Key message
We present the case of a female patient who delivered a healthy baby following in vitro fertilization and intracytoplasmic sperm injection (IVF-ICSI), despite the absence of zona pellucida (ZP). Our case report provides information on the possible genetic basis of ZP-free oocytes and the critical role of ZP during oocyte manipulation, and illustrates the possibility to obtain a viable offspring by IVF-ICSI from ZP-free oocytes.
Introduction
Despite marked developments in assisted reproductive technology (ART) over the past 4 decades, clinicians and embryologists commonly face challenging case scenarios in routine clinical practice. Gamete abnormalities, particularly oocyte anomalies, are mainly observed in individuals with idiopathic infertility (Asa et al., Reference Asa, Tabatabaee, Farrokhi and Nejatbakhsh2017). Previous studies have shown that different oocyte morphological abnormalities, including shape abnormalities (Esfandiari et al., Reference Esfandiari, Ryan, Gotlieb and Casper2005; Balaban and Urman, Reference Balaban and Urman2006; Ebner et al., Reference Ebner, Moser and Tews2006, Reference Ebner, Shebl, Moser, Sommergruber and Tews2008) and cytoplasmic abnormalities might be associated with a poor prognosis for pregnancy in ART (Ezra et al., Reference Ezra, Simon and Laufer1992; Rankin and Dean, Reference Rankin and Dean1996). Some rare abnormalities are difficult to detect and diagnose, and may lead to misdiagnosis and suboptimal management. Among them, zona pellucida (ZP) abnormalities have been associated with less than 5% of oocyte anomalies (Balaban et al., Reference Balaban, Brison, Calderon, Catt, Conaghan, Cowan, Ebner, Gardner, Hardarson and Lundin2011).
The ZP, a thick membrane surrounding the ovum, is essential for binding sperm and protection from polyspermy (El-Mestrah et al., Reference El-Mestrah, Castle, Borossa and Kan2002). Furthermore, it protects eggs before hatching and embryos from mechanical stress before implantation.
Intracytoplasmic sperm injection (ICSI) is used as a possible solution for patients with oocyte and ZP abnormalities (Yu et al., Reference Yu, Ahn, Lee, Jee and Kim2015). Studies have discussed the ZP in empty follicle syndrome, which is diagnosed and treated differently from the complete absence of the ZP (Vassena et al., Reference Vassena, Blazquez, Guillén, Colomé, Mataró and Vernaeve2014; Dai et al., Reference Dai, Chen, Hu, Du, Gong, Dai, Zhang, Wang, Chen and Guo2019a). However, to the best of our knowledge, there is just one study on the management of ZP-free oocytes during in vitro fertilization (IVF) (Stanger et al., Reference Stanger, Stevenson, Lakmaker and Woolcott2001).
Mechanical manipulation of oocytes during collection or denudation may contribute to the accidental loss of ZP and cause the complete expulsion of the oocyte from the ZP. However, other mechanisms, including genetic factors, might explain the occurrence of oocytes without the ZP. Here, we report the delivery of a healthy infant using ICSI in a patient whose oocytes had a genetic mutation characterized by the complete absence of the ZP.
Case history
A 35-year-old female patient was admitted to the fertility clinic with a history of primary unexplained infertility over the preceding 10 years. Her husband had undergone a full male infertility examination and was diagnosed normozoospermic (World Health Organization, 2010).
The patient had a normal hormonal profile on day 2 of her menstrual cycle. The ultrasonography results were unremarkable. The hysterosalpingography results were also normal and showed that the fallopian tubes were patent. The couple was offered ART treatment, as they had experienced several previous unsuccessful intrauterine insemination attempts.
Controlled ovarian hyperstimulation (COS) was started on day 2 of her menstrual cycle. Human menopausal gonadotropin 225 IU/day (Menogon HP®, Ferring GmbH, Kiel, Germany) was administered subcutaneously for 5 days. Ultrasound monitoring performed on day 7 showed four follicles in each ovary; the patient’s oestrogen level was 1406 pg/ml. Gonadotropin therapy was continued using the same dosage for another 4 days, and 0.25 mg cetrorelix acetate (Cetrotide®, Merck, Germany) was administered subcutaneously daily from day 6 until the trigger day. Repeated ultrasound on day 11 showed that the diameter of the dominant follicle reached 18 mm. Human chorionic gonadotropin (hCG) (Choriomon®; IBSA Pharmaceuticals) at a dose of 10,000 IU was administered intramuscularly to trigger oocyte maturation; oocyte pick-up (OPU) was performed transvaginally 36 h later. The OPU was performed at a vacuum pressure of 120 mmHg. Nine cumulus–oocyte complexes (COCs) were collected and incubated in a pre-equilibrated bicarbonate-buffered cleavage medium (Sage Cleavage Medium, Cooper Surgical, Trumbull, CT, USA) for 1 h in oil before denudation using 20 IU/ml of hyaluronidase enzyme in human tubal fluid (HTF)-buffered HEPES (SAGE InVitro Fertilization, Trumbull, CT, USA). In our unit, the standard operation protocol for denudation (Mahadevan and Trounson, Reference Mahadevan and Trounson1985) involves the use of a glass pipette of internal diameter 400 µm to remove the corona radiata cells and a 170-µm stripper to remove remnant granulosa cells around the ZP. After complete removal of the COCs, neither the oocytes nor ZPs were observed. A senior embryologist confirmed these results.
Thereafter, we immediately pipetted granulosa tissue from another patient into the same denudation dish to confirm the efficacy of the hyaluronidase enzyme used. The cells were intact after processing with a 170-µm stripper. After adding old, non-fertilized oocytes (arranged for discarding) to the same dish, we observed that the oocytes were intact even after 15 min of incubation, which validated the composition of hyaluronidase used.
The patient was informed that oocytes were not observed after collection and denudation. The couple returned for counselling and was advised to undergo further investigation. We requested karyotyping and whole exome sequencing (WGS) analysis. Consent was obtained from the patient before performing and reporting the research. As the karyotype was normal (46,XX), we referred the patient to genetic testing and counselling. WES was performed on DNA extracted from the patient blood sample. Approximately 45 Mb of the consensus coding sequence were enriched from fragmented DNA by probes designed from the human genome (Illumina) before being sequenced on a NextSeq 550 system (Illumina). Raw sequencing data analysis, including base calling, demultiplexing, alignment to the hg19 human reference genome (Genome Reference Consortium GRCh37) and variant calling was performed using Burrows Wheeler Aligner (BWA) and Genome Analysis Toolkit (GATK). Variant pathogenicity was determined by referencing the guidelines established by the American College of Molecular Genetics (ACMG) (Richards et al., Reference Richards, Aziz, Bale, Bick, Das, Gastier-Foster, Grody, Hegde, Lyon and Spector2015). Results obtained 2 months later revealed the presence of a mutation affecting the ZP glycoprotein 1 gene (NM_207341.2; c.706T > C; p. Cys236Arg).
Diagnostic intracytoplasmic sperm injection (D-ICSI) procedure
Two months after the first IVF–ICSI cycle, the patient contacted our centre again, seeking further treatment. We advised the couple to undergo a natural ICSI cycle to gather more information about oocyte quality and embryo development potential. We termed this procedure ‘diagnostic ICSI (D-ICSI)’. The couple agreed, and we carried out the procedure as follows. In the natural menstrual cycle, we performed a transvaginal ultrasound scan on cycle day 10, which revealed the presence of two growing follicles, the largest being 16 mm. The scan was repeated 2 days later when the largest follicle measured 20 mm. The final follicle maturation was triggered with 5000 IU IM of hCG (Choriomon® IBSA Pharmaceuticals). The OPU was carried 36 h later.
Two cumulus–oocyte complexes (COCs) were collected using a vacuum suction pressure of 80 mmHg. The COCs were placed into a dish with culture medium (G-MOPS buffer system; VitroLife, Gothenburg, Sweden) and were covered with oil. The COCs were then transferred to a new dish with cleavage medium (Origio® Sequential Cleav™, with phenol red; Origio Specialty Pharma, Denmark), and again covered with oil (Ovoil™-VitroLife, Sweden). Photographs of the COCs were taken using a stereomicroscope (Nikon SMZ 1500, Japan) at ×40 magnification for confirmation and documentation purposes (Figs 1 and 2).

Figure 1. Cumulus–oocyte complex showing a complete structure.
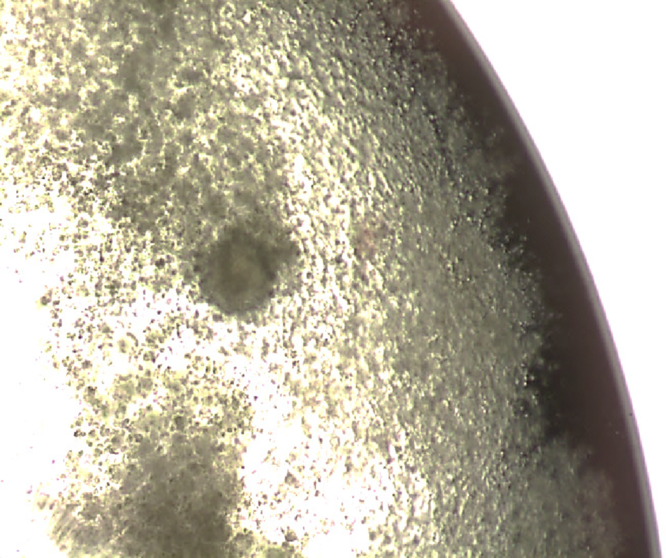
Figure 2. Cumulus–oocyte complex with normal looking structure.
The COCs were incubated for 30 min at a CO2 concentration of 6.0%. We started denudation using a 400-µm stripper in a droplet containing ICSI Cumulase® Origio (Origio Specialty Pharma, Denmark) (de Vos et al., Reference de Vos, van Landuyt, van Ranst, Vandermonde, D’Haese, Sterckx, Haentjens, Devroey and van der Elst2008; Evison et al., Reference Evison, Pretty, Taylor and Franklin2009). After carefully removing the granulosa tissue, we monitored the oocytes closely, and denudation was carried out using a 300-µm stripper, with each oocyte individually denuded. This step was completed using a 170-µm stripper. After denudation, only one oocyte was observed; however, polar bodies (PB) and the ZP were not observed (Fig. 3). ICSI was performed with this oocyte, and successful fertilization was observed 18 h after insemination (Fig. 4). The developed embryo was cultured using cleavage medium, and a day-4 early blastocyst was vitrified successfully (Fig. 5). We opted not to replace the embryo to the uterus in the same cycle because the endometrium was very thin (6.0 mm).
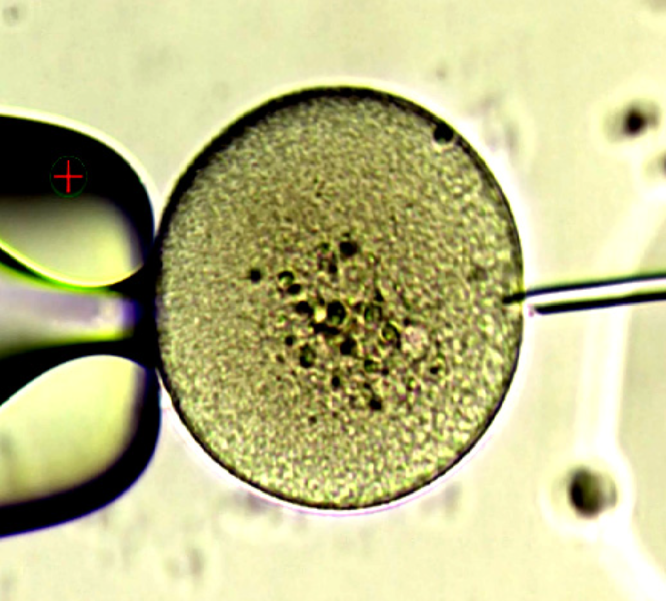
Figure 3. Oocyte during intracytoplasmic sperm injection; after decumulation, polar body was lost due to the absence of zona pellucida.

Figure 4. Zona-free zygote; 2PN seen 18 h post intracytoplasmic sperm injection.

Figure 5. Developed embryo in the early blastocyst stage on day 4. Zona pellucida-free embryo before embryo transfer.
Therapeutic ICSI procedure
Two months later, based on the results of the diagnostic ICSI, the patient started a new IVF cycle after reducing her body mass index from 29 kg/m2 to 26 kg/m2. Controlled ovarian stimulation was started on day 2 of her menstrual cycle, with a recombinant follicle-stimulating hormone (Gonal-F; Serono Inc., Rockland, MA, USA) at a dose of 300 IU/day for 9 days. Cetrorelix acetate (0.25 mg) was administered subcutaneously on the last 4 days of stimulation, and 10,000 IU of hCG was used for triggering. Changes in the ovarian stimulation protocol in the new IVF cycle were made at the doctor’s discretion. The oestradiol (E2) level was 1177 pg/ml on the trigger day, and the endometrium was 13.5 mm thick. Eight follicles were aspirated using a pressure of 80 mmHg and the COCs were placed under oil in cleavage medium (Origio® Sequential Cleav™, with phenol red) in a separate well within a four-well dish for 120 min before denudation. Luteal support was started on the same day as OPU with 100 mg vaginal progesterone administered intravaginally twice daily.
After 30 min, ICSI Cumulase® Origio (Origio Specialty Pharma) was added to each well, and denudation was performed, starting with a 400-µm stripper and transitioning to a 170-µm stripper. The collected oocytes did not have a ZP, as previously observed, and PBs were also missing. Eight zona-free oocytes (ZFO) were transferred to a new pre-equilibrated cleavage medium-containing dish (Origio® Sequential Cleav™, with phenol red) to maintain the denuded oocytes until ICSI, which was carried out gently to hold and immobilize the ZFOs during sperm injection. The husband’s semen was prepared using the density gradient procedure (Mortimer, Reference Mortimer1991). ICSI was successfully carried out in eight ZFO.
The injected oocytes were incubated individually in microdrops of pre-equilibrated cleavage medium (Cooper Surgical, Trumbull, CT, USA) covered with prewashed culture oil. At 18 h after insemination, two pronuclei (PN) were observed in six of the eight injected oocytes. A continuous culture was sustained for 4 days, with a single embryo per drop. We then shifted four morulae and one cleaving embryo to a new culture dish with blastocyst culture medium (Origio® Sequential Blast™). On day 5, we transferred three embryos without the ZP (EWZ) of the following grades: EWZ-4AA, EWZ-3AA, and EWZ-3BA. The other embryos, including one morula and one cleaving embryo, were observed until day 6 without further development and were discarded.
Fresh embryos were transferred using a Labotect embryo transfer catheter (Labotect Embryo Transfer Catheter Set, Ref. 320201; Labotect GmbH, Labor-Technik-Göttingen, Germany). On day 10 after embryo transfer, a positive serum hCG was reported. Six weeks later, a singleton was confirmed by a positive foetal heartbeat detected via a transvaginal ultrasound. The pregnancy progressed uneventfully, and the patient delivered a 3100 g healthy baby boy at 38 weeks of gestation by caesarean section. No neonatal intensive care (NICU) was required.
Discussion
Unexplained infertility is a common clinical problem. However, despite advances in the field of reproductive medicine, the aetiological basis of this condition remains a challenge. One of the complexities in the fertility diagnostic process is associated with the inherent uncertainty due to multifactor-dependent decisions. As noted in the committee’s conceptual model of the diagnostic process for the case under discussion, an overarching question throughout the process was whether sufficient information had been collected to make a proper diagnosis. Additional diagnostic procedures, such as diagnostic ICSI (D-ICSI) in the natural cycle as reported here, and ICSI combined with minimal stimulation, might be used in similar cases of unexplained infertility to obtain more information about oocytes and potential embryo development (Dai et al., Reference Dai, Chen, Hu, Du, Gong, Dai, Zhang, Wang, Chen and Guo2019a).
Here, we presented the case of a 35-year-old woman diagnosed with the complete absence of the ZP who gave birth to a healthy baby. The migration of oocytes inside the fallopian tube, and sperm fertilization following normal penetration are dependent on the presence of the ZP. Although the ZP partially functions as a sperm receptor and a protective sheath for developing embryos during the cleavage stage, our case illustrates that in the IVF settings, the ZP is not an essential component for oocyte maturation and embryogenesis, despite its importance during oocyte assessment and handling.
The oolemma is a soft and fragile structure that is weaker and less elastic compared with the ZP. During oocyte manipulation and ICSI procedures, gentle handling is required. The ZP is important for allowing the proper assessment of oocyte maturation. In the presence of ZFOs, the first PB is lost during oocyte denudation. Consequently, the determination of maturation status and meiotic spindle location is challenging. Hence, sperm injection should be random, unlike routine injections in patients with normal oocytes. In is noteworthy that sperm injections could be carried out using a spindle viewer system to determine the location of the meiotic spindle, thereby allowing a more accurate sperm injection. Our study and other previous studies show that fertilization and embryo development can be achieved irrespective of the presence of PBs (Mortimer, Reference Mortimer1991; Dai et al., Reference Dai, Chen, Hu, Du, Gong, Dai, Zhang, Wang, Chen and Guo2019a).
Non-aggressive and non-mechanical denudation procedures can be more effective compared with aggressive and mechanical procedures, even in patients with a history of fragile oocytes or poor embryogenesis quality (Evison et al., Reference Evison, Pretty, Taylor and Franklin2009). In our study, we used the human-cloned hyaluronidase enzyme, which is reported to be less traumatic to unprotected oocytes and oolemmas (de Vos et al., Reference de Vos, van Landuyt, van Ranst, Vandermonde, D’Haese, Sterckx, Haentjens, Devroey and van der Elst2008), and a diagnostic ICSI to both confirm the diagnosis of complete ZFO and assess the feasibility of ICSI and embryo development using ZP-free oocytes. Different manipulation procedures for genetically ZP-free oocytes and successful ICSI fertilization using non-denuded and partially denuded oocytes have been reported previously (Paz et al., Reference Paz, Amit and Yavetz2004; Vajta et al., Reference Vajta, Rienzi and Bavister2010; Hu and Trolice, Reference Hu and Trolice2016).
Our patient was found to have a new ZP1 mutation (NM_207341.2; c.706T > C; p. Cys236Arg), probably causing the absence of ZP. The mutation identified was homozygous, that is, both parents were carriers, due to the consanguinity of the patient’s family. Fortunately, ZP1 is an autosomal recessive trait, therefore the mutation should occur in both paternal and maternal chromosomes to cause the absence of the ZP. A homozygous mutated ZP1 gene from one parent (the mother in this case) passes on one mutated allele to the embryos, whereas a normal allele from the other parent (the father was presumably not a carrier of the ZP1 gene in our case) would ensure a normal ZP in the eggs of future female generations. This IVF case was challenging but resulted in normal pregnancy and delivery of a healthy baby. To the best of our knowledge, there has been just one study on a compound heterozygous mutation in ZP1 (Yang et al., Reference Yang, Luan, Peng, Chen, Su, Zhang, Wang, Cheng, Zhang and Wang2017; Dai et al., Reference Dai, Hu, Gong, Tan, Cai, Zhang, Dai, Lu, Chen and Chen2019b). A homozygous mutation in the same gene (Zhang et al., Reference Zhang, Shangguan, Li and He2018), as reported here, is most likely to be the main reason for this type of anomaly. Although the use of D-ICSI was beneficial in this particular case, the cost, benefit, ethics, potential consequences and drawbacks of this approach should be analyzed on an individual basis.
Conclusion
We illustrated the utilization of ‘diagnostic ICSI’ for a patient with unexplained infertility undergoing ART and characterized by an abnormal oocyte structure. The diagnostic ICSI in the natural cycle allowed the acquisition of information about oocytes and potential embryo development, and its results were useful to plan the subsequent ICSI cycle. We report the successful delivery of a healthy infant after ICSI using ZP-free oocytes from a patient with a homozygous mutated ZP1 gene.
Acknowledgements
None.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
We declare that there are no conflicts of interest.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from the patients for being included in the study.
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) have provided their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Animal studies
This article does not contain any studies with animal subjects.







