Introduction
In order for mammalian fertilization to proceed sperm must enter the female reproductive tract, where the selection processes submit them to strict controls (Hunter, Reference Hunter2005; Suárez, Reference Suárez2007). The subpopulation of sperm that reach the oviduct can bind to the epithelial cells of the isthmus, as occurs in several mammals such as human, sheep, cow, horse, pig and mouse (Hunter, Reference Hunter1981; Flechon & Hunter, Reference Hunter1981; Hunter & Nichol, Reference Hunter and Nichol1983; Hunter & Wilmut, Reference Hunter and Wilmut1984; Demott & Suárez, Reference Demott and Suárez1992; Thomas et al., Reference Thomas, Ball and Brinsko1994; Pacey et al., Reference Pacey, Davies, Warren, Barratt and Cooke1995; Suárez, Reference Suárez1998); such bound sperm show decreased movement and prolonged survival (Fazeli et al., Reference Fazeli, Elliott, Duncan, Moore, Watson and Holt2003; Suárez, Reference Suárez2008). It has been demonstrated that oviductal proteins can modulate those sperm functions (Avilés et al., Reference Avilés, Guitiérrez-Adán and Coy2010; Holt & Fazeli, Reference Holt and Fazeli2010; Hung & Suárez, Reference Hung and Suárez2010; Killian, Reference Killian2011). Oviductal annexin A2 has been involved in the attachment of sperm to oviductal epithelium in pig (Teijeiro et al., Reference Teijeiro, Ignotz and Marini2009) and cow (Ignotz et al., Reference Ignotz, Cho and Suárez2007) and oviductal porcine deleted in malignant brain tumour 1 (DMBT1) has been related to sperm selection (Teijeiro & Marini, Reference Teijeiro and Marini2012a; Teijeiro et al., Reference Teijeiro, Cabada and Marini2008, Reference Teijeiro, Dapino and Marini2011, Reference Teijeiro, Roldan and Marini2012).
However, the female genital tract is a complex biosystem that is constantly exposed to a multitude of bacterial, fungal and viral agents (Mildner et al., Reference Mildner, Stichenwirth, Abtin, Eckhart, Sam, Gläser, Schröder, Gmeiner, Mlitz, Pammer, Geusau and Tschachler2010). Importantly, anti-microbial peptides (AMPs) are small proteins produced by epithelial surfaces and inflammatory cells that have broad-spectrum anti-microbial and immunomodulatory activities and are present in a number of sites throughout the female reproductive tract (Frew & Stock, Reference Frew and Stock2011). S100A7 (Psoriasin), a protein that belongs to a multigenic family of calcium-modulated proteins of the EF-hand type, has been identified as an AMP with strong activity against Escherichia coli (Gläser et al., Reference Gläser, Harder, Lange, Bartels, Christophers and Schröder2005). Moreover, S100A7 has been proposed as the major Escherichia coli-cidal factor in the female genital tract, and is expressed in vulva, vagina and ectocervical epithelia (Mildner et al., Reference Mildner, Stichenwirth, Abtin, Eckhart, Sam, Gläser, Schröder, Gmeiner, Mlitz, Pammer, Geusau and Tschachler2010). In addition, expression of S100A7 has been found in reproductive tissues such as prostate, testicle, ovary and normal cervical epithelium (Shadeo et al., Reference Shadeo, Chari, Vatcher, Campbell, Lonergan, Matisic, van Niekerk, Ehlen, Miller, Follen, Lam and MacAulay2007). The present authors have identified S100A7 in human and boar sperm and have demonstrated the interaction of porcine sperm S100A7 with porcine oviductal DMBT1 (Teijeiro & Marini, Reference Teijeiro and Marini2012b).
As AMPs are likely to have important functions in the female reproductive tract, and the expression of S100A7, an AMP, was not evaluated in Fallopian tubes, which are considered to be ‘sterile sites’ (Quayle, Reference Quayle2002), it was decided to search for the presence of this protein in the human oviduct.
Comparatively, the presence of S100A7 in porcine reproductive tissues was also evaluated. Because the Escherichia coli-cidal activity of S100A7 has been proposed in mammals other than human, such as cows (Regenhard et al., Reference Regenhard, Leippe, Schubert, Podschun, Kalm, Grötzinger and Looft2009), anti-microbial activity and further sequence homology analysis of porcine S100A7 were performed.
Materials and methods
Chemicals
Chemicals were obtained from Sigma-Aldrich (Buenos Aires, Argentina), unless otherwise stated. The following primary antibodies were used: polyclonal anti-human S100A7 purified by affinity to His-6-S100A7 immobilized on nitrocellulose membranes (Teijeiro & Marini, Reference Teijeiro and Marini2012b) (dilution 1:1000 for western blot and 1:100 for immunofluorescence) and polyclonal anti-porcine S100A7 (dilution 1:1000). The following secondary antibodies were used: horseradish peroxidase-conjugated anti-rabbit IgG (dilution 1:10,000) (GE Healthcare, Buenos Aires, Argentina); Cy3-conjugated anti-rabbit immunoglobulin (dilution 1:1000) (Chemicon International Inc., Temecula, USA).
Sample collection
Boar seminal plasma was collected from adult fertile boars from different breeds by the glove-handed method. The sperm-rich fraction was separated from seminal plasma by centrifugation. Porcine tongues, testicles, epididymis, seminal vesicles, ovaries, oviducts and uteri were obtained from a local abattoir and transported immediately to the laboratory in ice-cold phosphate-buffered saline (PBS). Each tissue was dissected and cut into pieces 1 × 1 cm. Then, each fragment was resuspended in buffer that contained 7 M urea, 2 M thiourea, 4% (w/v) CHAPS, 1% NP40 and dithiothreitol (DTT) 100 mM, and disaggregated by forceps and scissors. The tissue was homogenized in a Potter homogenizer and then sonicated and centrifuged 15 min at 4°C at 16,000 g. The supernatant was collected and stored at –80°C until use. Human seminal plasma and Fallopian tubes were gifted by Dr Sergio Ghersevich from the Clinical Biochemistry Area, Facultad de Ciencias Bioquímicas y Farmacéuticas, Universidad Nacional de Rosario, Argentina. For human samples, study protocols were approved by the Institutional Bioethical Board of Facultad de Ciencias Bioquímicas y Farmacéuticas, Universidad Nacional de Rosario, Argentina and written consent was obtained from all donors. Human oviductal tissue was obtained from premenopausal women (n = 6, average age: 42.9 years) with no clinical history of infection or neoplasic diseases, scheduled for hysterectomies as a result of uterine fibromyomas or hypermenorrhea (Hospital Provincial del Centenario, Rosario, Argentina). Samples for western blot were processed in accordance with Zumoffen et al. (Reference Zumoffen, Gil, Caille, Morente, Munuce and Ghersevich2013), briefly, fragments of the oviducts were opened longitudinally and the epithelial layer of cells was carefully scraped out with a scalpel and homogenized in homogenization buffer that consisted of 50 mM Tris–HCl (pH 7.4), 0.1 mM EDTA, 0.1 mM EGTA, 0.1M phenylmethanesulfonyl fluoride (PMSF), 2 μg/ml Aprotinin and 0.1% v/v 2-mercaptoethanol, in an ice bath. The oviductal cells homogenates were then centrifuged at 15,000 g for 30 min at 4°C, and the supernatants stored at −20°C until further use. Fallopian tube samples for immunofluorescence were provided as paraffin blocks.
Immunofluorescence
Human Fallopian tube tissues were sectioned, deparaffinized in xylene, and then rehydrated through graded dilutions of ethanol (100, 95, 70 and 35%), followed by two washes in Tris-buffered saline (TBS) (25 mM Tris–HCl pH 7.5, 150 mM NaCl). Slides were gently rinsed twice with TBS, blocked with 2% BSA, 0.2% Triton X-100 in TBS for 1 h and treated with the primary antibodies overnight at 4°C. After rinsing twice with TBS, the slides were treated with Cy3-conjugated anti-rabbit immunoglobulin (1:2,000) for 1h. After rinsing twice with TBS, slides were covered with 4′,6-diamidino-2-phenylindole (DAPI) for 30 min, then were washed with TBS, covered with 0.22 M 1,4-diazabicyclo[2,2,2]octane dissolved in glycerol:TBS (9:1) and cover slips were applied. The preparations were examined under a confocal microscope (Nikon Model Eclipse TE-2000-E2, Melville, USA).
Subcloning and expression of porcine S100A7, and development of anti-porcine S100A7 antibodies (anti-His6-pS100A7)
The coding sequence for porcine S100A7 (pS100A7) was cloned into pBluescript II by Gorodkin et al. (Reference Gorodkin, Cirera, Hedegaard, Gilchrist, Panitz, Jørgensen, Scheibye-Knudsen, Arvin, Lumholdt, Sawera, Green, Nielsen, Havgaard, Rosenkilde, Wang, Li, Li, Liu, Hu, Dong, Li, Yu, Wang, Staefeldt, Wernersson, Madsen, Thomsen, Hornshøj, Bujie, Wang, Wang, Bolund, Brunak, Yang, Bendixen and Fredholm2007) and was a gift from Dr Claus Jørgensen (Faculty of Life Sciences, University of Copenhagen, Denmark). This sequence was subcloned into pET-tev vector as histidine fusion and the recombinant protein (His6-pS100A7) was expressed in E. coli BL21. Protein expression was induced with isopropyl-1-thio-β-d-galactopyranoside (IPTG) for 20 h and purification was performed with Ni-NTA agarose (QIAGEN, Hilden, Germany) in accordance with the manufacturer's instructions. His6-pS100A7 was eluted five times sequentially with 250 mM imidazole; 0.5 ml fractions were collected and subsequently tested for purity by silver-stained SDS-PAGE (Rabilloud et al., Reference Rabilloud, Vuillard, Gilly and Lawrence1994). Fractions that contained His6-pS100A7 were pooled and concentrated by ultrafiltration using Ultracel-3 K (Amicon® Ultra, Millipore. Massachusetts, USA) and re-buffered into PBS (130 mM NaCl, 2.68 mM KCl, 6.46 mM Na2HPO4, 1.47 mM KH2PO4), pH 7.4.
Purified recombinant protein was used for antiserum development in rabbit as described by Pérez et al. (Reference Pérez, Roma, Cabada and Marini2006). Polyclonal anti-human S100A7 antibodies (anti-hS100A7) were developed in rabbit using recombinant GST-hS100A7 expressed and purified as described by Teijeiro & Marini (Reference Teijeiro and Marini2012a).
Western blots
Protein extracts obtained as mentioned above were supplemented with loading buffer (25 mM Tris–HCl, pH 6.8, 30% glycerol, 4% SDS) boiled for 5 min, used for 15% SDS-PAGE and then proteins were transferred to nitrocellulose membranes (GE Healthcare, Buenos Aires, Argentina). Non-specific binding sites were blocked by incubation with 5% dried non-fat milk in TBS (25 mM Tris–HCl, pH 7.4, 150 mM NaCl). Membranes were treated 1 h with the corresponding antibodies. After washing (three times for 10 min each), the blots were incubated with peroxidase-conjugated anti-rabbit IgG for 1 h and washed again. Labelled proteins were revealed using enhanced chemiluminescence detection with ECL Kit (GE Healthcare, Buenos Aires, Argentina).
Microdilution susceptibility assay
The microdilution susceptibility assay was performed as described by Regenhard et al. (Reference Regenhard, Leippe, Schubert, Podschun, Kalm, Grötzinger and Looft2009) with minor modifications. Only Escherichia coli strain ATCC 35218 was used for the microdilution assay. In brief, cells were grown for 2 h in Luria-Bertali (LB) medium at 37°C. Then the bacterial culture was adjusted to 104 colony-forming units (CFU)/ml in 10 mM sodium phosphate buffer (pH 7.4).
Aliquots (100 μl) of the microbial suspension were mixed with 10 μl of His6-pS100A7 solution (range of final concentration from 50–150 μg/l) and incubated at 37°C. After 3 h, the total volume of test suspensions was plated onto LB-Agar. After overnight incubation at 37°C, CFU were counted. Results are given as CFU/ml. Two-way analysis of variance (ANOVA) was applied and individual means were further tested by least significant difference (LSD) test.
Radial diffusion assay
A radial diffusion assay was performed as in Lehrer et al. (Reference Lehrer, Rosenman, Harwig and Eisenhauer1991) with modifications. Bacteria were grown to mid-log phase in LB medium and plated into Petri dishes (10 ml). After plating, a 5 μl aliquot of each protein solution was pipetted into wells formed with a biopsy punch (diameter: 3 mm), and plates were incubated overnight at 37°C. Ampicillin and kanamycin were used as positive growth inhibition controls.
Phylogeny and sequence analysis of S100A7 proteins in mammals
In order to broaden the study to other mammals, the presence of S100A7 proteins from other mammalian species was sought. The proteins that were manually annotated and reviewed were used for comparison in their annotated form. The proteins that were automatically annotated and not reviewed were compared with the previous ones using a BLAST (Basic Local Alignment Search Tool) (Altschul et al., Reference Altschul, Madden, Schaffer, Zhang, Zhang, Miller and Lipman1997) program to discard redundant data. Accession numbers for S100A7 proteins used in this analysis were: gi|190668 (Homo sapiens), gi|114559708 (Pan troglodytes), gi|27807077 (Bos taurus), gi|110224792 (Equus caballus), gi|46397569 (Mus musculus), gi|426216655 (Ovis aries), EW648856.2 (Sus scrofa) and gi|350583366 (Sus scrofa). Phylogenetic analyses were carried out using http://www.phylogeny.fr. Multiple sequence alignments were performed using the MUSCLE algorithm. Curation was performed using G-blocks with non-stringent selection parameters. A phylogenetic tree was reconstructed using maximum of likelihood (PhyML) method. Bootstrap values were estimated with 100 replications.
Molecular weight of proteins was estimated from the sequences using bioinformatics tools from http://expasy.org/
Results
Presence of S100A7 in Fallopian tubes
To evaluate the presence of S100A7 in Fallopian tubes (human S100A7, hS100A7), tissue protein extracts were subjected to western blot assays. Antibodies generated against recombinant GST-hS100A7 were purified by affinity to recombinant His6-hS100A7 and used for this assay (Teijeiro & Marini, Reference Teijeiro and Marini2012b). Fig. 1A shows the presence of hS100A7 in protein extracts from Fallopian tubes and in seminal plasma. Immunofluorescence analysis showed that hS100A7 is located at the apical cytoplasm of Fallopian epithelial cells (Fig. 1B, white arrows, left panel). Negative controls were developed with pre-immune serum (Fig. 1C. right panel).

Figure 1 Human S100A7 expression in Fallopian tube and seminal plasma. (A) Western blot analyses of Fallopian tube tissue extracts and seminal plasma. Line 1: 2 μg of GST-hS100A7; line 2: Fallopian tube tissue extract (protein content 50 μg); line 3: seminal plasma (protein content 30 μg). Data are representative of six Fallopian tubes and five seminal plasma samples. (B) Immunofluorescence showing hS100A7 in the apical cytoplasm of epithelial cells (white arrows). Inset shows ×2.5 magnification. (C) Control was performed with pre-immune serum. Nuclei were stained with DAPI. Bar indicates 20 μm.
Presence of S100A7 in porcine tissues
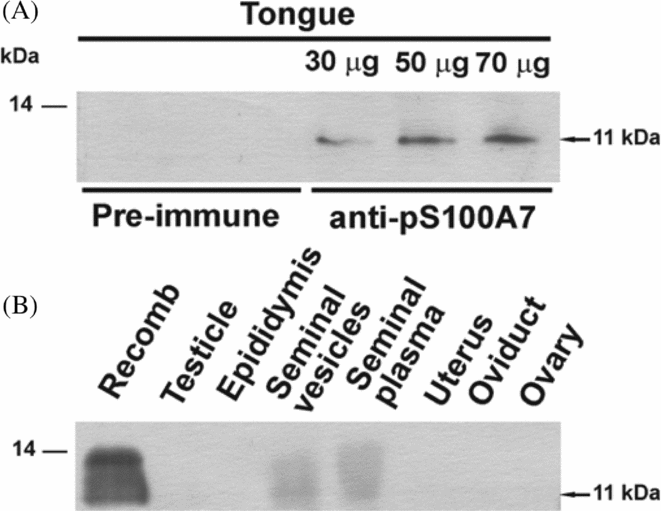
Porcine S100A7 was initially cloned from tongue RNA (Gorodkin et al., Reference Gorodkin, Cirera, Hedegaard, Gilchrist, Panitz, Jørgensen, Scheibye-Knudsen, Arvin, Lumholdt, Sawera, Green, Nielsen, Havgaard, Rosenkilde, Wang, Li, Li, Liu, Hu, Dong, Li, Yu, Wang, Staefeldt, Wernersson, Madsen, Thomsen, Hornshøj, Bujie, Wang, Wang, Bolund, Brunak, Yang, Bendixen and Fredholm2007). To corroborate the protein expression in its original tissue resource and to evaluate the quality of the antiserum prepared using His6-pS100A7, protein extracts from this organ were subjected to western blot. As shown in Fig. 2A, different amounts of a band of 11 kDa that corresponded to pS100A7 were observed for different quantities of tongue extracts revealed with anti-His6-pS100A7. Pre-immune serum showed no signal. Having checked the quality of anti-His6-pS100A10 antiserum, the presence of pS100A7 in different reproductive tissues was evaluated by western blot. From all the assayed reproductive tissues, only the seminal vesicles showed the presence of pS100A7. Seminal plasma also showed signal. S100A7 was not detected on porcine female organs under these conditions.

Figure 2 Presence of S100A7 in porcine tissues. (A) Expression of pS100A7 in tongue. Different amounts of tissue extracts were assayed by western blot showing increased amounts of pS100A7. Control was performed with pre-immune serum. (B) Analysis of expression of pS100A7 in porcine reproductive tissues by western blot. Only seminal vesicles and seminal plasma showed signal. Tissue extracts containing 50 μg of protein were used. Recomb: recombinant pS100A7 (2 μg).
His6-pS100A7 anti-microbial activity analyses
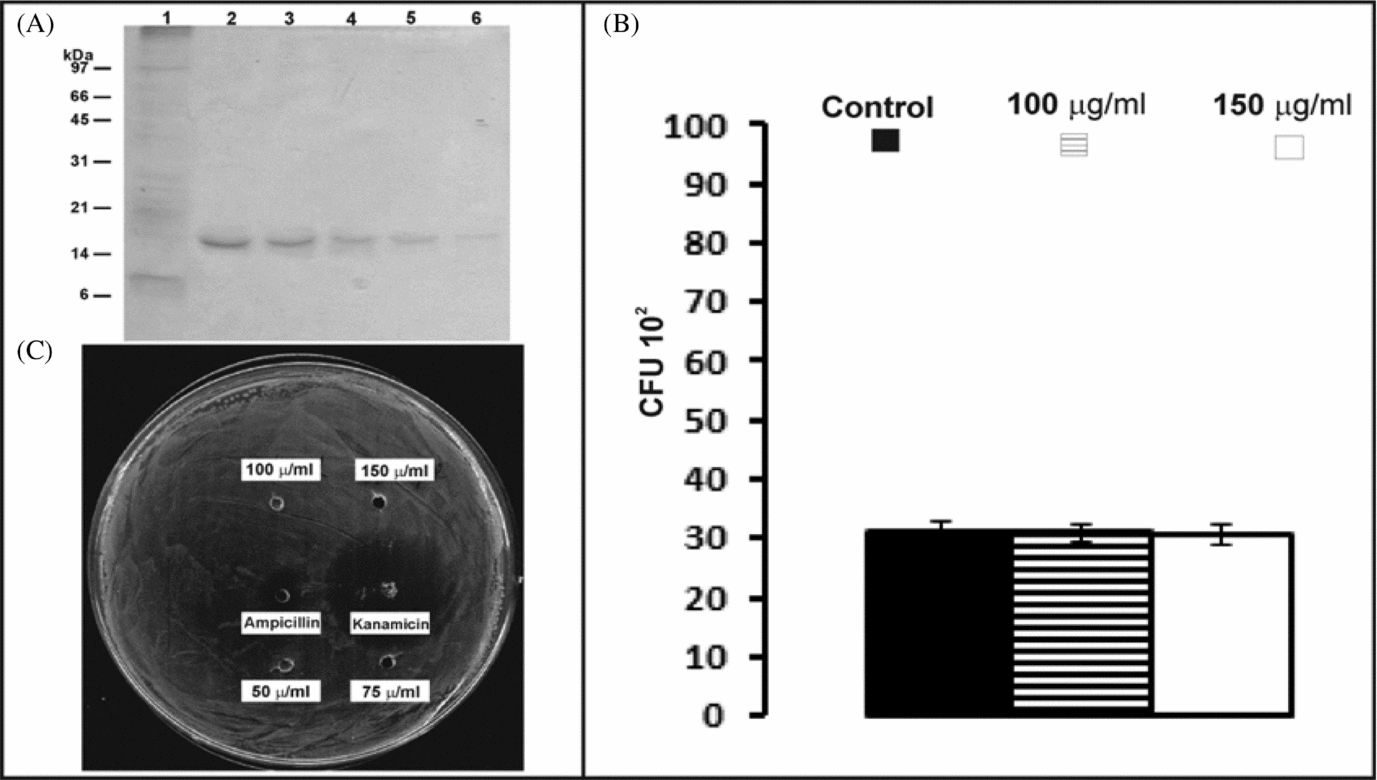
Previous reports indicate that cow S100A7 and human S100A7 have anti-microbial activity, preferentially against E. coli. Sequentially eluted His6-pS100A7 fractions were purified with the efficacy illustrated in Fig. 3A, pooled and re-buffered with PBS. Purified His6-pS100A7 was then used for anti-microbial activity assays. Testing the effect on the same E. coli strain, ATCC 35218, by microdilution susceptibility and radial diffusion assays, no anti-microbial activity for recombinant pS100A7 could be detected. There was no significant difference between CFU in E. coli treated with His6-pS100A7 and controls (Fig. 3B). Also, complementary radial diffusion assay showed no clear zones in wells that contained different concentrations of recombinant pS100A7, which indicated no inhibition of E. coli growth by pS100A7, in comparison with well known anti-microbial compounds (Fig. 3C).

Figure 3 Analyses of His6-pS100A7 anti-microbial activity. (A) Purification of recombinant pS100A7: 10 microliter samples from purified aliquots sequentially eluted of His6-pS100A7 were analysed by silver-stained SDS-PAGE (lines 2–6). Line 1: 10 μg E. coli BL21 IPTG induced culture extract. (B) E coli ATCC 35218 was treated with purified His6-pS100A7. Only two of the highest concentrations used are shown. Microdilution susceptibility assay shows no differences in CFU counting between treatments and control. (C) Radial diffusion assay shows no clear zone surrounding the wells loaded with different concentrations of His6-pS100A7. Ampicillin and kanamycin were used as positive controls.
Phylogeny analysis of S100A7 proteins in mammals
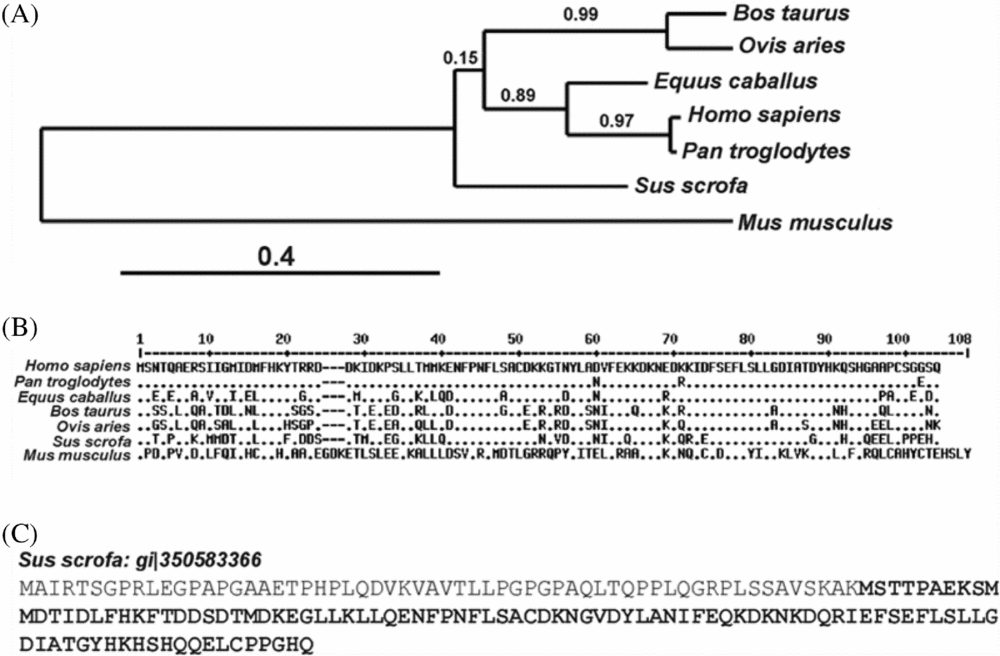
A phylogenetic tree was reconstructed using all the sequences indicated in the Materials and methods section. The phylogenetic reconstruction and the multiple alignment data showed that the amino acid sequence of Mus musculus (gi|46397569) and Sus scrofa (EW648856.2) S100A7 are highly divergent from the other sequences (Fig. 4A, B). As would be expected from patterns of mammalian evolution, primate and ruminant S100A7 cluster together. The phylogenetic tree reconstructed using the PhyML method suggests that the equine homologue is more closely related to primate sequences, while porcine and murine proteins form different branches.

Figure 4 Phylogenetic reconstruction and alignment of S100A7 proteins. (A) Phylogenetic tree reconstructed by the PhyML method. Branch lengths indicate rates of evolution. (B) Alignment of sequences used for the phylogenetic tree reconstruction. (C) Sequence of porcine S100A7 predicted by automated computational analysis (gi|350583366). The sequence of porcine S100A7 derived from the cDNA cloned by Gorodkin and co-workers (Reference Gorodkin, Cirera, Hedegaard, Gilchrist, Panitz, Jørgensen, Scheibye-Knudsen, Arvin, Lumholdt, Sawera, Green, Nielsen, Havgaard, Rosenkilde, Wang, Li, Li, Liu, Hu, Dong, Li, Yu, Wang, Staefeldt, Wernersson, Madsen, Thomsen, Hornshøj, Bujie, Wang, Wang, Bolund, Brunak, Yang, Bendixen and Fredholm2007) is showed in bold.
Analysis of molecular weights, calculated using bioinformatics tools, showed that the weight for the automatically derived porcine S100A7 sequence (gi|350583366) was 17621.97 Da, while that for tongue cDNA cloned porcine S100A7 (EW648856.2) was 11653.99 Da (Fig. 4C, in bold). The latter was confirmed to be present in tongue tissue by western blot in the current study (Fig. 2A). It should be noted that the amino acid at position 99 of the polypeptide derived from tongue cDNA is glutamic acid, while that from the automatically derived sequence is glycine.
Discussion
The mammalian oviduct is the natural site where final gamete maturation, fertilization and early embryo development take place (Harper, Reference Harper, Knobil and Neill1994). The oviduct is a dynamic organ that modulates gamete's functions and provides a proper environment for early embryo development (Pulkkinen, Reference Pulkkinen1995). In the past, functions have been identified and established for oviductal proteins, such as annexin A2 and DMBT1 (previously called SBG (sperm-binding glycoprotein)) (Teijeiro & Marini, Reference Teijeiro and Marini2012a; Teijeiro et al., Reference Teijeiro, Cabada and Marini2008, Reference Teijeiro, Ignotz and Marini2009, Reference Teijeiro, Dapino and Marini2011, Reference Teijeiro, Roldan and Marini2012), and S100A7 has been identified as a boar sperm protein that interacts with DMBT1 (Teijeiro & Marini, Reference Teijeiro and Marini2012b). Further, S100A7 has been identified and localized on human sperm head (Teijeiro & Marini, Reference Teijeiro and Marini2012b). The current work searched for S100A7 on the female reproductive tract, where it could have important functions in innate immunity.
S100A7 was originally cloned from unfractionated uncultured psoriatic keratinocytes (Madsen et al., Reference Madsen, Rasmussen, Leffers, Honore, Dejgaard, Olsen, Kiil, Walbum, Andersen, Basse, Lauridsen, Ratz, Celis, Vandekerckhove and Celis1991) and functions such as transglutaminase substrate/cornified envelope precursor, signal transduction protein, chemokine and anti-bacterial protein in normal epidermis were assigned to it (Eckert & Lee, Reference Eckert and Lee2006). The oviductal epithelium is the tissue involved in oviductal fluid secretion, in which gametes and embryo development are supported (Leese et al., Reference Leese, Tay, Reischl and Downing2001). The current results demonstrate that S100A7 is present in the epithelium of Fallopian tubes (Fig. 1). This finding is important because the Fallopian tube is considered to be a ‘sterile site’ (Quayle, Reference Quayle2002) and an eventual infection in this organ could cause tubal infertility or tubal ectopic pregnancy (Frew & Stock, Reference Frew and Stock2011). It has been demonstrated that human S100A7 has anti-bacterial properties against a spectrum of bacteria (Gläser et al., Reference Gläser, Harder, Lange, Bartels, Christophers and Schröder2005) and thus hS100A7 may contribute to the ‘sterility’ of the Fallopian tube. Its presence in human seminal plasma (Fig. 1) and in the sperm head (Teijeiro & Marini, Reference Teijeiro and Marini2012b) may be a product of secretion from the male epithelial reproductive tract and also function on anti-bacterial activity. In pigs, western blot expression analyses allowed the observation of pS100A7 in seminal vesicles and seminal plasma, in accordance with an origin for pS100A7 in seminal vesicles. However, in contrast with human, the protein was not detected in female porcine tissues.
Originally cloned from the tongue (Gorodkin et al., Reference Gorodkin, Cirera, Hedegaard, Gilchrist, Panitz, Jørgensen, Scheibye-Knudsen, Arvin, Lumholdt, Sawera, Green, Nielsen, Havgaard, Rosenkilde, Wang, Li, Li, Liu, Hu, Dong, Li, Yu, Wang, Staefeldt, Wernersson, Madsen, Thomsen, Hornshøj, Bujie, Wang, Wang, Bolund, Brunak, Yang, Bendixen and Fredholm2007), porcine S100A7 was detected by western blot in tongue tissue extracts, and confirmed the expression of the protein in this tissue (Fig. 2A). It is interesting to note that pS100A7 shares localization with hS100A7 in this organ. Unlike hS100A7, no pS100A7 was found in the oviductal epithelium (Fig. 2B). The presence of hS100A7 was detected in human testicles and ovaries (Shadeo et al., Reference Shadeo, Chari, Vatcher, Campbell, Lonergan, Matisic, van Niekerk, Ehlen, Miller, Follen, Lam and MacAulay2007) but pS100A7 was not detected in the same tissues from pigs. Thus, S100A7 seems to exhibit a conserved role in the tongue that is not observed between human and porcine reproductive tissues.
Anti-microbial activity for His6-pS100A7 was not detected either by microdilution susceptibility assay or by radial diffusion assay (Fig. 3B, C); however, this activity cannot be ruled out.
As expected from mammalian pattern evolution, the current phylogenetic reconstruction shows that primates and ruminants cluster together (Fig. 4). Although other primate S100A7 sequences are available, for example Gorilla gorilla (gi|426331624), they show extremely high homology and thus chimpanzee was chosen as representative of the group. Equine S100A7 is more related to S100A7 from primates, but no information could be found about its functions or localizations. In agreement with Wolf et al. (Reference Wolf, Voscopoulos, FitzGerald, Goldsmith, Cataisson, Gunsior, Walz, Ruzicka and Yuspa2006), the current phylogenic reconstruction suggests that murine S100A7 may be the ancestral gene for human S100A7 and, moreover, for the rest of the species analysed. Porcine S100A7 forms a different branch from that of most species, and this might be related to the lack of the anti-microbial activity shown, in contrast to the bovine and human proteins. Mutation of the carboxyterminal region of human S100A7 slightly reduces psoriasin's anti-bacterial activity (Lee & Eckert, Reference Lee and Eckert2007). In this regard, the lack of homology between the porcine and human proteins at the carboxyterminal end might be responsible for the lack of anti-microbial activity of pS100A7, and indicates that innate immunity may not be the main function of S100A7 in porcine reproductive tracts. Bioinformatic tools derived the molecular weight for the automatically annotated porcine protein (gi|350583366) (Fig. 4) as 17621.97 Da, while that of S100A7 cloned from pig tongues was 11653.99 Da. The tongue-cloned molecular weight is in agreement with the weight of the actual protein observed by western blot. The apparent molecular mass corresponds to the sequence obtained from tongue cDNA instead of the one deduced from the DNA sequence (gi|350583366) (Fig. 4C).
Acknowledgements
We thank Dr Sergio Ghersevich for Fallopian tube samples and Dr Claus Jørgensen for pig EST clone. We also thank Frigorífico Palidini S.A. This work was supported in part by the ANPCyT-BID program PICT 01–15092 of Argentina.