Introduction
Increase in fertility is due to the anabatic pressure of work, environmental pollution, lifestyle changes, and so on. Therefore, human superovulation, in vitro fertilization and embryo transplantation have been used in combination worldwide. Propofol is a common intravenous anaesthetic used for oocyte retrieval during ultrasound monitoring. However, little information is known about whether its use affects growth and development of the oocyte, as this area has been minimally researched and is not controversial. A previous study in Chinese hamster ovary cells reported that culture medium containing 0.2–20 μg/ml propofol had no effect on sister chromatid segregation during the first meiotic division and meiosis I (Tomioka & Nakajo, Reference Tomioka and Nakajo2000). An increased propofol concentration in oocytes from human transvaginal oocyte retrieval had no significant effects on the oocytes, possibly due to low concentration and short operating time (Ben-Shlomo et al., Reference Ben-Shlomo, Moskovich, Katz and Shalev1999). When oocytes and cumulus cell were cultured in medium containing low propofol concentrations, subsequent cleavage was clearly affected. Unhatched oocytes exposed to propofol had a high rate of parthenogenetic activation (Jarahzadeh et al., Reference Jarahzadeh, Jouya, Mousavi, Dehghan-Tezerjani, Behdad and Soltani2014). In total, 551 cumulus–oocytes complexes and 222 cumulus-free oocytes were collected in culture medium containing a propofol concentration of 0.1 or 10,000 ng/ml. Although there was no statistical difference for fertilization and cleavage compared with the control group, the mature ratio of cumulus–oocytes complexes and cumulus-free oocytes declined significantly in culture medium containing a propofol concentration of 10,000 ng/ml (Ma et al., Reference Ma, Shen and Zhang2008). We aimed to research the effects of propofol at different doses on mice oocyte maturation in vivo or in vitro, and in pregnancy and childbirth as well as for progeny.
Materials and Methods
Ethics statement
All studies adhered to procedures consistent with the National Research Council’s Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Inner Mongolia University.
Apparatus, chemicals and reagents
Electronic scales (CPA224S, Sartorius, Germany), pipettes (Eppendorf, Germany), benchtop (DL-CJ-1ND II, Beijing Donglian Har Instrument Manufacture Co., Ltd), ultracold storage freezer (HFU486, ThermoFisher, USA), disposable syringes (KangFulai Medical Equipment Ltd), inverted fluorescence microscope (IX71, Olympus, Japan), 35 mm and 60 mm culture dishes (BD Falcon, USA), Spot Test Plates (PYREX®), propofol (Xi’an Libang Pharmaceutical Co., Ltd), Giemsa stain (Beijing Solarbio Technology Co., Ltd), iodophor (Beijing Sihuan health YaoXie Factory Co., Ltd), bovine serum albumin (BSA; Amresco, Solon, OH, USA), human chorionic gonadotropin (hCG; Sansheng, NingBo, China), pregnant mare serum gonadotropin (PMSG; Sansheng), hydroxyethyl piperazine ethanesulfonic acid (HEPES), water for embryo culture, NaCl, phenol red, CaCl2.2H2O, EDTA.2Na, d-glucose, KH2PO4, Na lactate, NaHCO3 were used All other reagents were purchased from Sigma Aldrich (St. Louis, MO, USA) except when noted otherwise.
Laboratory animal and grouping
In total, 48 pairs of Kunming mice and another 104 female mice, and 20 male mice at 8–10 weeks of age were provided from the Specific Pathogen Free (SPF) experimental animal breeding room at Inner Mongolia University. Female weight was 35±1 g, male weight was 41±1 g and animal feeding and breeding conditions were controlled by standard methods.
Animals were grouped for part 1: the effects of propofol on pregnancy, childbirth and progeny; and for part 2: the effects of propofol on mice oocyte maturation and development. Mice were divided randomly into four groups (each group of 12). For the control (Ctrl) group, 2 ml physiological saline was injected intraperitoneally; in the P-2 group, propofol was given at 2 mg/kg; in the P-50 group, propofol was given at 50 mg/kg; in the P-100 group, propofol was given at 100 mg/kg.
For Part 3: the effect of propofol on the oocyte maturation and development in vitro was studied. Mice were divided randomly into seven groups (56 female mice in total, each group had eight mice). Cells in the Ctrl group were cultured in Chatot–Ziomek–Bavister (CZB) medium; in the P-0.01 group cells were cultured in milrinone 2.5 μmol/l+propofol 0.01 mg/ml+CZB; in the P-0.03 group cells were cultured in milrinone 2.5 μmol/l+propofol 0.03 mg/ml+CZB; in the P-0.1 group cells were cultured in milrinone 2.5 μmol/l+propofol 0.1 mg/ml+CZB; in the P-0.3 group cells were cultured in milrinone 2.5 μmol/l+propofol 0.3 mg/ml+CZB; in the P-1 group cells were cultured in milrinone 2.5 μmol/l+propofol 1 mg/ml+CZB; and in the P-3 group cells were cultured in milrinone 2.5 μmol/l+propofol 3 mg/ml+CZB.
The effect of propofol on pregnancy, childbirth and progeny
Oestrus detection
Successive dioestrus occurs in female mice when bred in a single cage. When male mice or their urine is placed in the cage, most of the female mice will go into heat and synchronize of oestrus, a phenomenon known as the ‘Dr Whitten effect’, and is used for artificial breeding. Female mice will climax in oestrus when mating with male mice or after exposure to male mice faeces for 2 days. At artificial oestrus synchronization, the mouse vaginal orifice opens, tissue around the vaginal orifice is pink, secreta is wet, and vaginal orifice swelling, obvious wrinkles, and stria are clearly seen around the vaginal orifice.
Propofol injection
The study used conventional doses (1–2 mg/kg) of propofol to observe its effect on mice reproduction. The drugs were injected when mouse vaginal smears were detected in proestrus: Ctrl group: physiological saline intraperitoneal injection of 2 ml; P-2 group: intraperitoneal injection of propofol 2 mg/kg; P-50 group, intraperitoneal injection of propofol 50 mg/kg; P-100 group, intraperitoneal injection of propofol 100 mg/kg. The mice in the P-50 and P-100 groups were placed in the lateral position until they could freely move after propofol injection to prevent propofol-induced respiratory depression caused by an increase in respiratory secretions.
Cohabitating in ovulatory period
The mice in estrus were mated at a ratio 1:1 male:female. A milky, slightly hard vaginal plug formed close to the vaginal orifice after successful mating. The vaginal plug normally persisted for 24 h after mating. Mice were checked for vaginal plug after 12 h to determine mating success (Mester et al., Reference Mester, Ritter, Pitman, Bibby, Gilchrist, McNatty, Juengel and McIntosh2015), however this method was not always foolproof (Ratky et al., Reference Ratky, Brussow, Egerszegi, Torner, Schneider, Solti and Manabe2005). A detected vaginal plug indicated a half day of pregnancy. Occasionally mice did not form the vaginal plug or the plug fell off, then a vaginal smear was performed to detect the presence of sperm and confirm that mating had occurred (Tanaka, Reference Tanaka1962).
Detection index
Vaginal smears were treated with Giemsa stain to detect the oestrus. The performance of anaesthetized female mice after propofol injection; the number of positive vaginal plugs; the number of births; litter size; the number of stillbirths; survival rate (%); and postpartum weight of progeny after 1 week were recorded.
The effect of propofol injection on oocyte maturation
Germinal vesicle oocyte collection and culture in vitro
Next, 0.1 IU/g PMSG was injected by hypodermic needle into the experimental mice. At 47.5 h after injection mice were given: Ctrl group, a physiological saline intraperitoneal injection of 2 ml; P-2 group, an intraperitoneal injection of propofol at 2 mg/kg; P-50 group, an intraperitoneal injection of propofol at 50 mg/kg; and P-100 group, an intraperitoneal injection of propofol at 100 mg/kg.
Mice were sacrificed by cervical dislocation 48 h after injection, and cumulus–oocyte complexes at the GV stage were obtained. The oocytes were placed into CZB drops containing milrinone 2.5 μmol/l in 60 mm Petri dishes after washing three times (10 oocytes/20 μl). Petri dishes were placed in a CO2 in air incubator for 30 min. Oocytes were washed in CZB medium without milrinone three times, then 60 mm Petri dish containing CZB droplets were placed in the CO2 incubator. The rates of polar body extrusion in oocytes at the metaphase II (MII) stage after culture for 14 h were observed and recorded (Wang et al., Reference Wang, Racowsky and Deng2011).
In vitro fertilization, embryo collection, and culture
Six hour capacitation medium (T6) and fertilization medium were balanced overnight in a CO2 incubator (Han et al., Reference Han, Liang, Cheng, Duan, Zhong, Potireddy, Moncada, Merali and Latham2010). Adult males at 8–10 weeks of age were sacrificed by cervical dislocation. Spermatozoa were collected from the cauda epididymis using a germfree glass needle. Spermatozoa vigour was confirmed under the microscope, then sperm were transferred to a dish containing 200 μl capacitation medium for 2 h. MII cumulus–oocytes complexes were obtained after superovulation, and the cumulus-free oocytes were abandoned. Cumulus–oocytes complexes were placed in insemination medium, and 10 μl sperm suspension was added after capacitation. Sperm suspensions at a concentration of 1 × 106 cells/ml were added to MII stage oocytes for fertilization. After 6 h, the fertilized oocytes were washed with CZB medium and cultured in CZB medium without glucose in a humidified atmosphere with 5% CO2 in air at 37°C.
Detection index
The ratios of pronucleus (PN), polar body emission, 2-cell and 4-cell embryo development were observed at 0, 24, and 48 h after fertilization. A positive PN ratio showed that the male and female pronuclei had formed, and these were transferred to new medium drops for culture. A negative ratio of 2-cell or 4-cell developing embryos indicated abnormal development or oocyte nucleus division. Other abnormalities were serious imbalance in cell size after division, irregular edged oocyte as seen under the microscope, disappearance of the oocyte nucleus, abnormal nucleus structure, oocyte nucleus solidification or contraction, or high densification of the nuclear region. The day of fertilization was designated day 0, the development ratio of morula and blastocyst was measured at days 3 and 4 after fertilization. Positive or negative examples were detected at each stage of maturation and development (Fig. 1).
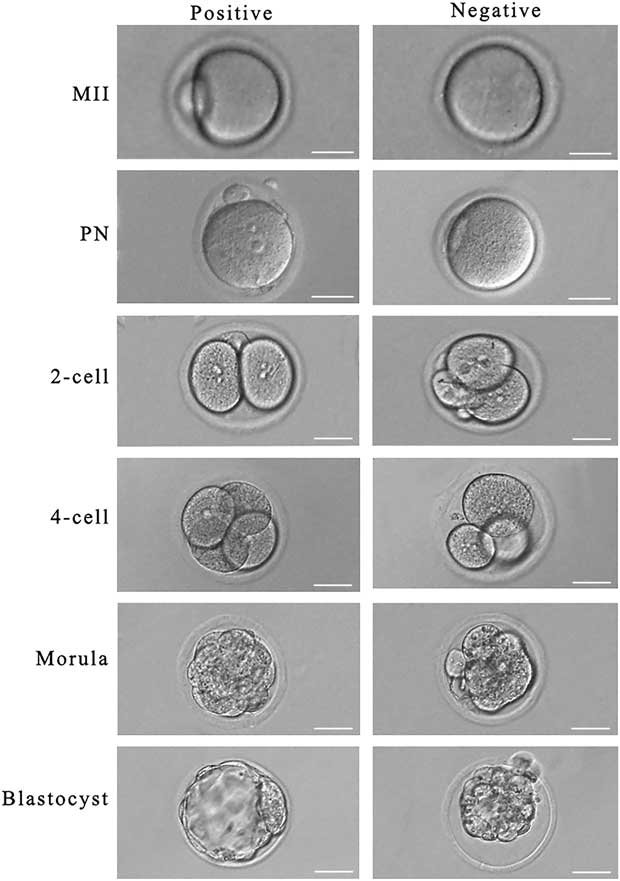
Figure 1 Detection standard of each stage in oocyte maturation and development (magnification ×200). Scale bar=50 μm.
The effect of propofol on oocyte maturation and development in vitro
Collection of mouse oocyte in GV stage and cultured in vitro
GV oocyte collection was performed as above. GV cumulus–oocyte complexes were washed three times in CZB medium containing milrinone 2.5 μmol/l, then transferred either to CZB medium (Ctrl group); milrinone 2.5 μmol/l+propofol 0.01 mg/ml+CZB (P-0.01 group); milrinone 2.5 μmol/l+propofol 0.03 mg/ml+CZB (P-0.03 group); milrinone 2.5 μmol/l+propofol 0.1 mg/ml+CZB (P-0.1 group); milrinone 2.5 μmol/l+propofol 0.3 mg/ml+CZB (P-0.3 group); milrinone 2.5 μmol/l+propofol 1 mg/ml+CZB (P-1 group); or milrinone 2.5 μmol/l+propofol 3 mg/ml+CZB (P-3 group), respectively. Twenty-five oocytes was transferred to 50 μl culture medium, then incubated in a CO2 in air incubator for 30 min. The oocytes were then washed three times in CZB medium without milrinone, and transferred to CZB medium in a 60 mm Petri dish and cultured in a CO2 incubator. The ratio of polar body extrusion at the MII stage was observed and recorded after culture for 14 h (Wang et al., Reference Wang, Racowsky and Deng2011).
In vitro fertilization, culture and collection of early embryo
The method of early embryo collection was performed as above.
Detection index
The ratio of polar body extrusion, rate male and female pronuclear formation, and development of 2-cell and 4-cell stages, morula and blastocyst were all detected.
Statistical analysis
SPSS 21.0 statistical software was used for statistical analysis. The effect of propofol at different doses on mouse pregnancy and birth was measured. Comparisons of mean value of samples and F-test and sample percentage using χ2 test were made, including mean value±standard deviation. A P-value<0.05 indicated statistical significance.
Results
The mice in propofol injected group with no deaths
In the P-2 group, the mice displayed uncoordinated movements, avoided external stimuli, and exhibited conjunctival reflex 2 or 3 min after propofol injection. In the P-50 group, the mice respiration rates decreased, and there was no voluntary movement 2 or 3 min after propofol injection. In the P-100 group, mice respiration rates decreased, mouth secretions increased, and there was totally no voluntary movement 2 or 3 min after propofol injection, movement was restored after 2 or 3 h.
The effect of propofol on mouse pregnancy and birth at different doses
Typical proestrus (Figs 2 and 3) was detected in each group by Giemsa staining of vaginal smears (Fig. 4). At the second day of mating, vaginal plugs were detected in mice including one in the Ctrl group. There was no statistically significant difference in the number of positive vaginal plugs between groups (P>0.05). One female mouse in the P-50 group did not produce a litter, there was no statistically significant difference in the rate of parturition between each group (P>0.05) and there was no statistically significant difference in litter size between each group (P>0.05). One mouse died after delivery in the control and in the P-100 group, respectively. There was no statistically significant difference in fetal death and survival rate between each group (P>0.05).
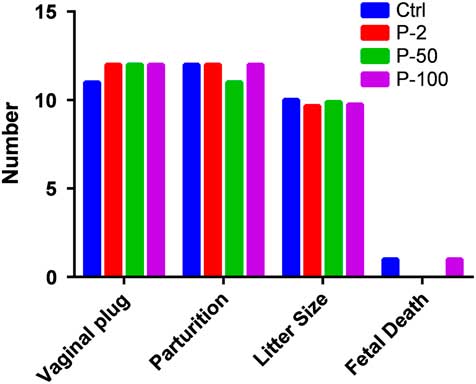
Figure 2 Effect of different doses of propofol on mouse pregnancy and parturition. There was no difference in the positive vaginal plugs in each group (P>0.05), or the number of delivery parturitions in each group (P>0.05). The number of litter sizes in each group (P>0.05). There was no difference in fetal death, the survival rate in each group (P>0.05).

Figure 3 Pups survival rate (%), there was no difference in pups survival rate in each group (P>0.05).

Figure 4 Giemsa-stained light microscopy of vaginal smears. Scale bar=20 μm.
The effect of propofol on progeny weight in different doses
A female mouse (Fig. 5) in the P-50 group did not produce a litter, and these data were discarded. One mouse died after delivery in the Ctrl and P-100 groups, respectively, therefore the progeny weight was calculated as zero. A correlation test for each group displayed P-values<0.05, not satisfying distributional assumptions; multivariable analysis of variance needed to be carried out. From birth days 1 to 7, there was no statistically significant difference in body weight between the groups given different doses of propofol (P-2, P-50, P-100 and Ctrl group) (P>0.05).
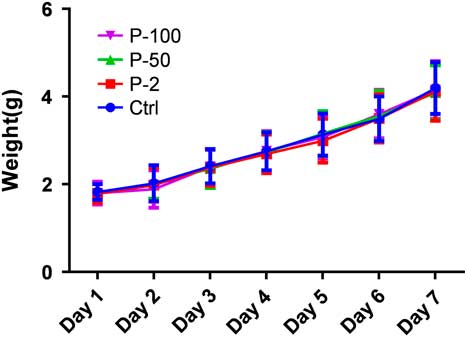
Figure 5 Effect of different doses of propofol on pups weight (g). There was no difference in pups weight in each group (P>0.05).
The effect of propofol injection on the ratio of oocyte maturation and development in vitro
Comparison of the Ctrl group with other groups for the ratio of GV oocyte maturation and development in vitro after injection of propofol (Fig. 6). Oocytes at the GV stage (1326) were collected, of which there were 301 in the Ctrl group, 338 in the P-2 group, 357 in the P-50 group, and 330 in the P-100 group. The ratio of oocyte maturation was high in each group, the ratio of polar body extrusion in the Ctrl group was 92.4%, in the P-2 group was 93.2%, in the P-50 group was 93.0%, and in the P-100 group was 93.0%. P-values were all greater than 0.05 compared with the Ctrl group (P-2 group, P=0.15; P-50 group, P=0.09; P-100 group, P=0.10), therefore there was no statistically significant difference. The development ratio of 4-cell embryos was low in each group, in the Ctrl group it was 53.9%, in the P-2 group its was 51.8%, in the P-50 group it was 50.2%, in the P-100 group it was 52.0%. P-values were all greater than 0.05 compared with the Ctrl group (P-2 group, P=0.10; P-50 group, P=0.30; P-100 group, P=0.08); therefore there was no statistically significant difference.
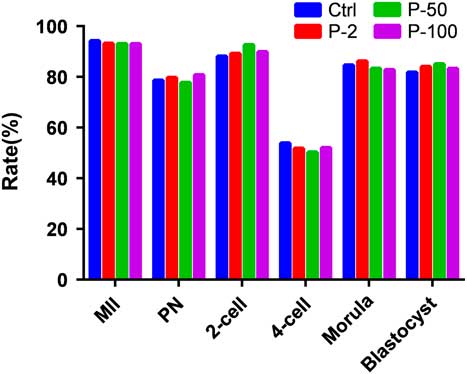
Figure 6 Effect of different doses of propofol on semi-vitro oocytes meiotic maturation and early embryo development rates in mouse. The maturation rates, fertilization rate, and the embryo development of 2-cell, 4-cell, morula, and blastocyst for different concentrations of propofol on oocyte culture in vitro were no different when compared with Ctrl group (P>0.05).
The effect of propofol on a ratio of oocyte maturation and development in vitro at different doses
Comparisons of the Ctrl group with other groups for the ratio of GV oocyte maturation and development in vitro after injection of propofol (Figs 7 and 8) were made. In our study, oocytes at the GV stage (1866) were collected, of which 265 were in the Ctrl group, 282 were in the P-0.01 group, 274 were in the P-0.03 group, 276 were in the P-0.1 group, 269 were in the P-0.3 group, 248 were in the P-1 group, and 252 were in the P-3 group. The ratio of oocyte maturation was high in each group, the ratio of polar body extrusion in the Ctrl group was 93.2%, in the P-0.01 group was 56.0%, in the P-0.03 group was 59.1%, in the P-0.1 group was 55.8%, in the P-0.3 group was 62.5%, in the P-1 group was 63.7%, in the P-3 group was 58.3%. P-values were all less than 0.05 compared with Ctrl group (P-0.01 group, P=0.000; P-0.03 group, P=0.001; P-0.1 group, P=0.000; P-0.3 group, P=0.002; P-1 group, P=0.005; P-3 group, P=0.001). Pearson chi-squared test for each group for the ratio of polar body extrusion after propofol treatment gave P=0.914, the P-value was >0.05, therefore there was no statistically significant difference. The development ratios of the 4-cell embryo were low in each group, and P-values were all greater than 0.05 compared with the Ctrl group, so there was no statistically significant difference. P-values were all greater than 0.05 compared with the Ctrl group for the ratios of male and female pronuclear formation, 2-cell and 4-cell stages, and morula and blastocyst development, so there was no statistically significant difference.
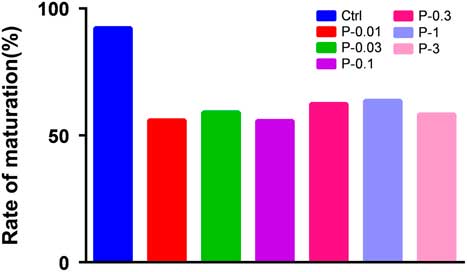
Figure 7 Effect of different concentrations of propofol on in vitro oocytes meiotic maturation rates in mouse. The maturation rate for different concentrations of propofol on oocyte culture in vitro was different compared with Ctrl group (P<0.05). There was no difference in the ratio of oocyte maturation among the different concentration propofol supplementation groups (P>0.05).
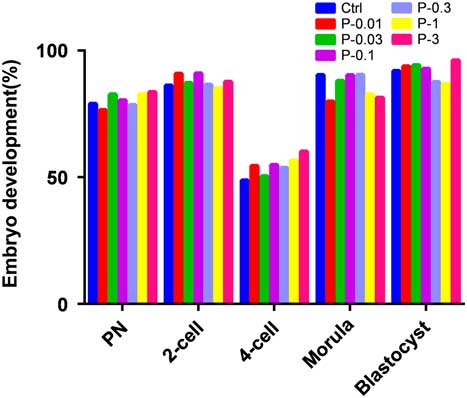
Figure 8 Effect of different concentrations of propofol on in vitro early embryo development rates. There was no difference in the development rate of early embryo for different concentrations in the propofol supplementation groups when compared with Ctrl group (P>0.05).
Discussion
The dose of propofol for mouse was a critical issue in this study. Alves et al. reported that a dose of 75 mg/kg of propofol met surgery needs for mice, as the anaesthesic dose was safe and effective for mice, and could be maintained for 15–30 min (Alves et al., Reference Alves, Valentim, Olsson and Antunes2007). An injection dose of propofol of 50 mg/kg, 100 mg/kg or 150 mg/kg was used to explore the effects and mechanisms of reperfusion of a complete cerebral ischaemia lesion (Cong et al., Reference Cong, Wang, Li and Li1999). Propofol dose in these experiments is consistent with theoretical calculations. There were no deaths of mice in this study after propofol injection, indicating that the used dose was safe and effective.
There was no statistically significant difference for parturition (P>0.05). Thaete et al. reported that embryonic development was limited when the pregnant mice were given conventional anaesthetics as well as analgesics, but no effect was found on placenta development and litter size; these results were consistent with those in our study (Thaete & Malkinson, Reference Thaete and Malkinson1991). There was no significant difference in the group for each time period indicating that length of time (1 to 7 days) did not have an effect between groups (P-2, P-50, P-100 and the Ctrl group). This result maybe due to the short half-life of propofol, which is quickly metabolized in vivo, having an influence on reproductive organs and embryos in the short term. In our study, no significant effects of different doses propofol were found on mating, pregnancy, childbirth, litter size, stillborn and survival rate. Propofol was safe and effective for anaesthetised, pregnant mice and can be the theoretical basis for the clinical use of propofol.
Transvaginal oocyte retrieval was performed after intermittent injection with propofol in nine patients, the concentration of propofol in the follicular fluid was positively correlated with injected total dose of propofol (Coetsier et al., Reference Coetsier, Dhont, De Sutter, Merchiers, Versichelen and Rosseel1992). The dose of propofol (100 mg/kg) used in the P-100 group was 50-fold that of the conventional dose (2 mg/kg), and was equivalent to 50 times the conventional dose in intermittent injection. This level is sufficient to simulate the maximum total injected dose in clinical use. The dose gradient established in our study contained the dose range used in medical practice. Relevant results showed that the ratio of oocyte maturation was high in each group, no significant difference is found when compared with the Ctrl group (P>0.05). The ratio of 4-cell development was low in each group, and there was no significant difference when compared with the Ctrl group (P>0.05). In total, 202 were placed in the propofol group (propofol 2 mg/kg) and the cervix local anaesthesia block group, as the ratio of fertilization and cleavage was not significantly different between the two groups (Christiaens et al., Reference Christiaens, Janssenswillen, Verborgh, Moerman, Devroey, Van Steirteghem and Camu1999). This finding is consistent with the results of our study, indicating that the conventional therapeutic dose of propofol used to obtain ova painlessly is safe and effective.
There are reports on transvaginal oocyte retrieval (Seyhan et al., Reference Seyhan, Ata, Son, Dahan and Tan2014), for which the operation process and time was the same (Zhang et al., Reference Zhang, Wang and Lu2013; Siristatidis et al., Reference Siristatidis, Chrelias, Alexiou and Kassanos2013). Each group was treated with propofol for 0.5 h, this time to act on the body is consistent with that used in clinical transvaginal oocyte retrieval. Between propofol group and Ctrl group, the ratio of oocyte maturation was significantly reduced, indicating that drug treatment is effective in our study.
The oocytes were treated with different concentrations of propofol to observe its effect on the ratio of maturation and development in vitro in our study. The ratio of oocyte maturation was high in each group compared with the Ctrl group, the ratio of oocyte maturation was significantly reduced after propofol treatment. The development ratio of the 4-cell embryo was low in each group, and there was no statistically significant difference. The ratio of male and female pronuclear formation for 2-cell and 4-cell stages, and morula and blastocyst development did not change significantly when compared with that in the Ctrl group (P>0.05). Propofol did not affect the ratio of oocyte development. Alsalili et al. collected 551 oocytes and 222 oocytes with cumulus–oocyte complexes by superovulation, to further explore maturation mechanisms in culture medium that contained propofol at 0 ng/ml, 100 ng/ml, 1000 ng/ml or 10,000 ng/ml, respectively (Alsalili et al., Reference Alsalili, Thornton and Fleming1997). In the 10,000 ng/ml propofol group, the ratio of oocyte and oocytes and cumulus maturation decreased significantly, however the ratios of fertilization and cleavage were not affected compared with the Ctrl group. This finding is consistent with the results of our study.
Previous research reported that a dose of 0.01 mg/ml propofol decreased the ratio of oocyte maturation, while no effect on the rate of development was found (Alsalili et al., Reference Alsalili, Thornton and Fleming1997). Therefore, the concentration gradient of each variable with dose observed:
(1) if higher concentrations of propofol interfere with oocyte maturation, and may even cause cell damage and apoptosis.
(2) if high concentrations of propofol in medium affect oocyte development, such as the ratio of male and female pronuclear formation, 2-cell and 4-cell stages, and morula and blastocyst development.
The results showed that the ratio of polar body extrusion was statistically different between each propofol treatment group and the Ctrl group (P<0.05), but comparison by Pearson chi-squared test for each group showed no statistically significant difference (P>0.05). The ratio of polar body extrusion did not reduce as propofol concentration increased for each propofol treatment group; there was no statistically significant difference for the ratio of oocyte maturation in each treatment group. There was no statistically significant difference for the ratio of male and female pronuclear formation, in 2-cell and 4-cell stages and morula and blastocyst development for each group (P>0.05). The ratio of cleavage and development did not correlate with propofol concentration for each propofol treatment group.
Oocytes at the MII stage were extracted for in vitro fertilization for clinical human reproduction (Gizzo et al., Reference Gizzo, Noventa, Quaranta, Venturella, Vitagliano, Gangemi and D’Antona2016; Pina-Aguilar, Reference Pina-Aguilar2008). Classic indicators for MII oocyte development are the rate of male, female pronuclear formation, 2-cell embryo development, 4-cell embryo development, morula development, and blastocyst development. Compared with the Ctrl groups, the P-values of rate of male and female pronuclear formation, 2-cell embryo development, 4-cell embryo development, morula development, and blastocyst development in the propofol treatment group were all greater than 0.05, meaning there was no statistically significant difference. Propofol used in clinical oocyte extract was found to be safe and had no adverse effect on oocytes. Li et al. (Reference Li, Xiong, Alhashem, Zhang, Tilak, Patel, Siegel, Ye and Bekker2014) gave continuous infusion of propofol into female rats for 1 h or 2 h, or gave saline and lipid emulsion without propofol to study the effect of propofol on pup growth and development. They examined pup general look and feeling as well as athletic ability as the detection index. Pups in each group were not different for birth weight, brain weight, weight at 10 days after the birth, but values in the continuous infusion of propofol for 2 h group were significantly lower than that in the Ctrl group. Mature eyes (eye opening), and reflex reactions (hind limb reflex, righting reflex, reflective gait, tilt table test) were delayed after the continuous infusion of propofol for 1 h or 2 h. Forelimb reflection of pups was delayed in development after continuous infusion of propofol for the 2 h group, all detected indexes were restored to normal levels at postpartum 28 days. Therefore, the use of propofol in pregnant rat caused physical growth changes and the nerve maturation reflex of pups was delayed. The propofol infusion time was significantly greater than the time oocytes were treated with propofol (0.5 h), therefore these finding do not contradict this experiment.
In conclusion, propofol has an effect on the rate of MII oocyte polar body extrusion and GV oocyte maturation but, with increasing propofol concentration, change in inhibition of oocyte maturation was not clear. Change in propofol concentration had no effect on the rates of male and female pronuclear formation, 2-cell and 4-cell stages, and morula and blastocyst development, or oocyte development culture in vitro. The mechanism of the effect of propofol on oocyte development is still not clear and should be studied further.
Declaration of interest
The authors declare no competing or financial interests.
Funding
This work was supported by the NSF of China (31371454, 31671560) and the NSF of Inner Mongolia in China (2015JQ02).










