and covered with a cover slip.
Images were obtained using an optical microscope (Axiophot2, Zeiss) with an immersion objective, with a digital camera (AxioCam MRC, Zeiss) attached to the microscope and to a computer, and were scanned using Zen Lite 2012 software using Thematic Laboratory of Optical and Electronic Microscopy (LTMOE).
Sperm cells were counted over a minimum of 200 spermatozoa from each male, classifying them into either normal cells or abnormal cells having only one anomaly. Primary and secondary abnormalities were analyzed according to the fish classification model proposed by Miliorini et al. (Reference Miliorini, Murgas, Rosa, Oberlender, Pereira and Costa2011) and the data were recorded as percentage of abnormal sperm cells.
Fixed samples of two males from each hormonal treatment group as well as from the control group were used to obtain the electromicrographs in scanning electron microscopy (SEM). The samples were washed in 0.1 M sodium cacodylate buffer and kept refrigerated at 5°C until dehydration, carried out using increasing concentrations of ethanol (30–95%) for 10 min each and in 100% ethanol, twice, for 10 min each. The coverslips were subjected to the Critical Point Dry Cleaner with liquid CO2, and later assembled into stubs, metallized and electromicrographed on a scanning electron microscope LEO 435VP, Zeiss, in the LTMOE laboratory.
Statistical analysis
The experiment was conducted using a completely randomized design with three treatments (two hormonal treatments groups and one control group without hormonal treatment) and four replicates (each male was considered as one replicate). Data were expressed as mean±standard error (mean±SE). To determine statistical significance (P<0.05), analysis of variance (ANOVA) and Holm–Sidak Test were applied to the morphological variables. The analyses were carried out using the SigmaStat 3.5 program.
Results
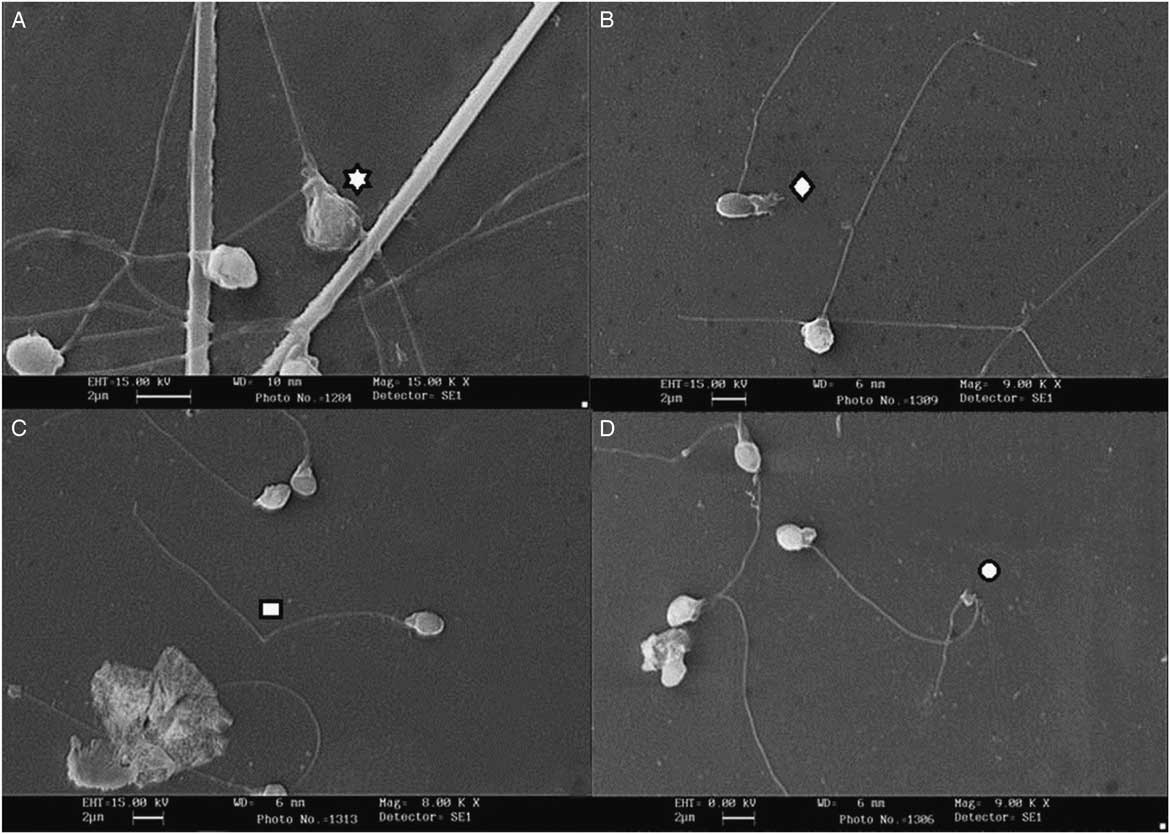
The percentage of normal spermatozoa was not significantly different between the treatment groups. No significant differences were detected between treatment groups in relation to the total primary abnormalities (Fig. 1) and secondary abnormalities (Fig. 2 and Table 1).

Figure 1 Scanning electromicrographs of B. amazonicus spermatozoa. (A) Degenerated head. (B) Degenerated midpiece. (C) Fractured tail. (D) Strongly curled tail.

Figure 2 Scanning electromicrographs of B. amazonicus spermatozoa. (A) Isolated heads (B) Folded tail.
Table 1 Percentage of normal spermatozoa, primary and secondary abnormalities (mean±SE) observed in B. amazonicus induced with Ovaprim™, carp pituitary extract (CPE) and control group

There were no significant differences between the treatment groups in relation to the primary abnormalities found in the head, the midpiece and the flagellum. Also, there were no significant differences between the treatment groups for secondary abnormalities located in the head and in the flagellum (Table 2)
Table 2 Percentage of abnormalities in the head, midpiece and flagellum (primary) and in the head and flagellum (secondary) verified in B. amazonicus induced with Ovaprim™, carp pituitary extract (CPE) and control group

When the sperm alterations were evaluated individually, only the percentage of degenerated head abnormalities presented a significant difference among the spermatozoa in the group treated with CPE compared with those in the control group (Figs 3 and 4 and Table 3).

Figure 3 Phase contrast optical microscopy photographs of B. amazonicus spermatozoa. (A) Sct: Strongly curled tail, Ft: Folded tail. (B) Dh: Degenerated head, Ih: Isolated head. (C) Dh: Degenerated head, Ft: Fractured tail. (D) Sct: Strongly curled tail, Dm: Degenerated midpiece.

Figure 4 Phase contrast optical microscopy photographs of B. amazonicus spermatozoa. (A) Sct: Strongly curled tail. (B) Ft: Folded tail, N: Normal. (C) Sct: Strongly curled tail. (D) Ct: Coiled tail; N: Normal.
Table 3 Percentage of sperm abnormalities (mean±SE) observed in B. amazonicus semen after induction with Ovaprim™, carp pituitary extract (CPE) and control group

*Different superscript letters on the same line indicate a significant difference (P<0.05).
Discussion
Several authors have related the morphological alterations of fish spermatozoa to the use of hormone therapies. Kavamoto et al. (Reference Kavamoto, Barnabe, Campos and Andrade-Talmelli1999) found that spermatozoa of Prochilodus lineatus presented abnormalities after being treatment with human chorionic gonadotropin. Moraes et al. (Reference Moraes, Streit, Ribeiro, Sakaguti, Souza and Povh2004) compared the effects of pituitary extracts of carp, chicken and rabbit, and found that Cyprinus carpio and Leporinus macrocephalus had high secondary sperm abnormalities when treated with rabbit pituitary extract. In Pseudoplatystoma reticulatum, there was a decrease in the occurrence of primary abnormalities after treating males with CPE (Streit Jr et al., Reference Streit, Sirol, Ribeiro, Digmayer, Galo, Moraes and Povh2012).
Colégio Brasileiro De Reprodução Animal (The Brazilian College of Animal Reproduction – CBRA) (2013), an organization that regularizes the semen quality criteria of breeding animals, established that abnormal sperm percentages above 30% for cattle, equine and swine, 20% for sheep and goats, and 10% for poultry, compromise artificial insemination. Although it has not been advocated by CBRA, it is estimated that the percentages of acceptable sperm defects are higher for fish because their sperm concentration is higher than the terrestrial species (Streit Jr et al., Reference Streit, Sirol, Ribeiro, Digmayer, Galo, Moraes and Povh2012). According to Miliorini et al. (Reference Miliorini, Murgas, Rosa, Oberlender, Pereira and Costa2011), it is probable that the critical percentage of sperm abnormalities of migratory fishes with external fertilization and artificial reproduction is about 50%, as artificial fertilization involves a large proportion of spermatozoa per oocyte in a controlled environment. In this study, although the percentage of abnormal sperm cells was elevated (39–50.4%), other species also had relatively high rates of deformities, varying from 32 to 65% (Streit Jr, Reference Streit2002; Moraes et al., Reference Moraes, Streit, Ribeiro, Sakaguti, Souza and Povh2004; Bombardelli et al., Reference Bombardelli, Mörschbächer, Campagnolo, Sanches and Syperreck2006; Streit Jr et al., Reference Streit, Sirol, Ribeiro, Moraes, Galo and Digmayer2008; Garcia et al., Reference Garcia, Vasconcelos, Povh, Ribeiro, Eloy and Streit2015).
Sperm abnormalities may limit sperm motility and vigour, interfering with fertilization rates (Cosson et al., Reference Cosson, Dreanno, Billard, Suquet and Cibert1999) and, according to Freneau (Reference Freneau2011), the primary abnormalities have a greater effect on fertilization. However, the high percentage of primary abnormalities observed may be insignificant, considering that the species presents a high sperm concentration. Fertilization tests are required to confirm this hypothesis.
The percentages of spermatozoa, observed with primary and secondary abnormalities, were similar to those found for other CPE-induced species. In Rhamdia quelen it was verified that 5.8% had primary alterations and 26.3% had secondary alterations (Bombardelli et al., Reference Bombardelli, Mörschbächer, Campagnolo, Sanches and Syperreck2006). In Piaractus mesopotamicus, 12.4% presented primary and 24.6% presented secondary alterations (Streit Jr, Reference Streit2002). Moraes et al. (Reference Moraes, Streit, Ribeiro, Sakaguti, Souza and Povh2004) reported the following percentages of abnormal sperms: Prochilodus lineatus 40.2%, Leporinus macrocephalus 49% and Cyprinus carpio 37.4%. In Salminus maxillosus, 56.7% were normal before treatment and 48% were normal after this (Streit Jr et al., Reference Streit, Sirol, Ribeiro, Moraes, Galo and Digmayer2008). Maria et al. (Reference Maria, Azevedo, Santos and Carneiro2012) reported 85% of normal spermatozoa after treatment with CPE and 75% normal in the non-hormone-treatment group for Colossoma macropomum. Martins et al. (Reference Martins, Streit, Abreu, Correa-Filho, Oliveira, Lopera-Barrero and Povh2017) in a study on the effects of CPE, Ovopel (GnRHa and metoclopramide) and control group, showed no difference between groups for C. macropomum. Garcia et al. (Reference Garcia, Vasconcelos, Povh, Ribeiro, Eloy and Streit2015) reported no significant changes for Brycon insignis comparing groups before and after treatment with CPE or GnRH analogues. Possibly, the defects observed are related to factors other than spawning inducing hormones.
There was no observation in the samples of tails with proximal or distal cytoplasmic droplets. The same finding was observed by Moraes et al. (Reference Moraes, Streit, Ribeiro, Sakaguti, Souza and Povh2004) in Prochilodus lineatus, Leporinus macrocephalus, Cyprinus carpio and by Streit Jr et al. (Reference Streit, Sirol, Ribeiro, Moraes, Galo and Digmayer2008) in S. maxillosus.
According to Hafez & Hafez (Reference Hafez and Hafez2004) and Herman et al. (Reference Herman, Mitchell and Doak1994), the secondary abnormalities may be related to the preparation of the smears and primary abnormalities are related to failures during spermatogenesis in mammals. These same authors related the origin of sperm abnormalities with nutritional deficiency, age, consanguinity and males diseases, besides the ambient temperature and problems in the spermatic duct. Changes in fish sperm can be caused by xenobiotics, genetic mutation or ageing, or by conservation protocols (Fauvel et al., Reference Fauvel, Suquet and Cosson2010). The origin of the sperm abnormalities is not fully understood, and it is only possible to assume the same origin (Araújo et al., Reference Araújo, Streit, Ribeiro, Martins, Souza, Oliveira, Ribeiro, Lopera-Barrero and Povh2014; Streit Jr et al., Reference Streit, Sirol, Ribeiro, Moraes, Galo and Digmayer2008; Streit Jr et al., Reference Streit, Benites, Moraes, Ribeiro, Sakaguti and Caldieri2006).
Conversely, the collection of semen by extrusion can force the spermatozoa to exit at different stages of development. In this case, some sperm cells may still be immature, and therefore unviable (Asturiano et al., Reference Asturiano, Marco-Jiménez, Pérez, Balasch, Garzon, Peñaranda, Vicente, Viudes-de-Castro and Jover2006). According to Maria et al. (Reference Maria, Azevedo, Santos and Carneiro2012), structural damage in spermatozoa can be attributed to increased abdominal pressure exerted at the time of semen extrusion.
The quality of fish gametes depends on the appropriate hormone environment during development, but this may be disturbed by stress (Kime & Nash, Reference Kime and Nash1999). Stress increases the level of cortisol and decreases steroid testosterone and ketotestosterone levels (Rurangwa et al., Reference Rurangwa, Kime, Ollevier and Nash2004). These hormones are involved in sperm maturation and testicular hydration (Schulz & Miura, Reference Schulz and Miura2002). Stress, in some species, induced changes in plasma osmolarity, which in turn affected sperm quality, reducing sperm motility after activation and also significantly decreased sperm count compared with non-stressed controls (reviewed in Rurangwa et al., Reference Rurangwa, Kime, Ollevier and Nash2004). According to Donaldson et al. (Reference Donaldson, Solar and Harvey2000), artificial reproductive management is a stressful factor and may influence spermiogenesis. Bromage (Reference Bromage1995) considers that stress caused by capture and manipulation in the induction tank may influence reproductive rates. Matrinxã is an extremely aggressive fish during capture and in the process of artificial propagation, however detailed studies are lacking to infer that the sperm deformities found could be attributed to stress during handling.
Considering the results of the present study, we can conclude that the hormonal therapy of matrinxã can be performed with both Ovaprim™ and CPE, without influencing the percentage of sperm abnormalities in B. amazonicus.
Acknowledgements
The author thanks Dr Elizabeth Gusmão Affonso for acting as an intermediary for Thematic Laboratory of Optical and Electronic Microscopy (LTMOE/INPA).
Ethics statement
The authors assert that all procedures contributing to this work comply with the Ethical Principles of Animal Experimentation, adopted by the Brazilian College of Animal Experimentation (COBEA), and were approved by the Ethics Committee on Animal Use (CEUA) – Federal University of Amazonas (protocol no. 019/2014).
Conflicts of interest
There are no conflicts of interest.