Introduction
Immature equine oocytes are capable of completing meiosis in vitro, but subsequent in vitro fertilization (IVF) and embryonic development of those oocytes are questionable (Dell’Aquila et al., Reference Dell’Aquila, Fusco, Lacandra and Mariato1997; Zhao et al., Reference Zhao, Taverne, Van Der Weijden, Bevers and Van Den Hurk2001; Hinrichs, Reference Hinrichs2010). The effect of growth hormone (GH) on mammalian oocytes and embryo development in vitro has been studied in human (Hassan et al., Reference Hassan, Azab, Rahman and Nafee2001), rat (Yoshimura et al., Reference Yoshimura, Iwashita, Karube, Oda, Akiba, Shiokawa, Ando, Yoshinaga and Nakamura1994) and bovine (Kolle et al., Reference Kolle, Stojkovic, Prelle, Waters, Wolf and Sinowatz2001; Reference Kolle, Stojkovic, Reese, Reichenbach, Wolf and Sinowatz2004). In addition, studies have also demonstrated that equine oocytes resumed meiosis in the presence of equine GH (eGH) in vitro (Marchal et al., Reference Marchal, Caillaud, Martoriati, Gerard, Mermillod and Goudet2003; Pereira et al., Reference Pereira, Lorenzo, Carneiro, Ball, Goncalves, Pegoraro, Bilodeau-Goeseels, Kastelic, Casey and Liu2012). Recently, we have reported the presence of eGH-R in equine ovarian follicular structures such as cumulus cells and oocyte, which may mediate a positive effect when eGH is used in culture during equine oocyte maturation in vitro (Pereira et al., Reference Pereira, Lorenzo, Carneiro, Ball, Bilodeau-Goeseels, Kastelic, Pegoraro, Pimentel, Esteller-Vico, Illera, Granado, Casey and Liu2013a). Furthermore, investigations are needed for a better understanding of the developmental competence of equine oocytes when eGH is added during in vitro maturation (IVM) to improve the efficiency and the use of assisted reproductive technologies (ART) in the horse.
Studies have demonstrated the critical roles of GH action, GH receptor (GH-R) and IGF-I in the mediation of cell growth (Liu & LeRoith, Reference Liu and LeRoith1999; Lupu et al., Reference Lupu, Terwilliger, Lee, Segre and Efstratiadis2001). The effects of rbGH on the IVM of bovine oocytes and cumulus cell expansion inhibition were exerted through kinase activity, thus indicating that rbGH action is not mediated by a tyrosine kinase pathway in bovine (Izadyar et al., Reference Izadyar, Colenbrander and Bevers1997a). In cattle, GH action on oocytes is not mediated by IGF-I but is mediated by the cyclic AMP (cAMP) signal transduction pathway (Izadyar et al., Reference Izadyar, Van Tol, Colenbrander and Bevers1997b). There have been no published reports on the level at which the eGH could be inhibited during IVM of equine oocytes, with this in mind we investigated if the possible eGH effect is mediated through the cAMP or adenylate cyclase (AC) cascade.
cAMP plays an important role in the control of oocyte maturation and development in mammals (Raper et al., Reference Raper, Kothary, Ishoo, Dikin, Kokudo, Hashimoto and DeMatteo1995; Liu et al., Reference Liu, Kong, Xia and Zhang2013). The AC is composed of two cytoplasmic domains and two membrane-spanning domains, which interact with G-protein to stimulate or inhibit its enzyme activity. The activation of G-protein in turn activates the AC resulting in conversion of adenosine triphosphate (ATP) to cAMP. Addition of a specific inhibitor (H-89) against cAMP-dependent protein kinase was used to determine at which point eGH could be inhibited through the cAMP pathway (Chijiwa et al., Reference Chijiwa, Mishima, Hagiwara, Sano, Hayashi, Inoue, Naito, Toshioka and Hidaka1990). A cell permeable adenosine analog that binds to a receptor located on the catalytic subunit of the AC blocks cAMP production, inhibiting the transduction pathway and the effect of GH on nuclear progression (Izadyar et al., Reference Izadyar, Colenbrander and Bevers1997a). 2′,3′-Dideoxyadenosine (DDA) is a specific adenylate cyclase inhibitor and is a cell permeable adenosine analog that binds to a receptor located on the catalytic subunit of the AC, inhibiting the transduction pathway and the effect of GH on oocyte nuclear progression in bovine species (Izadyar et al., Reference Izadyar, Colenbrander and Bevers1997a). These findings suggest that the cAMP pathway mediates the stimulatory effect of GH on IVM of bovine oocytes. However, little information is known about the role that eGH might play in the signal transduction mechanism by which GH exerts its cellular activities in equine oocytes matured in vitro.
Studies have suggested that the presence of GH-R mRNA in equine denuded oocytes may have a possible role for the eGH to act directly on the oocyte, confirming the importance of eGH in the ovarian physiology of the horse (Marchal et al., Reference Marchal, Caillaud, Martoriati, Gerard, Mermillod and Goudet2003; Pereira et al., Reference Pereira, Lorenzo, Carneiro, Ball, Bilodeau-Goeseels, Kastelic, Pegoraro, Pimentel, Esteller-Vico, Illera, Granado, Casey and Liu2013a,Reference Pereira, Lorenzo, Carneiro, Ball, Pegoraro, Pimentel and Liub). The addition of eGH to the maturation medium positively influences the in vitro nuclear and cytoplasmic maturation of equine oocytes (Pereira et al., Reference Pereira, Lorenzo, Carneiro, Ball, Goncalves, Pegoraro, Bilodeau-Goeseels, Kastelic, Casey and Liu2012). Therefore, the present study was conducted to investigate if this eGH effect is removed when specific AC and cAMP-dependent protein kinase A inhibitors are added to the IVM of equine oocytes.
Materials and methods
Source of cumulus–oocyte complexes (COCs)
Unless otherwise stated, all chemicals, reagents, and buffers were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Equine ovaries were obtained during the physiological breeding season from abattoirs located approximately 20 min from the Lethbridge Research Centre, Alberta, Canada. Immediately after collection, ovaries were transported in warmed saline solution to the laboratory in a thermal container at 25–30°C. The tunica albuginea was removed to prevent contamination and to allow the visualization of follicles on the surface. Ovaries selected for puncture were stripped and each follicle of <25 mm in diameter was punctured and aspirated with an 18-gauge needle connected to a 35 ml syringe. During aspiration, a scraping motion was performed to each follicle with a needle to improve oocyte recovery. The follicular fluid was flushed in and out of the follicle two to four times to optimize the retrieval of oocytes present in each follicle. The oocytes and follicular fluid from each follicle aspirated were placed into 15 ml polypropylene tubes. After 20 min of sedimentation, the pellet was collected using a Pasteur pipette and placed in 35-mm Petri dishes to examine the contents under a stereomicroscope and to locate the COCs.
Morphology and structural integrity of each oocyte were evaluated with its own follicular fluid. Retrieved COCs were classified as being compact (CP; having a tight, complete compact cumulus with a distinct, smooth hillock), expanded (EX; having a granular or expanded cumulus), or denuded (D; having a partial cumulus or only corona radiata present) (Hinrichs & Williams, Reference Hinrichs and Williams1997; Hinrichs & Schmidt, Reference Hinrichs and Schmidt2000). Only oocytes with a compact cumulus were used in this experiment. The basal medium for oocyte washing and maturation was tissue culture media 199 (TCM-199), 0.1% of bovine serum albumin (BSA; fraction-V), 100 IU/ml penicillin G and 50 μg/ml streptomycin sulfate. The medium was filtered and allowed to equilibrate for 1 h under 5% CO2 in air before being used. The interval between obtaining ovaries from the slaughterhouse to the beginning of oocyte culture ranged from 2–4 h.
Culture of COCs
Equine GH provided by The National Hormone & Peptide Program (NHPP; Harbor-UCLA, CA) was diluted in tissue culture medium and used at a concentration of 400 ng/ml, previously determined to be optimal for oocyte nuclear maturation (unpublished data) and according to Pereira et al. (Reference Pereira, Lorenzo, Carneiro, Ball, Goncalves, Pegoraro, Bilodeau-Goeseels, Kastelic, Casey and Liu2012; Reference Pereira, Lorenzo, Carneiro, Ball, Pegoraro, Pimentel and Liu2013b). Selected COCs were allocated randomly into basal media (control group) supplemented with 400 ng/ml of eGH alone (eGH group) and allocated into a four-well Petri dish (Nunc Intermed, Roskilde, Denmark) containing 500 μl of maturation medium and incubated at 38.5°C in air with a 5% CO2 atmosphere and >98% relative humidity for 30 h. Before the incubation period, COCs were washed three times in the basal medium for selection and also to prevent contamination during culture.
In addition to 400 ng/ml of eGH, a specific AC inhibitor (2′,3′-dideoxyadenosine; DDA) (288103; Calbiochem) was added in vitro at 100 μM/ml. Furthermore, a specific inhibitor of cyclic AMP-dependent protein kinase-A (N-[2-(p-bromociannamy-1)ethyl]-5-isoquinolinesulfonamide; H-89) (371963; Calbiochem) was added at 10 μM/ml to determine the possible role of eGH on the maturation of equine oocytes in vitro. DDA was used at 10−8, 10−10 or 10−14 M and H-89 at 10−9, 10−11 or 10−15 M in the IVM system. Both inhibitors were used at two different concentrations, one below, and the other above the recommended dilution in the literature to determine the level by which eGH would be inhibited (Chijiwa et al., Reference Chijiwa, Mishima, Hagiwara, Sano, Hayashi, Inoue, Naito, Toshioka and Hidaka1990; Izadyar et al., Reference Izadyar, Colenbrander and Bevers1997a).
Oocyte fixation and nuclear status assessment
Oocytes were removed from culture after 30 h of maturation and the cumulus cells were stripped with 1% hyaluronidase solution by a microcapillary glass pipette. After cumulus cell removal, the oocytes were incubated in 10 μg/ml of bis-benzamide (Hoechst 33342) for 20 min at 39°C, and pipetted onto a slide containing a strip of silicone sealant at the top and bottom. A coverslip was placed directly over the centre of the drop containing the oocytes within a 25-μl drop of mounting medium (Vector Labs. Inc., Burlingame, CA, USA). Oocytes were evaluated using an epifluorescence microscope (AE-30; Motic Co., Richmond, BC, Canada) with a 365-nm exciter filter to evaluate nuclear maturation.
Oocytes were classified on the basis of nuclear chromosomal status and by the presence of a polar body (PB). Oocytes showing no signs of nuclear development were classified as an oocyte at the germinal vesicle stage (GV). Oocytes having a condensed chromatin configuration were characterized as at the metaphase I (MI) stage of development. The presence of chromosomes aligned in the metaphase plate and the first PB were indicators of a complete nuclear maturation and were recorded as the percentage of oocytes that reached metaphase II (MII) (Fig. 1).
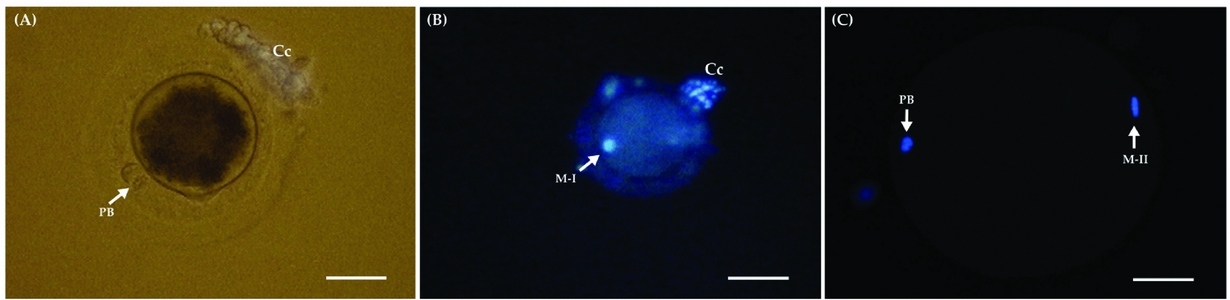
Figure 1 (A) Photomicrograph of an in vitro-matured equine oocyte showing PB after 30 h of culture. (B) Oocyte showing a condensed chromatin configuration were characterized as at the metaphase I (M-I) stage of development. (C) Oocyte showing the presence of chromosomes aligned in the metaphase plate and the first PB. Cumulus cells (Cc); polar body (PB); metaphase I (M-I); metaphase II (M-II). (A, B) Bars represent 10 μm. (C) Bar represent 20 μm.
Statistical analyses
The difference in oocyte maturation after treatment groups (control, eGH, DDA and H-89 treatment groups) on IVM was performed by chi-squared (χ2) test. Fischer's exact test was used when an expected value cell was less than 5. Values were considered to be statistically significant when the P-value was < 0.05.
Results
The effect of eGH on nuclear maturation of equine oocytes
Overall, we observed 11 (6.6%) degenerated oocytes, 78 (47%) had no evidence of development (GV), whereas 30 (18.1%) and 47 (28.3%) reached the MI and MII stages, respectively (Table 1). Oocytes reaching the MII stage of development showed a higher (P < 0.05) nuclear maturation rate when matured in the presence of eGH (29 of 84; 34.5%) compared with the control (18 of 82; 21.9%). The eGH-treated group (45 of 84; 53.5%) increased (P < 0.05) the number of oocytes reaching any stage of development when compared with the control (32 of 82; 39%), when data from MI and MII were combined.
Table 1 Effect of equine growth hormone (eGH) at 400 ng/ml on the nuclear maturation of equine oocytes in vitro matured after 30 h of culture

a,b Different superscripts within a column indicate differences (P < 0.05) among groups.
eGH, equine growth hormone; GV, germinal vesicle; MI, metaphase I; MII, metaphase II.
The inhibitory effect of H-89 on IVM of equine oocytes
The number of oocytes reaching maturity when the inhibitor H-89 used at 10−9 M in addition to eGH-treated group (4 of 29; 13.8%) decreased (P < 0.05) compared with the eGH group (11 of 29; 38%; Table 2). Addition of H-89 alone at 10−15 M into the COC culture did not inhibit (P > 0.05) equine oocyte development to MII (5 of 16; 31.3%) when compared wth H-89 10−11 M (2 of 18; 11.1%) and 10−9 M (4 of 17; 23.6%), although it differed (P > 0.05) from eGH alone (11 of 29; 38%). However, H-89 used at a concentration of 10−11 M decreased (P < 0.05) the number of oocytes reaching the MI stage (2 of 18; 11.1%) when compared with H-89 10−11 M concentration in combination with eGH (14 of 36; 38.9%). No differences were seen among the other treatments.
Table 2 The effect of a specific inhibitor against cyclic AMP-dependent protein kinase (H-89: 10−9, 10−11 or 10−15 M) on nuclear maturation of equine oocytes matured in vitro with equine growth hormone after 30 h of culture

a,b,c Different superscripts in the same column indicate differences (P < 0.05).
eGH, equine growth hormone; GV, germinal vesicle; MI, metaphase I; MII, metaphase II; H-89, N-[2-(p-bromociannamy-1)ethyl]-5-isoquinolinesulfonamide.
The inhibitory effect of DDA on IVM of equine oocytes
The DDA inhibitor at a concentration of 10−8 M in the presence of eGH (2 of 27; 7.4%) did reduce the number of oocytes reaching MII stage compared with the eGH group alone (9 of 30; 30%) and with the 10−14 M + eGH (9 of 26; 34.6%) treatment group (P < 0.05). No differences were seen among the other treatments. Data are shown in Table 3.
Table 3 The effect of a specific adenylate cyclase inhibitor (DDA: 10−8, 10−10 or 10−14 M) on nuclear maturation of equine oocytes matured in vitro with equine growth hormone after 30 h of culture

Different superscripts in the same column indicate differences (P < 0.05).
eGH, equine growth hormone; DDA, 2′,3′-dideoxyadenosine; GV, germinal vesicle; MI, metaphase I; M II, metaphase II.
Discussion
Results from this study demonstrated that the addition of eGH at 400 ng/ml increased the development of immature equine oocytes to the MII stage during IVM. In addition, we observed that 53.5% of compact equine COCs resumed meiosis in the presence of eGH. Moreover, we observed that the lower number of evaluated oocytes used for final interpretation of the results was due to the fact that some equine COCs were lost during cumulus cells removal prior to the staining procedure. The use of rbGH at 100 ng/ml in vitro has been demonstrated to improve nuclear and cytoplasmic maturation rates of bovine oocytes and stimulate subsequent embryonic development to the blastocyst stage (Izadyar et al., Reference Izadyar, Colenbrander and Bevers1996, Reference Izadyar, Hage, Colenbrander and Bevers1998). Growth hormone acts as a survival factor during in vitro culture and reduces apoptosis during early embryogenesis in the bovine species (Izadyar et al., Reference Izadyar, Van Tol, Hage and Bevers2000; Moreira et al., Reference Moreira, Paula-Lopes, Hansen, Badinga and Thatcher2002). In the horse, addition of eGH has shown an overall nuclear and cytoplasmic maturation rate of approximately 60% for oocytes collected from slaughterhouse ovaries (Pereira et al., Reference Pereira, Lorenzo, Carneiro, Ball, Bilodeau-Goeseels, Kastelic, Pegoraro, Pimentel, Esteller-Vico, Illera, Granado, Casey and Liu2013a, Reference Pereira, Lorenzo, Carneiro, Ball, Goncalves, Pegoraro, Bilodeau-Goeseels, Kastelic, Casey and Liu2012). In agreement with previous studies, our findings were important to determine if different IVM systems would have dissimilar effects on oocyte development after 30 h of culture.
The selection and use of only compact oocytes for IVM provided a homogeneous equine oocyte population to use during culture despite the lower maturation rates when compared with other mammalian species. Poor oocyte morphology and inadequate culture conditions during maturation may be responsible for the low success rate of in vitro fertilization and the reduced developmental competence of equine oocytes (Del Campo et al., Reference Del Campo, Donoso, Parrish and Ginther1990; Dell’Aquila et al., Reference Dell’Aquila, Fusco, Lacandra and Mariato1997). In vitro maturation rates of equine oocytes differ between laboratories as a result of the use of inappropriate methods for oocyte selection or poor in vitro culture conditions or both (Hinrichs & Williams, Reference Hinrichs and Williams1997; Hinrichs, Reference Hinrichs2010). Previous researchers have reported a high number of immature oocytes found in COCs at the time of collection, defining a more homogeneous population from slaughtered mares of unknown reproductive history to be used for IVM (Zhang et al., Reference Zhang, Muzs and Boyle1990; Torner et al., Reference Torner, Alm, Kanitz, Goellnitz, Becker, Poehland, Bruessow and Tuchscherer2007). Therefore, we used only COCs with a compact cumulus and homogeneous ooplasm in our studies.
Inhibition of spontaneous oocyte nuclear maturation in vitro would be desirable for evaluating the effects of hormones and growth factors on oocyte maturation and developmental competence (Mermillod et al., Reference Mermillod, Tomanek, Marchal and Meijer2000). The mechanisms involved in the control of oocyte maturation are not fully understood yet. Stabilization and dimerization of the GH/GH-R complex is responsible for inducing activation of the cytoplasmic tyrosine kinases (JAK-2), which are required for tyrosine phosphorylation of GH-R residues to initiate signalling (Rotwein et al., Reference Rotwein, Gronowski and Thomas1994; Smit et al., Reference Smit, Meyer, Billestrup, Norstedt, Schwartz and Carter-Su1996). Activation of protein kinases is the most common mode of signal transduction in biological systems; however, the second messenger cAMP plays an important role in the control of oocyte maturation and development (Guixue et al., Reference Guixue, Luciano, Coenen, Gandolfi and Sirard2001; Bevers & Izadyar, Reference Bevers and Izadyar2002). High levels of cAMP have been proposed as the regulatory mechanism to maintain the oocyte in meiotic arrest (Eppig & Downs, Reference Eppig and Downs1984; Downs & Hunzicker-Dunn, Reference Downs and Hunzicker-Dunn1995). In agreement with Izadyar et al. (Reference Izadyar, Colenbrander and Bevers1997a), we observed an inhibitory effect of eGH on nuclear progression when H-89 was added in our IVM system. The inhibitor H-89 used at a concentration of 10−9 M decreased the number of equine oocytes reaching maturity when compared with the eGH group, possibly by blocking cAMP production.
Activation of G-proteins serves to couple receptors to membrane-bound enzymes such as AC that induces the generation of secondary messengers such as cAMP. Addition of DDA, a specific inhibitor of AC, at the onset of maturation of bovine oocytes completely inhibited the stimulatory effect of GH on nuclear progression (Izadyar et al., Reference Izadyar, Colenbrander and Bevers1997a; Raper et al., Reference Raper, Kothary, Ishoo, Dikin, Kokudo, Hashimoto and DeMatteo1995). In our study, we observed that the presence of 10−8 M DDA did reduce the number of oocytes reaching maturation when eGH was added to culture for 30 h. In mouse oocytes, a high cAMP concentration is produced endogenously within the oocyte by ACs and constitutively activates G-protein-coupled receptors (Mehlmann et al., Reference Mehlmann, Jones and Jaffe2002). In addition, this spontaneous resumption of meiosis is accompanied by a rapid decrease in oocyte cAMP levels and in its competence. However, in equine oocytes, GH may use cAMP as a second messenger to signal its effect on oocyte nuclear maturation.
Results from this study demonstrate that a significant number of equine oocytes achieved nuclear maturation status in the presence of eGH into the IVM system at 30 h of culture. We conclude that the addition of either DDA or H-89 to the culture was accompanied by the inhibition of eGH-induced oocyte nuclear maturation. These findings provide strong evidence for cAMP signalling in GH-induced oocyte maturation in the presence of eGH in vitro. Our results suggest that the signalling transduction pathway in equine oocyte was inactivated possibly by the inhibition of the activation of protein kinases or by blocking cAMP production. These results provide a physiological and biological mechanism for the maturation and development of horse oocytes when in vitro.
Acknowledgements
The authors thank Paul Panich from the Lethbridge Research Centre for his technical assistance with the ovaries manipulation. This research was supported by a Del Amo Program Grant and by The Coordination for the Improvement of Higher Education Personnel/CAPES, Brazil.
Conflict of interest statement
The authors declare no conflicts of interest.






