Introduction
The gonadotropin, follicle stimulating hormone (FSH), is a heterodimeric glycoprotein produced within the adenohypophysis and is involved in the regulation of several reproductive processes in the female and male. In the female, FSH acts primarily at the level of the ovarian follicle.
There has been controversy over the role of gonadotropins in the regulation of preantral follicle development. In vivo, the growth of preantral follicles is considered to be independent of the gonadotropins because animal or human preantral follicles can develop to the antral stage in conditions with minimal levels of circulating gonadotropins (Gulyas et al., Reference Gulyas, Hodgen, Tullner and Ross1977; Halpin et al., Reference Halpin, Jones, Fink and Charlton1986; Hillier, Reference Hillier1994). However, results from infantile rodent studies suggest that the development of early follicles is under the influence of gonadotropins. When infantile rats were treated with eCG, their ovarian weight increased (Goldenberg et al., Reference Goldenberg, Reiter and Ross1973). Treatment of neonatal rats with a GnRH antagonist resulted in a decreased number of growing ovarian follicles found at puberty (Meijs-Roelofs et al., Reference Meijs-Roelofs, Van Cappellen, Van Leeuwen and Kramer1990). Moreover, a study in juvenile rats has indicated: (i) the important role of endogenous gonadotropins in preantral follicle development; and (ii) that preantral follicles are highly responsive to exogenous gonadotropins (McGee et al., Reference McGee, Perlas, Lapolt, Tsafriri and Hsueh1997a). Another experimental model supports gonadotropin responsiveness and dependence of preantral follicles, and shows that preantral follicles do not grow beyond the two-layer stage in human ovarian xenografts in severe combined immunodeficiency/hypogonadal (SCID/hyp) mice unless exogenous FSH is administered (Oktay et al., Reference Oktay, Newton, Mullan and Gosden1998).
In vitro, several studies have suggested that FSH plays an important role in preantral follicle growth. In mice (Nayudu & Osborn, Reference Nayudu and Osborn1992; Cortvrindt et al., Reference Cortvrindt, Smitz and Van Steirteghem1997; Spears et al., Reference Spears, Murray, Allison, Boland and Gosden1998), hamsters (Roy & Greenwald, Reference Roy and Greenwald1989) and human (Wu et al., Reference Wu, Chen and Li2002), antrum formation or the formation of antral-like cavities does not occur in preantral follicles unless FSH is present in the culture medium and FSH appears to be an essential survival factor, in the absence of FSH, follicles became clearly atretic. In rats in preantral follicles, FSH was shown either to stimulate an antrum-like reorganization of the granulosa cells (Gore-Langton & Daniel, Reference Gore-Langton and Daniel1990; Cain et al., Reference Cain, Chatterjee and Collins1995) or, in the presence of a cGMP analogue, FSH is a growth and differentiation factor (McGee et al., Reference McGee, Spears, Minami, Hsu, Chun, Billig and Hsueh1997b). Recently, studies in pigs have shown that FSH induces both preantral oocyte growth to nearly final size or rapid preantral growth and antral development (Hirao et al., Reference Hirao, Nagai, Kubo, Miyano, Miyake and Kato1994; Wu et al., Reference Wu, Emery and Carrell2001). In contrast, other studies have indicated that treatment with FSH does not enhance preantral follicle growth (Li et al., Reference Li, Phillips and Mather1995; Boland et al., Reference Boland, Humpherson, Leese and Gosden1993).
Epidermal growth factor (EGF) is a mitogen and is primarily localized in the granulosa cells of preantral follicles (Roy & Greenwald, Reference Roy and Greenwald1990). Because the majority of the cells of the preovulatory follicles have passed beyond the proliferation stage, EGF production and action are of more significance to the granulosa cells of growing preantral follicles. The results from quantitation of ovarian EGF by radioimmunoassay suggested that there is a close correlation between gonadotrophin action and the induction of ovarian EGF (Anderiesz et al., Reference Anderiesz, Ferraretti, Magli, Fiorentino, Fortini, Gianaroli, Jones and Trounson2000). Study of hamster ovaries indicated that hamster follicular cells express the EGF gene and that the expression is under FSH control (Roy & Harris, Reference Roy and Harris1994). However, little information exists on the action of EGF with FSH in the developmental competence of porcine preantral follicle oocytes grown in vitro.
The objective of this study was to assess roles of FSH, EGF or combination of EGF and FSH in porcine preantral follicle growth and subsequent embryonic development.
Materials and Methods
Animal and tissue collection
Ovaries from prepubertal gilts were collected at a local slaughterhouse and transported to the laboratory in Dulbecco's phosphate-buffered saline (DPBS, Gibco 11500–030) supplemented with 3 mg/ml BSA (A-8022, fraction V, Sigma). Blood from prepubertal gilts was collected and centrifuged at 300 g for 10 min. The serum was removed from the pellet of cells and stored at –20 °C until use.
Preantral follicle collection and in vitro culture
Preantral follicles were collected and cultured using a minor modification of our method as previously described (Wu et al., Reference Wu, Emery and Carrell2001). The ovaries were cut into small pieces (1–3 mm) and preantral follicles were isolated in DPBS with 3 mg/ml BSA, mechanically using forceps. Preantral follicles at a diameter of 296 ± 8 μm were collected into 4-well multidishes (Nunclon) containing collecting medium, NCSU23 supplemented with 3 mg/ml BSA. After collection, the follicles were transferred from the collecting medium into culture medium. The culture medium consisted of NCSU23 supplemented with 3.5 μg/ml insulin (I5523, Sigma), 10 μg/ml transferrin (T5391, Sigma), 100 μg/ml l-ascorbic acid (A4544, Sigma) and 7.5% porcine serum. Except for the control group, the culture medium was also supplemented with 1.5 ng/ml ovine FSH (oFSH-20, 4453 IU/mg, the National Hormone and Pituitary Program of National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK]) or 1.5 ng/ml oFSH and EGF (E 4127, Sigma) at various concentrations. The dose of 1.5 ng/ml oFSH was selected on the basis of our previous study showing a maximally stimulated in vitro porcine follicular growth at this dose (Wu et al., Reference Wu, Emery and Carrell2001).
The follicles were randomly distributed to different experimental groups and cultured for 3 days in 24-well cell culture clusters plates (3524, Costar, Corning, NY, USA) with three follicles per well in 280 μ1 of culture medium. Culture was carried out at 38.5 °C in an atmosphere of 5% CO2 in air. Culture medium was changed every day. Follicle diameter was measured every 24 h using a stereomicroscope ocular scale at a magnification of ×50.
Histological investigation of follicles
Cultured follicles were fixed in 3% paraformaldehyde and 2.5% glutaraldehyde in phosphate buffer. After washing, the fixed follicles were dehydrated in increasing concentration of ethanol. The follicles were then pre-infiltrated with a 1:1 mixture of 100% ethanol and Technovit 7100 together with Hardener I (Heraeus Kulzer GmbH). Infiltration was completed by placing the follicles into Technovit 7100 with Hardener I. Finally, the follicles were transferred to beem capsules in Technovit 7100 with Hardener I and II for embedding. The follicles were serially sectioned by 1 μm and stained with methylene blue solution (Wu et al., Reference Wu, Nayuyu, Kiesel and Michelmann2000).
Measurement of estradiol
Estradiol in culture media was measured immunoenzymatically using an ELISA method (Serono Diagnostics). The inter- and intra-assay coefficients of variation were ≤5% and the sensitivity was <20 pmol/ml.
In vitro maturation of cumulus–oocyte complexes (COCs)
Maturation of COCs was performed as described by Wu et al. (Reference Wu, Emery and Carrell2001) and Abeydeara et al. (1998). After culture, the follicles were opened by two needles and the COCs were flushed into DPBS supplemented with 3 mg/ml BSA. The COCs were collected and washed three times in NCSU23 medium supplemented with 0.23 m pyruvate and 10% porcine serum. For in vitro maturation, 0.12 μg/ml oFSH, 2.5 μg/ml ovine LH (oLH-26, the National Hormone and Pituitary Program), 20 ng/ml epidermal growth factor (EGF, E-4127, Sigma), 50 μg/ml l-ascorbic acid and 10–20 antral follicular shell pieces were added to the medium in which COCs were cultured for 48 h.
In vitro fertilization (IVF) and embryo culture
After maturation, oocytes were washed three times with IVF medium consisting of mTBM, 2 mM caffeine and 2 mg/ml BSA (A7888, Sigma). The oocytes were then transferred to 50 μ1 of IVF medium and incubated in the incubator for about 30 min until spermatozoa were added for fertilization. Porcine sperm (SGI) preparation was the same as that described previously (Yang et al., Reference Yang, Chen and Li1999; Wu et al., Reference Wu, Emery and Carrell2001). After sperm preparation, 50 μ1 of the sperm suspension was added to 50 μ1 of the medium that contained oocytes (final concentration of 5 × 105 cells/ml). The oocytes were incubated with spermatozoa for 5–6 h at 38.5 °C in an atmosphere of 5% CO2 in air. After insemination, oocytes were removed from fertilization drops and cultured in 500 μ1 of embryo culture medium (NCSU23 containing 3 mg/ml BSA and 0.5% [v/v] MEM amino acids) in a 60 × 15 mm centre-well organ culture dish (Becton Dickinson, Falcon) until examination. Cleavage rate was recorded at 48 h after IVF. One-cell oocytes were mounted, fixed in 25% (v/v) acetic acid in ethanol, stained with 1% (w/v) orcein in 45% (v/v) acetic acid and examined under a phase-contrast microscope for penetration by a sperm and pronuclear development. Fertilization rates were calculated by adding the cleaved embryos and one-cell oocytes that had been penetrated by spermatozoa. At 168 h after IVF, blastocyst formation was observed. The blastocysts were then mounted, fixed in acetic alcohol and stained with 1% aceto-orcein.
Statistical analysis
Experiments were repeated at least three times. One-way ANOVA were used for statistical comparisons. p-values of <0.05 were considered significant.
Table 1 Effect of FSH, EGF or EGF with FSH on development of porcine preantral follicles in vitro

aFollicle diameters under different conditions were the same on day 0.
bp < 0.05 as compared with control.
Results
Role of FSH, EGF or combination of EGF and FSH in promoting preantral follicle growth
Porcine preantral follicles were cultured in the absence (control) or in the presence of FSH, EGF or a combination of EGF and FSH to determine the role exerted by FSH, EGF or a combination of EGF and FSH on the follicle growth. In the absence of FSH or EGF, follicle grew initially at the rate of 31 μm/day, but this growth was not sustained by days 2 and 3 (14 μm/day) and the final mean follicle size was only 357 ± 5 μm. The resulting follicles were non-antral. In contrast, the presence of FSH produced an average growth rate of 58 μm/day. The growth was most apparent over the first 2 days in which the growth rate was 66 μm/day. This resulted in a mean final diameter of 471 ± 7 μm with a high proportion (89%) of antrum formation and healthy appearing oocytes. The final follicle size was significantly larger than that of the control. With EGF alone, follicles reached final size of 368 ± 6 to 381 ± 10 μm but did not develop to antral stage. The three different amounts of EGF (0.75, 1.5, 3.0 ng/ml) did not reveal significant differences in their end size. Combined addition of EGF and FSH produced the rapid growth of follicles and resulted in the mean final diameters of 480 ± 9 to 486 ± 8 μm. However, the final follicle sizes were not significantly larger than was achieved with FSH alone (Table 1).
Role of FSH, combination of EGF and FSH in inducing estradiol secretion by follicles
The secretion of estradiol by follicles (three follicles per well) was assessed under cultures with FSH, a combination of EGF and FSH or control. Without FSH or EGF supplementation, follicles secreted progressively reducing amounts of estradiol. By contrast, follicles cultured with FSH or a combination of EGF and FSH supplementation, released progressively increasing amounts of estradiol. Moreover, the amount of estradiol on days 2 and 3 of the culture was significantly higher in follicles cultured with FSH (49 pg/ml, 71 pg/ml, respectively) or a combination of EGF and FSH (67.25 ± 15 pg/ml, 78.5 ± 16 pg/ml) than in those with controls (16 pg/ml, 6 pg/ml, respectively) (Fig. 1). There was no significant difference in estradiol secretion between follicle culture with FSH or a combination of EGF and FSH.

Figure 1 Comparison of the effects of medium containing FSH, EGF with FSH or control on production of estradiol by follicles in vitro. * indicates p < 0.05 as compared with other conditions.
Maturation, fertilization and embryonic development of oocytes from preantral follicles under culture with FSH or combination of EGF and FSH
The capacity of the oocytes to mature, fertilize and develop to embryo stage was evaluated after culture of the preantral follicles either with FSH or a combination of EGF and FSH and compared with the control,. The meiotic competence of oocytes was assessed by the presence of the first polar body (PB). No oocytes from the control preantral follicles reached PB stage and moreover no oocytes were fertilized. Oocytes from preantral follicles cultured with FSH or combination of EGF and FSH reached the PB stage similar proportions (52 ± 8 and 55 ± 6%, respectively) (Table 2). For fertilization, no significant difference was observed between treatment with FSH or a combination EGF and FSH. However, when preantral follicles were cultured with a combination of EGF and FSH, 60 ± 5% of the fertilized oocytes derived from these follicles developed to the 2-cell stage, which was significantly higher than that achieved with FSH alone (45 ± 7%). No significant difference was observed in the percentage of the 2-cell stage follicles with either FSH or FSH plus EGF treatment that developed to blastocyst stage (14 ± 5% and 22 ± 4%, respectively). The blastocysts contained an average of 29 ± 3 nuclei (Fig.2a, b).
Table 2 Maturation, fertilization and embryonic development of oocytes from porcine preantral follicles under different conditions

a p > 0.05 as compared with other conditions.
b p < 0.05 as compared with FSH.
Histological observations of cultured follicles
Histological examination of sections of follicles cultured for 3 days under three different conditions (0 control, FSH, or EGF with FSH) showed that oocytes were at the germinal vesicle (GV) stage and the cytoplasm had a homogeneous structure (Fig. 3). For the control, a few granulosa cells had proliferated, but no antrum formation was observed (Fig. 3a). With FSH treatment, the oocytes were healthy and surrounded by one to two layers of granulosa cells (cumulus cells). Granulosa cells have proliferated, have normal organization and antrum formation was seen (Fig. 3b). Finally, with a combination of EGF and FSH, oocytes were surrounded by granulosa cells (cumulus cells). Following treatment, granulosa cells had proliferated and had normal organization and antrum formation was evident (Fig. 3c).
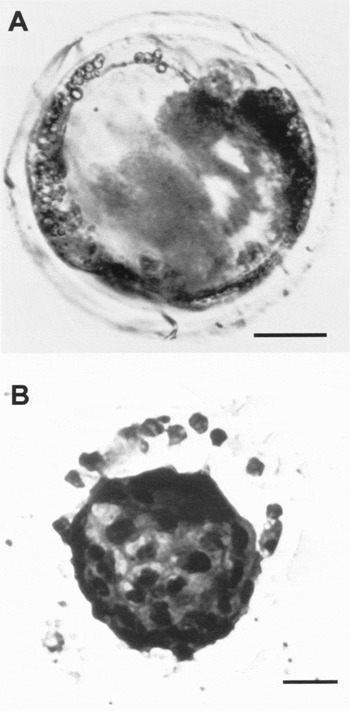
Figure 2 Representative examples of embryonic development from oocytes of porcine preantral follicles after culture with a combination of EGF and FSH. A. Blastocyst; B. The blastocyst was stained with aceto-orcein. Bar = 25 μm.

Figure 3 Examples of porcine follicles fixed after 3 days of culture with different conditions A, With control. B, With FSH. C, With a combination of FSH and EGF (1.5 ng/ml). Scale bar represents 50 μm.
Discussion
In this study, preantral follicles from prepubertal gilts were subjected to four culture conditions: with control, with FSH, with EGF or with a combination of EGF and FSH. When FSH was added to the culture medium, preantral follicles grew rapidly to the antral stage, half of the oocytes matured and subsequently developed to the blastocyst stage after IVF. In contrast, without FSH preantral follicles failed to grow to the antral stage and none became a mature oocyte. The results demonstrate that addition of FSH is essential for: (i) the growth of preantral follicles; (ii) antrum formation; (iii) the competence of oocytes to resume meiosis and to undergo fertilization and (iv) embryonic development.
The current study supports previous studies (Roy and Greenwald, Reference Roy and Greenwald1989; Gore-Langton and Daniel, Reference Gore-Langton and Daniel1990; Nayudu and Osborn, Reference Nayudu and Osborn1992; Hirao et al., Reference Hirao, Nagai, Kubo, Miyano, Miyake and Kato1994; Cain et al., Reference Cain, Chatterjee and Collins1995; Cortvindt et al., 1997; McGee et al., Reference McGee, Spears, Minami, Hsu, Chun, Billig and Hsueh1997b; Spears et al., Reference Spears, Murray, Allison, Boland and Gosden1998; Wu et al., Reference Wu, Emery and Carrell2001) that suggest that FSH plays an important role in the development of preantral follicles in vitro. Yuan et al. (Reference Yuan, Lucy and Smith1996) and Liu et al. (Reference Liu, Aronow, Witte, Pope and La Barbera1998) have shown that messenger RNA for FSH receptor was expressed in granulosa cells of porcine preantral follicles. The biochemical action of FSH on follicles is mediated by the binding of this hormone to the cell-surface FSH receptor on granulosa cells, resulting in the activation of a guanine nucleotide-binding protein (G protein), which stimulates adenylate cyclase (Richards et al., Reference Richards, Jahnsen, Hedlin, Lifka, Ratoosh, Durica and Goldring1986, Johnson & Dhanasedaran, 1999). One result of this action is that FSH promotes preantral follicle growth by inducing granulosa cell proliferation and differentiation, as the granulosa cell growth accounts for the majority of this follicular expansion (Gougeon, Reference Gougeon1982).
Furthermore, recent studies have reported that kit ligand (KL) expression is stimulated by (Bu)2 cAMP in mouse granulosa cells (Packer et al., Reference Packer, Hsu, Besmer and Bachvarova1994) and that FSH stimulates gene expression of KL in purified bovine granulosa cells (Parrott & Skinner, Reference Parrott and Skinner1998). These results suggest that FSH may in part promote follicular development by directly stimulating KL expression in granulosa cells. Parrott & Skinner (Reference Parrott and Skinner1998) found that KL stimulates keratinocyte growth factor (KGF) and hepatocyte growth factor (HGF) expression in thecal cells. Previous work has also shown that granulosa cell-derived KL acts on thecal cells to promote cell proliferation and differentiation (Parrott & Skinner, Reference Parrott and Skinner1997). Therefore, FSH may regulate thecal cell function indirectly and induce thecal cell proliferation and differentiation. In the present study, we have shown that the amount of estradiol on days 2 and 3 of culture was significantly higher in follicles cultured with FSH than in those cells without FSH. FSH may indirectly drive the theca cells to make the androgen substrate, which is converted to estrogen by the granulosa cell enzyme aromatase. FSH and the secreted estrogen coordinate to induce antrum formation in preantral follicles in culture. This study has shown that a high proportion of preantral follicles grew to the antral stage when FSH was added to the culture medium. The result was corroborated by histological observations.
An ideal follicle functions: (i) to provide the micro-environment necessary for the growth and maturation of an oocyte; and (ii) to produce sex steroids. The development and maturation of a follicle are highly involved processes. In this study, most of preantral follicles cultured with FSH grew to morphologically normal antral follicles with higher estradiol secretion. The oocytes from these antral follicles can mature, fertilize and develop to blastocyst stage. Without FSH, preantral follicles were unable to develop to the antral stage. These results suggest that FSH can induce functional follicular development and that the follicle is a developmental unit.
In contrast, it is considered that FSH may not be required element for in vitro follicular development (Boland et al., Reference Boland, Humpherson, Leese and Gosden1993; Li et al., Reference Li, Phillips and Mather1995). Recently, Eppig et al. (Reference Eppig, O'Brien, Pendola and Watanabe1998) reported that FSH treatment of cultured granulosa cell– oocyte complexes did not significantly affect oocyte growth, oocyte competence to resume meiosis or undergo fertilization and preimplantation development. Furthermore, treatment of the complexes with both FSH and insulin produced a highly deleterious effect on their competence to undergo development from the 2-cell stage to blastocyst.
In the present study, treatment of preantral follicles with EGF plus FSH significantly increased the percentage of their oocytes that were competent to undergo cleavage to the 2-cell stage after IVF, when compared to those given FSH alone. However, no significant difference was observed in the percentage of the 2-cell oocytes that developed to the blastocyst stage after treatment either with FSH plus EGF or FSH alone. In addition, EGF alone treatment of preantral follicles produced a final size that was significantly smaller than that of follicles that had received a combination of EGF plud FSH or FSH alone. The results indicate that EGF acts synergistically with FSH to promote follicular development and induce a beneficial effect on embryo developmental competence. Previous work (Roy & Harris, Reference Roy and Harris1994) showed that hamster follicular cells express the EGF gene, that the expression is under FSH control and that, in hamsters, FSH action is mediated, at least in part, via EGF. Downs et al. (Reference Downs, Daniel and Eppig1988) reported that EGF and FSH induced nuclear maturation in mouse COCs in vitro through a mechanism mediated by cumulus cells.
Histological observation from this study revealed that a combination of EGF and FSH produced a large antral cavity compared with treatment of FSH alone. In contrast, FSH may be considered to interfere with the mitogenic stimulus provided by EGF. Recent studies suggested that both EGF and FSH can effectively activate the mitogen-activated protein kinase (MAPK) cascade in granulosa cells (Maizels et al., Reference Maizels, Cottom, Jones and Hunzicker-Dunn1998). However, increases in cyclic adenine monophosphate (cAMP) synthesis induced by FSH interfered with both activation of the MAPK signalling pathway in response to EGF (Wu et al., Reference Wu, Dent, Jelinek, Wolfman, Weber and Sturgill,1993) and its mitogenic effects in rat fibroblasts (Cook & McCormick, Reference Cook and McCormick1993).
Oocyte maturation is often conceptually divided into nuclear and cytoplasmic processes. Nuclear maturation is a term that refers to the resumption of meiosis and progression to metaphase II. Cytoplasmic maturation is a more general term that refers to other maturation events not directly related to meiotic progression that prepare the oocyte for fertilization and preimplantation development (Eppig et al., Reference Eppig, Schultz, O'Brien and Chesnel1994; Eppig, Reference Eppig, O'Brien and Wigglesworth1996). Both EGF and FSH induce nuclear maturation in mouse COCs in vitro through a mechanism mediated by cumulus cells (Downs et al., Reference Downs, Daniel and Eppig1988). There are reports that EGF promotes nuclear maturation in humans (Das et al., Reference Das, Stout, Hensleigh, Tagatz, Phipps and Leung1991), bovine (Kobayashi et al., Reference Kobayashi, Yamashita and Hoshi1994) and porcine (Singh et al., Reference Singh, Barbe and Armstrong1993) oocytes, as well as cytoplasmic maturation of mouse (Das et al., Reference Das, Stout, Hensleigh, Tagatz, Phipps and Leung1991).
In summary, this study has shown that FSH is essential for the growth of preantral follicles, antrum formation, estradiol secretion and their oocyte-acquiring competence to resume meiosis and to undergo fertilization and embryonic development. EGF with FSH treatment of porcine preantral follicles can improve the quality of oocytes by a higher frequency of embryonic development.
Acknowledgements
The work was supported by the Specialized Research Fund for the Doctoral Programme of Higher Education (SRFDP) and sponsored by shanghai pujiang program.







