Introduction
Testicles are the main organ of the male reproductive system and constitute a suitable environment for spermatogenesis and other fertility states. Testicles are composed of tubular ducts and have complicated networks for germ and Sertoli cells. Germ cells are located in seminiferous tubules, and initiate spermatogenesis and other crucial processes responsible for inheritance. Specific types of somatic cells have key roles in normal fertility by supporting chemical and physical processes. In addition, germ cells and Sertoli cells are significantly involved in the treatment of male infertility and other diseases (Griswold, Reference Griswold1995; Mikuz, Reference Mikuz2019).
Sertoli cells function in nutrition, as structural supports, and in immunity (Rato et al., Reference Rato, Alves, Socorro, Duarte, Cavaco and Oliveira2012). Each Sertoli cell can maintain a limited number of germ cells (Rebourcet et al., Reference Rebourcet, Darbey, Monteiro, Soffientini, Tsai, Handel, Pitetti, Nef, Smith and O’Shaughnessy2017), therefore the correct growth of Sertoli cells, based on their number and function, determines spermatogenic capacity until adulthood. During spermatogenesis, distinct sperm cells become more specialized, and their biochemistry machinery is insufficient for their metabolic needs. Sertoli cells meet these needs by responding to several metabolic stimuli through signalling cascades to maintain the energy for sperm-producing tubule homeostasis. Sertoli cells play an important role in testicular immunity by creating a physical barrier called the blood–testis barrier (BTB) (Qu et al., Reference Qu, Ogawa, Kuramasu, Nagahori, Sakabe and Itoh2020).
Despite innate immune responses (Rato et al., Reference Rato, Alves, Socorro, Duarte, Cavaco and Oliveira2012) and this front-line protection, some pathogens possess mechanisms that can lead to infection. Genital infections are caused by bacteria, parasitic and viral pathogens (Almeida et al., Reference Almeida, Braz-de-Melo, Santos, Corrêa, Kobinger and Magalhaes2020) and the BTB is not completely impermeable to microbial agents (Cheng and Mruk, Reference Cheng and Mruk2012). To date, more than 27 viruses, including human immunodeficiency virus (HIV), herpes simplex virus (HSV), and severe acute respiratory syndrome coronavirus (SARS-CoV) can penetrate semen and negatively affect spermatogenesis, producing a risk to fertility and to spread between couples (Rebourcet et al., Reference Rebourcet, Darbey, Monteiro, Soffientini, Tsai, Handel, Pitetti, Nef, Smith and O’Shaughnessy2017).
In December 2019, the SARS-CoV-2 virus, with symptoms similar to pneumonia, spread in Wuhan, China; this disease was named coronavirus-2019 (COVID-19) by the World Health Organization (WHO). SARS-CoV-2 virus has a c. 76% amino acid content similar to SARS-CoV. The virus can enter the host cell using the ACE2 receptor (Wang and Xu, Reference Wang and Xu2020). Coronaviruses make up the largest family of single-stranded RNA viruses and this family currently has 30 members (Huang et al., Reference Huang, Ji, Zhou, Huang, Peng, Fan, Lin and Zhu2021). The ACE2 receptor is found in several parts of the body, such as the gastrointestinal tract, heart, respiratory system, and reproductive system. This receptor is expressed in large quantities in the male reproductive system on Sertoli and Leydig cells and on spermatogonia (Wang and Xu, Reference Wang and Xu2020). If a man becomes infected with COVID-19, the chances of damaging these cells are high and, as a result, spermatogenesis could be impaired. It should be noted that the BTB may serve as a repository for viruses, protecting them from antiviral agents. Therefore, it is important to study testicles as targeted organs (Abobaker and Raba, Reference Abobaker and Raba2021).
Our study aimed to understand the role of COVID-19 in male infertility and the destructive effect of its virus on Sertoli cells, aiding future studies for the treatment of male infertility and reproductive biology advances.
Sertoli cells
Sertoli cells were first identified in the 19th century by Enrico Sertoli, who in his original article described a type of somatic cell with an irregular large shape and speculated that these cells were involved in the formation of viable spermatozoa in spermatogenesis (Oliveira and Alves, Reference Oliveira and Alves2015; Griswold, Reference Griswold2018). Sertoli cells are polarized epithelial cells that extend from the base of the seminiferous epithelium to the lumen of the seminiferous tubule. This means that the basal part of the Sertoli cell is attached to the membrane part of the seminiferous tubule (Berger and Nitta-Oda, Reference Berger and Nitta-Oda2018). Sertoli cells expand to surround germ cells and provide a support network such that most of the surfaces of the Sertoli cells are in contact with germ cells (Crisóstomo et al., Reference Crisóstomo, Alves, Gorga, Sousa, Riera, Galardo, Meroni, Oliveira, Alves and Oliveira2018). Therefore, an accurate understanding of the morphology of Sertoli cells gives us a clear view of their role in spermatogenesis (Tindall et al., Reference Tindall, Rowley, Murthy, Lipshultz and Chang1985). Tarulli et al. (Reference Tarulli, Stanton and Meachem2012) likened spermatogenesis to a symphony in which germ cells occur in regulated waves. Based on this analogy, the population of Sertoli cells that surround germ cells can be considered as a concert hall that provides the environment to maximize sound dynamics. Sperm could be considered the output sound, and their quality depends on Sertoli cells, seen as the size of the concert hall, and Sertoli cell maturity, as the quality of the concert hall.
Morphological research on Sertoli cells has progressed through three phases. First, routine light microscopy (LM) examination continued until the 1960s. The invention of the transmission electron microscope (TEM) led to the second phase of research, which lasted until 2000 and this microscope was very useful for taking high-quality images of Sertoli cell membranes and organelles. The last phase of research, immunohistochemistry and immunofluorescence, began before 2000, but gradually became the main tool for three-dimensional imaging of interactions between Sertoli cells and germ cells, and for localization of specific testicular proteins. Main immunohistochemical nuclear markers for Sertoli cells commonly used for morphological studies include SRY-box containing gene 9 (SOX9), androgen receptor (AR), Wilms tumour protein-1 (WT1), GATA-binding protein 1 (GATA1), GATA4 and cyclin-dependent kinase inhibitor 1B (p27kip1) (França et al., Reference França, Hess, Dufour, Hofmann and Griswold2016).
In mammals, Sertoli cells have been observed as different sizes, with an individual volume of c. 2000–7000 µm2. Sertoli cell volume density relative to total seminiferous tubular epithelium is also highly variable, ranging from approximately 15% in mice to 40% in humans. These cells also have an irregularly shaped cytoplasm that varies from species to species (Oliveira and Alves, Reference Oliveira and Alves2015). The first important step in the development of the testis from other glands, resulting in fetal masculinity, is the differentiation of Sertoli cells and Leydig cells. In mammals, during puberty, Sertoli cells undergo profound modifications in their morphology and function, and are morphologically and biochemically different from other undifferentiated cells. The formation of the male reproductive system and the degeneration of the female reproductive system depend on this (Baker and O’Shaughnessy, Reference Baker and O’Shaughnessy2001; Oliveira and Alves, Reference Oliveira and Alves2015).
Electron micrographs have shown that Sertoli cells have a clear and irregular cytoplasm. Sertoli cells contain many organelles such as several mitochondria that explain their high metabolic activity (Wang et al., Reference Wang, Wen, Yuan, Sun, Niu and He2016). The shape of the mitochondria varies depending on species but most mitochondria are spherical or elongated, but can also be cup shaped or doughnut shaped (Gautam et al., Reference Gautam, Rao, Gothwal, Garg and Bhattacharya2019).
In most species, Sertoli cells contain large, irregular nucleoli that, depending on a person’s age, can deform during the seminiferous epithelium cycle. The nucleus is usually found near the basement membrane but in some species the nucleus can be found in the higher parts of the epithelium near the lumen. The nuclear envelope contains indentations due to the accumulation of vimentin and intermediate filaments. These indentations indicate the maturation of Sertoli cells and are not present during growth or in patients with fertility disorders. In addition, pores and fissures are seen whose density depends on the stage of the spermatogenesis cycle (Oliveira and Alves, Reference Oliveira and Alves2015; França et al., Reference França, Hess, Dufour, Hofmann and Griswold2016). Adjacent Sertoli cells attach to each other with tight junctions that create a continuous lock between adjacent Sertoli cells and separates the basal (containing spermatogonia and preleptotene spermatocytes) from the adluminal compartments of the seminiferous epithelium (containing advanced germ cells), also creating the BTB or Sertoli cell barrier. This name is misleading because this barrier is between two intratubular compartments not between blood and testicle tissues (Ritzén, Reference Ritzén1983; Shi et al., Reference Shi, Li, Ren, Xie, Yin and Mo2018). This junctional structure has a porosity of c. 1000 Daltons, which means that anything with a higher molecular weight cannot pass from the outside to the inside of the lumen. The movement of germ cells through the BTB involves the reconstruction of these cell junctions at different stages of the seminiferous epithelial cycle. However, this barrier is stable and it is never completely disassembled or assembled. It is dynamic between these two states. This means that, as the germ cells cross the BTB toward the lumen, the junction in front of the germ cells separates, while the previous cells regroup. This protect germ cells in the adluminal compartments from release of foreign matter and from the immune system (Chojnacka et al., Reference Chojnacka, Zarzycka and Mruk2016; Shi et al., Reference Shi, Li, Ren, Xie, Yin and Mo2018) (Figure 1).
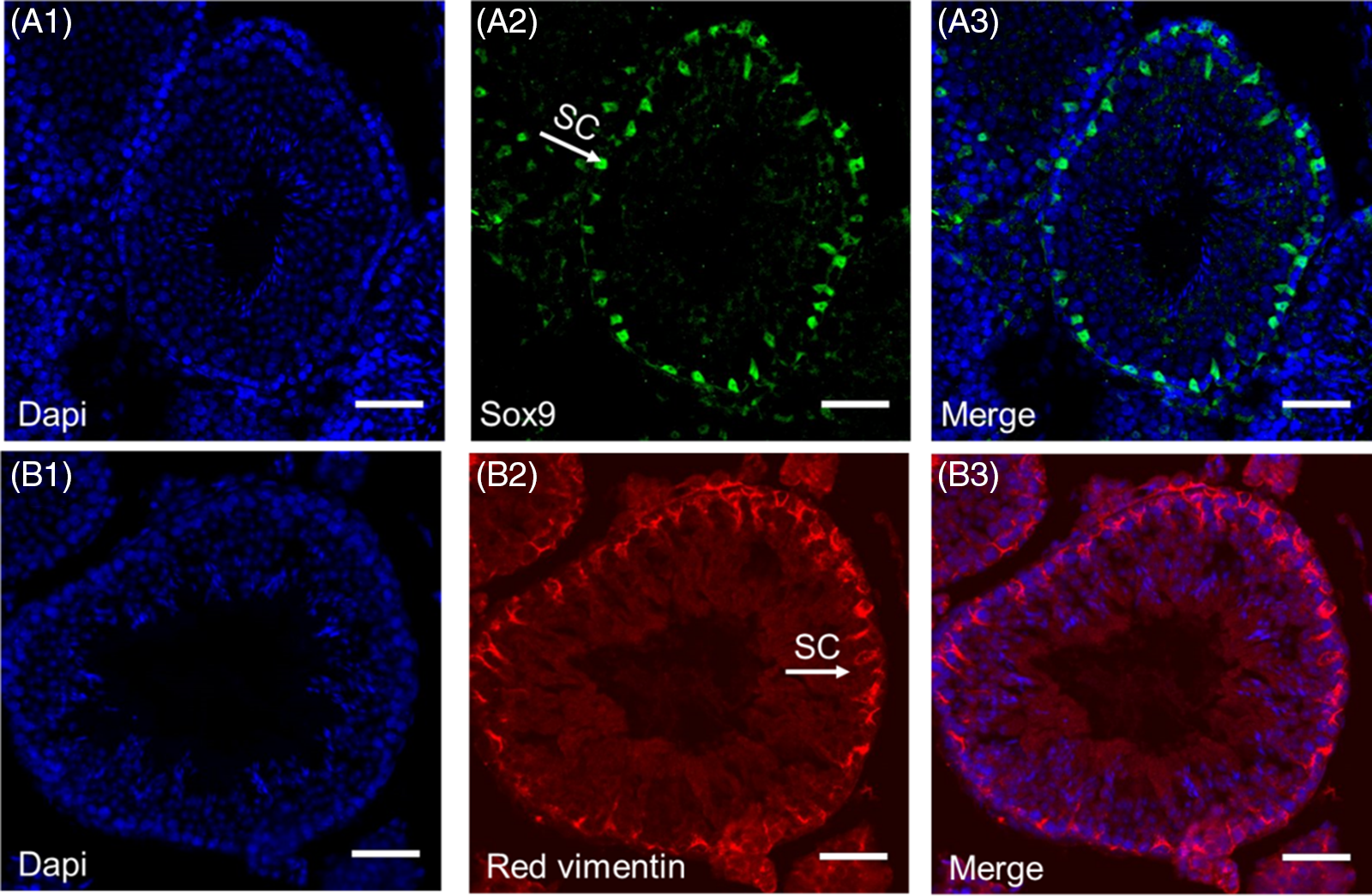
Figure 1. Immunohistochemistry staining of Sertoli cells in mice. Blue 4′,6-diamidino-2-phenylindole (DAPI) staining of all cells within the seminiferous tubule (A1, B1). Sertoli cell staining with Sox9 (A2) and red vimentin (B2). Merged images of DAPI and Sox9 (A3) and vimentin (B3) (scale bars, 50 μm) (Azizi et al., Reference Azizi, Niazi Tabar, Mohammadi and Skutella2021).
At the beginning of meiosis, germ cells outside the BTB become dependent on Sertoli cells as soon as they cross it, in order to survive and continue the process of spermatogenesis. Sertoli cells provide the factors needed for germ cell metabolism and development, therefore are also called nurse cells, which are vital for the normal development of germ cells (Siemann et al., Reference Siemann, Strange, Maharaj, Shi and Verma2017; Crisóstomo et al., Reference Crisóstomo, Alves, Gorga, Sousa, Riera, Galardo, Meroni, Oliveira, Alves and Oliveira2018).
Throughout the Sertoli cell cytoplasm are lysosomes, multivascular bodies (MVB) and lipid droplets that indicate the phagocytic activity of these cells and their frequency depends on the stage of spermatogenesis (Gautam et al., Reference Gautam, Rao, Gothwal, Garg and Bhattacharya2019). Sertoli cells phagocytose germ cells that have degenerated during spermatogenesis In addition, the cytoplasm remaining after mature spermatozoa are released can be digested by Sertoli cells and phagocytic lysosomes. Autophagy is also active in Sertoli cells and is a lysosomal pathway that removes defective organelles and proteins in the cell. Autophagy functions in cell viability or with apoptosis in cell death (Chen et al., Reference Chen, Zhou, Wang, Qian and Han2013; Yefimova et al., Reference Yefimova, Messaddeq, Harnois, Meunier, Clarhaut, Noblanc, Weickert, Cantereau, Philippe, Bourmeyster and Benzakour2013).
Seminiferous tubule adluminal fluid is needed to carry sperm and for spermatogenesis; this fluid is made by Sertoli cells and its composition differs from plasma and testicular interstitial fluid in testicular cells. One of the main differences is that the adluminal fluid of seminiferous tubule has a higher potassium content but a lower calcium content than found in plasma (Vogl et al., Reference Vogl, Lyon, Adams, Piva and Nassour2018) (Figure 2).
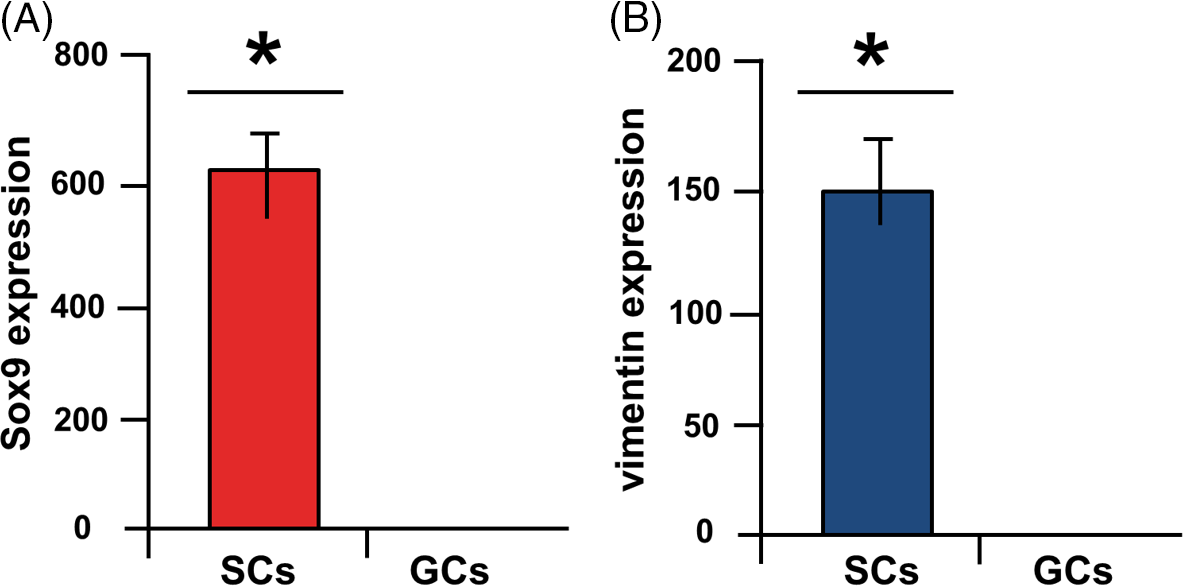
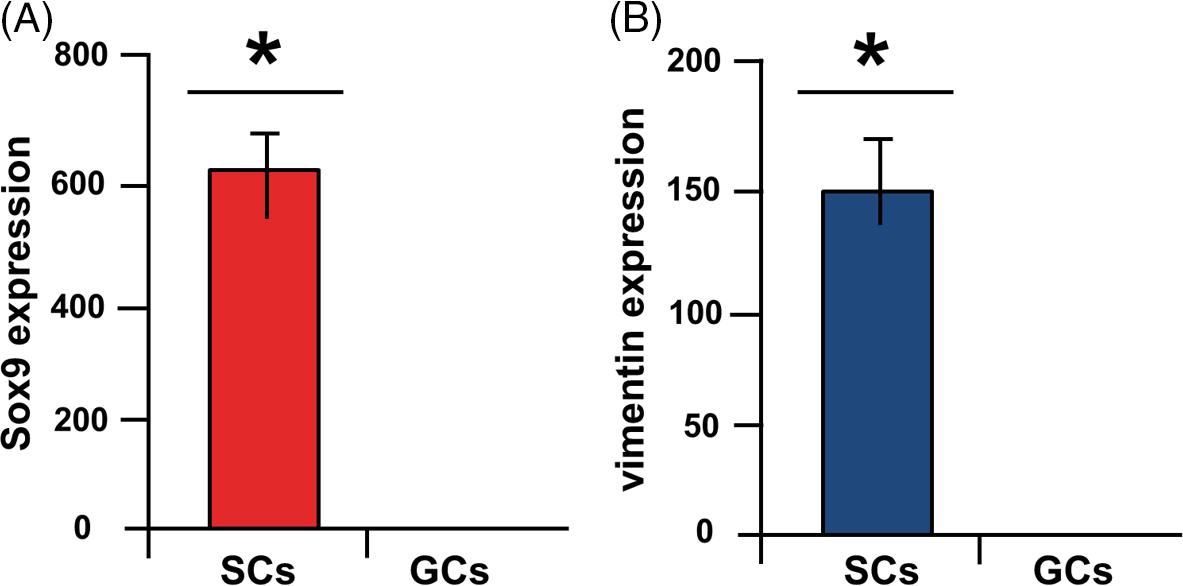
Figure 2. Fluidigm real-time RT-PCR. Highly significant expression of SOX9 (A) and vimentin (B) in Sertoli cells (SCs) compared with germ cells (GCs) (*P < 0.05) (Azizi et al., Reference Azizi, Niazi Tabar and Mohammadi2020).
The main regulators of Sertoli cells are hormones, which include follicle-stimulating hormone (FSH), sex steroid hormones, and thyroid hormones (TH). Sertoli cells express the FSH receptor and men with smaller testicles have not expressed the FSH receptor, so their spermatogenesis has been impaired. FSH is secreted from the anterior pituitary gland in response to gonadotropin-releasing hormone (GnRH), which is released from the hypothalamus (Figure 3). This hormone stimulates Sertoli cells to secrete androgen binding protein (ABP) (Petersen and Söder, Reference Petersen and Söder2006).
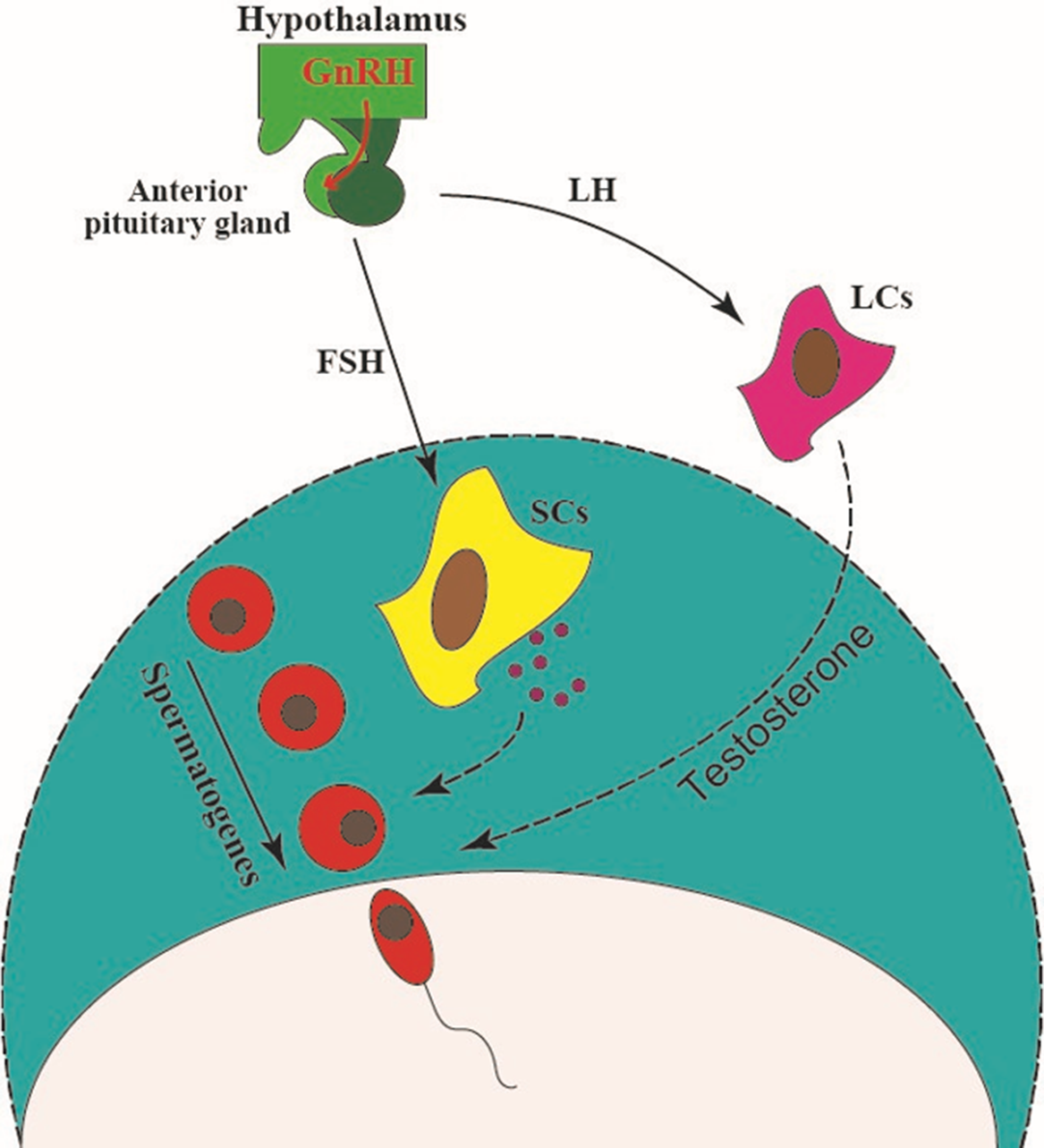
Figure 3. The hypothalamus secretes a hormone called gonadotropin-releasing hormone (GnRH), which triggers the release of FSH and LH hormones from the pituitary gland. FSH stimulates Sertoli cells to secrete factors such as transferrin and lactate. It also stimulates the secretion of inhibin B from Sertoli cells, which results in negative feedback of FSH secretion. LH stimulates testosterone secretion by acting on Leydig cells, and directly affects the process of spermatogenesis (Gurung and Jialal, Reference Gurung and Jialal2020).
FSH also causes the release of both inhibin B and activin from Sertoli cells that create negative and positive feedbacks for FSH secretion. FSH also inhibits testosterone (T) secretion from Leydig cells by stimulating the production of estradiol (E2) from Sertoli cells (Steinberger et al., Reference Steinberger, Heindel, Lindsey, Elkington, Sanborn and Steinberger1975). In addition, FSH and insulin increase the lipid metabolism of Sertoli cells by increasing acetate compounds in Sertoli cell lipids (Guma et al., Reference Guma, Wagner, Martini and Bernard1997).
Unlike germ cells, Sertoli cells also express androgen receptors. Androgen is needed to start and continue the process of spermatogenesis, and if its receptor is not expressed in Sertoli cells, spermatogenesis stops at the spermatocyte or primary spermatid stage. The presence of this hormone is also essential for Sertoli cell maturation and therefore formation of the BTB (Walker, Reference Walker2003). Thyroid hormone receptors (TRs) are also expressed on Sertoli cells, and T3 is likely to bind directly to these receptors. Consequently, TRs can mediate the possible role of THs in sperm production. This receptor is at its highest concentration at the postnatal and pre-pubertal periods in Sertoli cells and stimulates their differentiation and maturation (Singh et al., Reference Singh, Hamada and Agarwal2011).
Effects of COVID-19 on Sertoli cells
In December 2019 in Wuhan, China, a new type of coronavirus was discovered in patients who had pneumonia-type symptoms, this virus was named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and this new coronavirus disease was officially named by the WHO as coronavirus disease 2019 (COVID-19), which is currently a significant constraint to public health (Shen et al., Reference Shen, Xiao, Aierken, Yue, Wu, Liao and Hua2020; Zhou et al., Reference Zhou, Niu, Jiang, Zhang, Zheng, Wang, Zhu, Gao, Huang, Wang and Sun2020). By November 2020, based on WHO data, approximately 60,000,000 people were reported to have had COVID-19 disease. Data from China also showed that older people, especially those with serious underlying diseases, are more at risk of dying than younger people. In total, 80% of deaths have occurred in adults over 60, and only 1% of deaths have occurred in people under 19 years of age (CDC COVID-19 Response Team, 2020). Although many COVID-19 patients are asymptomatic, some patients develop pneumonia and 10% of patients require intensive care unit (ICU) care and mechanical ventilation. Symptoms of the virus vary from patient to patient but are usually accompanied by fever and dry cough, shortness of breath, lethargy, and muscle and bone pain. Less common symptoms include sore throat, diarrhoea, nausea, dizziness, haemoptysis and chest pain, and progression to pneumonia usually occurs 1–2 weeks after the initial symptoms (Ragab et al., Reference Ragab, Salah Eldin, Taeimah, Khattab and Salem2020).
The SARS-CoV-2 pathogen is a positive single-stranded large RNA virus (Younis et al., Reference Younis, Abassi and Skorecki2020), which belongs to the Sarbecovirus subgenus, and the Betacoronavirus genus (Lu et al., Reference Lu, Zhao, Li, Niu, Yang, Wu, Wang, Song, Huang, Zhu, Bi, Ma, Zhan, Wang, Hu, Zhou, Hu, Zhou, Zhao, Chen, Meng, Wang, Lin, Yuan, Xie, Ma, Liu, Wang, Xu, Holmes, Gao, Wu, Chen, Shi and Tan2020). Virus sequencing has shown that SARS-CoV-2 has many similarities with the original SARS-CoV, and has 76.5% identity with SARS-CoV in its amino acid sequence (Wang and Xu, Reference Wang and Xu2020; Younis et al., Reference Younis, Abassi and Skorecki2020). This suggests that SARS-CoV and SARS-CoV-2 proteins have almost identical three-dimensional structures in the receptor-binding domain (Younis et al., Reference Younis, Abassi and Skorecki2020). Therefore, SARS-CoV-2 probably uses the SARS-CoV receptor, namely angiotensin-converting enzyme (ACE2), to enter the host cells (Shen et al., Reference Shen, Xiao, Aierken, Yue, Wu, Liao and Hua2020). The reason for the rapid spread of SARS-CoV2 is that it has a higher affinity for binding to the ACE2 receptor than SARS-CoV (Ou et al., Reference Ou, Liu, Lei, Li, Mi, Ren, Guo, Guo, Chen, Hu, Xiang, Mu, Chen, Chen, Hu, Jin, Wang and Qian2020). COVID-19 has four main structural proteins, which include N (nucleocapsid), E (envelope), S (spike) and M (membrane) (Figure 4). COVID-19 binds to ACE2 through its receptor-binding domain (RBD), which is present in its S protein (Chen et al., Reference Chen, Liu and Guo2020). The spike is a homotrimer glycoprotein that binds to the integral membrane type I in ACE2, then pH-dependent endocytosis occurs. The acidic pH of lysosomes and endosomes activates the enzymes cathepsin B and cathepsin L and breaks down the S glycoprotein into two subunits, S1 and S2. Subunit S1 is used for binding and S2 for integration with the host cell membrane (Figure 5). Host cells also bind to neighbouring cells to form syncytia by expressing spike protein on their surface. Creating syncytia not only disrupts the function of the organ involved but also allows the virus to spread further and escape the immune system (Hikmet et al., Reference Hikmet, Méar, Edvinsson, Micke, Uhlén and Lindskog2020; Kearney, Reference Kearney2020; Walls et al., Reference Walls, Park, Tortorici, Wall, McGuire and Veesler2020). In general, structural and functional studies have shown that SARS-CoV-2 uses the SARS-CoV receptor ACE2 to enter and infect cells (Hoffmann et al., Reference Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler, Erichsen, Schiergens, Herrler, Wu, Nitsche, Müller, Drosten and Pöhlmann2020), so ACE2 analysis is necessary to determine the route of infection.
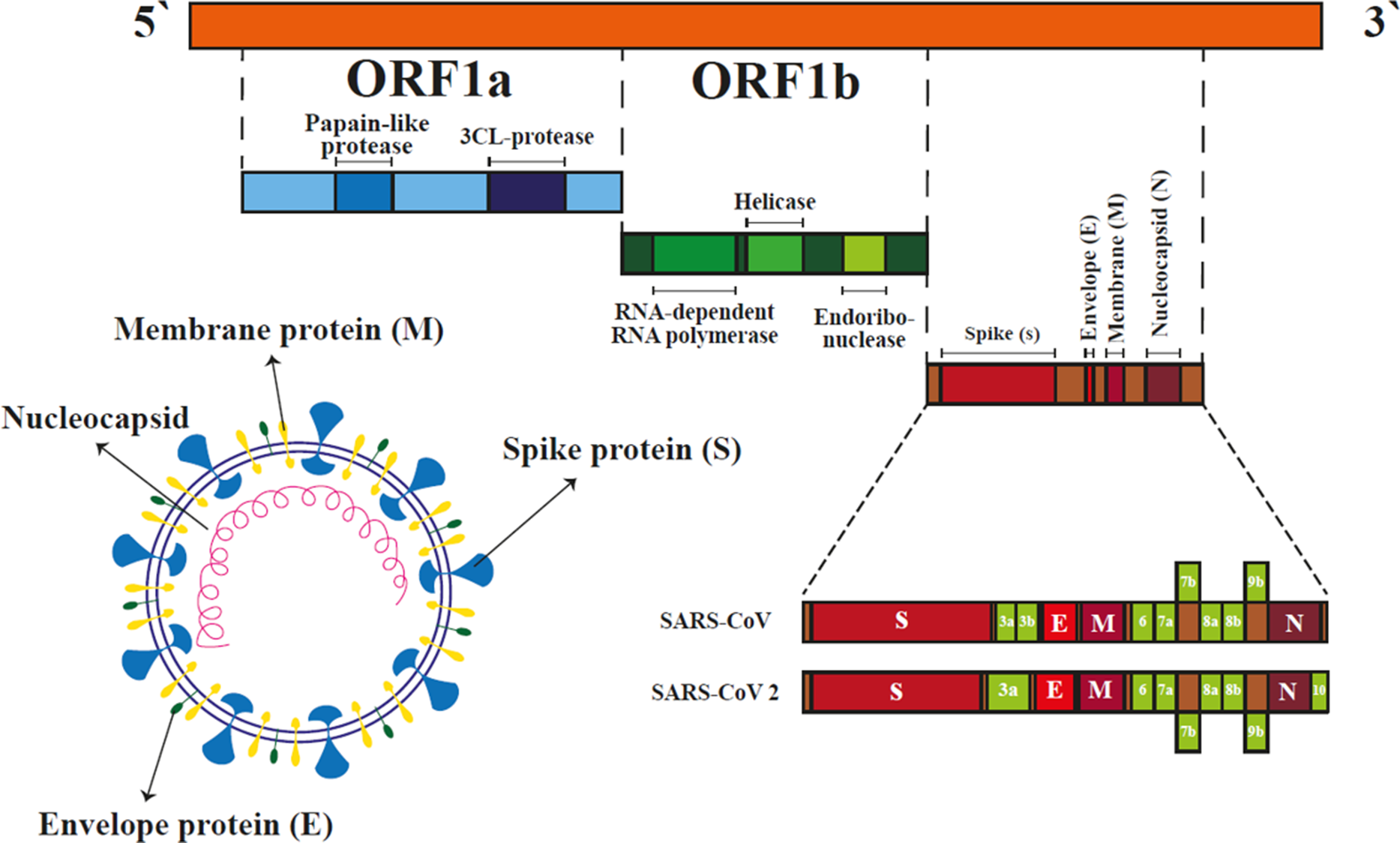
Figure 4. Coronavirus structure and features. COVID-19 is an enveloped single-stranded RNA virus composed of envelope (E), nucleocapsid (N), spike (S) and membrane (M) proteins. Two long sequences of SARS-CoV-2 RNA encode special proteins such as an RNA-dependent RNA polymerase that is crucial for viral spread during infection. Other parts of the RNA sequence represent structural proteins including envelope, spike and nucleocapsid (Chen et al., Reference Chen, Liu and Guo2020).
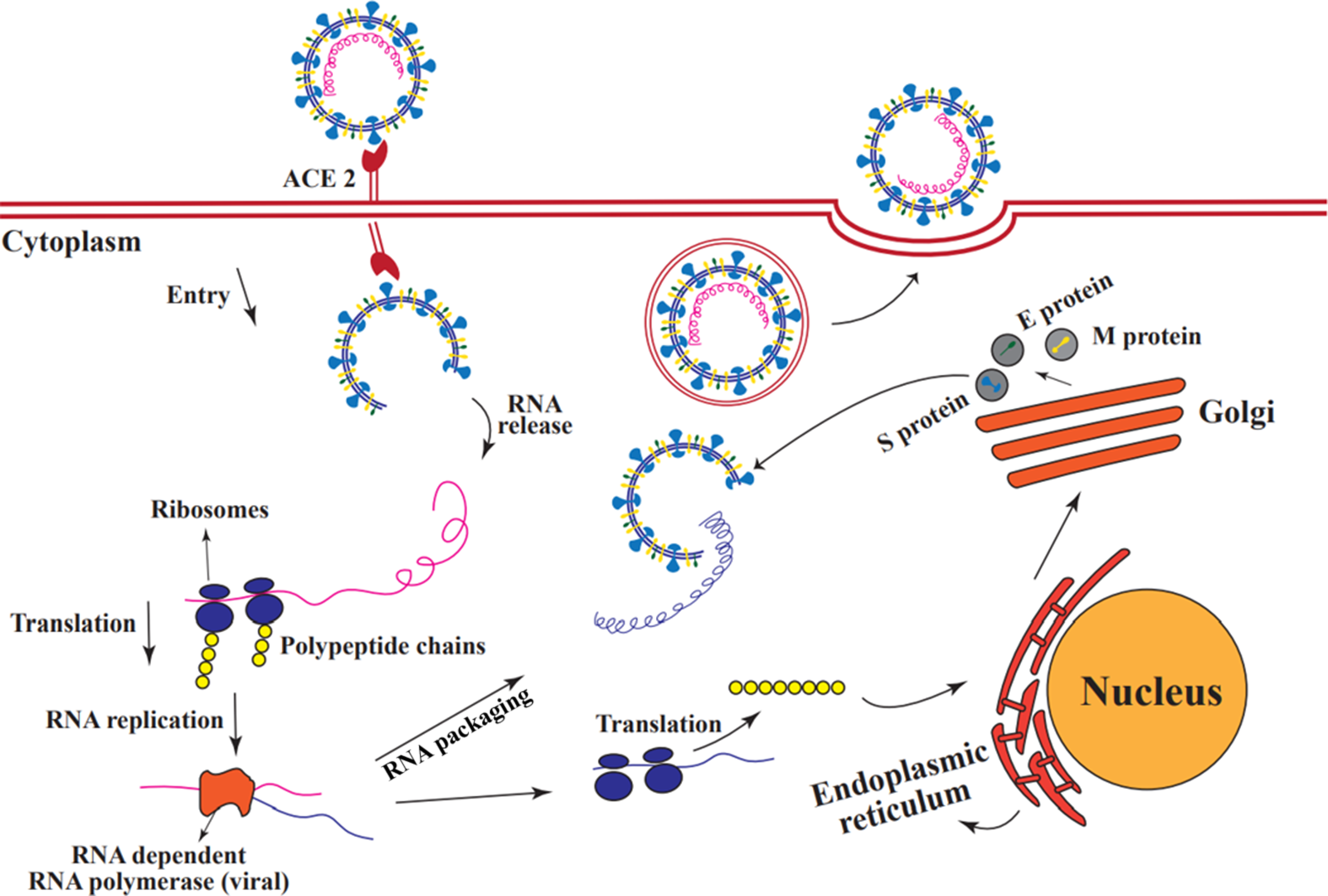
Figure 5. Coronavirus replication in host cells. The S protein in SARS-CoV-2 allows the virus to bind to the ACE2 receptor on the surface of the host cell. Once the virus enters the host cell, viral RNA is released, and then the virus uses the host cell’s ribosome as a protein-making machine to translate its genes. One of the translated proteins is an RNA-dependent RNA polymerase that is not found in eukaryotic cells and which the virus needs it for its RNA replication. Some replicated RNAs are re-translated by the host ribosome to produce the structural proteins of the virus. These then move to the rough endoplasmic reticulum and from there to the Golgi apparatus. In the Golgi apparatus, these proteins undergo modifications and combine with replicated RNAs. After packaging, a new virus is created that exits the cell by exocytosis (Millet and Whittaker, Reference Millet and Whittaker2018).
Angiotensin-converting enzyme2 (ACE2) is a carboxypeptidase enzyme that contains zinc in its structure. ACE2 is a single-pass type I membrane protein that was identified in 2000 as an angiotensin-converting enzyme (ACE) homologue (Turner, Reference Turner, Unger, Steckelings and Souza dos Santos2015; Hikmet et al., Reference Hikmet, Méar, Edvinsson, Micke, Uhlén and Lindskog2020). This protein negatively regulates the renin–angiotensin system (RAS), which is a hormonal system that is involved in regulating multiple functions of tissues and organs. It is particularly critical in maintaining blood pressure homeostasis, electrolyte balance and inflammatory responses. ACE2 performance in this system is the opposite and moderator of ACE action. ACE catalyzes angiotensin I (ANG I) to angiotensin II (ANG II) during the RAS, and results in narrowing of the arteries. By contrast, ACE2 is responsible for the generation of angiotensin 1-7 (ANG 1-7) from ANG II; this results in dilation of the arteries (Karnik et al., Reference Karnik, Unal, Kemp, Tirupula, Eguchi, Vanderheyden and Thomas2015; Jia, Reference Jia2016).
Based on previous reports, there are no gender differences in ACE2 activity between the lungs and hearts of normal and healthy mice but, in male kidneys, ACE2 is more active than in female kidneys. Therefore, ACE2 expression patterns may differ in male and female gonads (Liu et al., Reference Liu, Chen, Tang, Zhang, Chen, Yan, Yuan, Yang, Kong, Yan and Qiao2020).
Previous studies have also shown that male patients infected with SARS-CoV-2 experience widespread destruction of germ cells, have few or no spermatozoon in the seminiferous tubules, and testes basement membrane begins to thicken. This indicated that there are many SARS-CoV-2 receptors, ACE2, in the testes (Shen et al., Reference Shen, Xiao, Aierken, Yue, Wu, Liao and Hua2020; Wang and Xu, Reference Wang and Xu2020).
Based on single-cell RNA sequencing (scRNA-seq) analysis of testicular tissue, ACE2-expressing cells are found in almost all cell types in testis. Analyses of ACE2 RNA expression profiles at single-cell resolution in adult human testicles have shown that spermatogonial stem cells (SSC), leptotene spermatocytes type1 (L1), leptotene spermatocytes type 3 (L3), zygotene spermatocytes (Z), diplotene spermatocytes (D), spermatocyte 7 (SPC7), spermatid stage 1 (S1) and all three types of testicular somatic cells, namely Sertoli cells, Leydig cells, and myoid cells, are susceptible to SARS-CoV-2 infection. Previous experiments have also shown that more than 90% of Sertoli cells express ACE2, suggesting that testicular somatic cells, especially Sertoli cells, may be more susceptible to SARS-CoV-2 infection than germ cells (Babushkina et al., Reference Babushkina, Belokopytova, Grachev, Meko and Vaganov2017; Fan et al., Reference Fan, Li, Ding and Lu2020; Liu et al., Reference Liu, Chen, Tang, Zhang, Chen, Yan, Yuan, Yang, Kong, Yan and Qiao2020). Somatic cells are a main component of the testicular microenvironment and play an important role in maintaining SSC and performing spermatogenesis. In particular, Sertoli cells are in direct contact with spermatogenic cells to control differentiation. Therefore, the dysfunction of these cells disrupts testicular function (Chen and Liu, Reference Chen and Liu2015; Shen et al., Reference Shen, Xiao, Aierken, Yue, Wu, Liao and Hua2020).
Discussion
Viruses have a greater effect on human fertility than previously thought. Over the years, several major viral epidemics have endangered male fertility. To date, more than 27 viruses have been found in semen (Crespillo-Andujar et al., Reference Crespillo-Andujar, Díaz-Menéndez and Mora-Rillo2018), some of which such as HIV/AIDS, Zika, and hepatitis can be transmitted through semen (Atkinson et al., Reference Atkinson, Thorburn, Petridou, Bailey, Hewson, Simpson, Brooks and Aarons2017).
Global health is now affected by the COVID-19 pandemic. The SARS-CoV-2 virus is very similar to SARS-CoV but has become much more widespread than previous SARS-CoV outbreaks (Shen et al., Reference Shen, Xiao, Aierken, Yue, Wu, Liao and Hua2020). In addition, SARS-CoV-2 may remain in the body longer than SARS-CoV and, based on Kissler et al. (Reference Kissler, Tedijanto, Goldstein, Grad and Lipsitch2020) predictions, SARS-CoV2 can recur even after its elimination. Although COVID-19 is associated with gastrointestinal symptoms and infections in other parts of the body, this disease is often identified with pneumonia symptoms. The incubation period of this virus can vary from 3 to 15 days (Chan et al., Reference Chan, Yuan, Kok, To, Chu, Yang, Xing, Liu, Yip, Poon, Tsoi, Lo, Chan, Poon, Chan, Ip, Cai, Cheng, Chen, Hui and Yuen2020). SARS-CoV-2 is a virus with a positive-sense RNA genome that varies in length from 26 to 36 kilobases. It is a member of the Coronaviridae family and uses ACE2 receptors to enter the host cell (Illiano et al., Reference Illiano, Trama and Costantini2020). SARS-CoV-2 viral glycoprotein includes an exocellular domain, a transmembrane domain and an intracellular domain. The extracellular domain consists of units S1 and S2. S1 binds to the ACE2 peptidase domain, while S2 facilitates membrane and receptor fusion (Dimitrov, Reference Dimitrov2003).
Available data on world mortality show that the mortality rate is higher in men than women (Lai et al., 2020). Based on recent studies, male infertility has also been attributed to SARS-CoV-2 (Shen et al., Reference Shen, Xiao, Aierken, Yue, Wu, Liao and Hua2020). In COVID-19 disease, the availability of angiotensin II increases due to saturation of ACE2 receptors following virus binding. Excess angiotensin II can be a cause of pulmonary symptoms, which are characteristic of COVID-19 disease. The conversion of angiotensin II to angiotensin (1-7) by ACE2 blocks this process (Illiano et al., Reference Illiano, Trama and Costantini2020). Recent studies by scientists have shown that the human testis is an organ enriched with ACE2. Based on scRNA-seq analyses on testicular cells, Sertoli and Leydig cells express ACE2 at higher levels than alveolar type II (AT2) cells (Fan et al., Reference Fan, Li, Ding and Lu2020). Sertoli cells have the highest expression of ACE2 compared with other cells in the seminiferous tubule epithelium; this indicates that these somatic cells are more sensitive to SARS-CoV-2. This finding is important because Sertoli cells are in direct contact with germ cells and function as nurse cells (Dutta and Sengupta, 2020). Yang et al. (Reference Yang, Chen, Huang, Zhong, Su, Chen, Cao, Ma, He, Li, Li, Zhou, Fan, Luo, Chang, Arkun, Zhou and Nie2020) showed that possible interaction of Sertoli cells and SARS-CoV-2 can cause swelling, vacuolation, cytoplasmic rarefaction, and detachment from tubular basement membranes. Special junctions between Sertoli cells are damaged, therefore causing dysfunction of the BTB (Archana et al., Reference Archana, Selvaraju, Binsila, Arangasamy and Krawetz2019). The BTB is a barrier against cytotoxic agents in the blood and also protects the male gonads from immune system responses, leading directly to maintenance of spermatogenesis (Hedger and Meinhardt, Reference Hedger and Meinhardt2003). Inflammation caused by the virus can lead to widespread secretion of cytokines such as interferon (IFN), interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α) (Ma et al., Reference Ma, Xie, Li, Shi, Mao, Xiong, Zhang and Zhang2020). Overproduction of cytokines can lead to infiltration of leukocytes into the testis, thereby damaging the BTB. Leukocytes can also affect Leydig cells and therefore affect T production and secretion (Xu et al., Reference Xu, Qi, Chi, Yang, Wei, Gong, Peh and Gu2006). Because sperm surface proteins are considered foreign to the immune system, the body produces antibodies against them called anti-sperm antibodies (ASA), which are produced following leukocyte entry into the testicles. Several studies have shown that the development of ASA usually causes sperm to stick together, therefore impeding sperm motility and fertility (Marconi and Weidner, Reference Marconi, Weidner, Krause and Naz2017). Recent studies to investigate the effect of SARS-CoV-2 on hormonal change and gonadal dysfunction in men have shown that there was a significant increase in serum luteinizing hormone (LH) levels in men with COVID-19. No noticeable changes were observed in FSH and T levels. Subsequently, men with COVID-19 had a substantial decrease in T/LH and FSH/LH compared with healthy men. LH stimulates Sertoli cells to produce and secrete inhibin B, which causes negative feedback for FSH. LH also stimulates the production of T by Leydig cells (Ma et al., Reference Ma, Xie, Li, Shi, Mao, Xiong, Zhang and Zhang2020). In general, the BTB is not impermeable to viruses, especially with onset of virus inflammation and excessive secretion of cytokines and some viruses are found in semen (Vishvkarma and Rajender, Reference Vishvkarma and Rajender2020). The destructive effects of these viruses can include obvious damage to testicles, impaired spermatogenesis and regulation of sex hormones, as well as irregular production of inflammatory cytokines (Puggioni et al., Reference Puggioni, Pintus, Melzi, Meloni, Rocchigiani, Maestrale, Manunta, Savini, Dattena, Oggiano, Palmarini and Ligios2018). The presence of coronavirus in semen has not yet been conclusively confirmed. As Sertoli cells are in direct contact with the blood, the effect of COVID-19 disease on these cells can be very significant. Damage to these cells, as they are never replaced and are vital for spermatogenesis, is enough to cause infertility (Johnson et al., Reference Johnson, Thompson and Varner2008; Vishvkarma and Rajender, Reference Vishvkarma and Rajender2020).
Conclusion
From the evidence gathered in this article, it is plausible to state that SARS-CoV-2 can affect Sertoli cells in several ways, although research and findings in this context have been very limited. Sertoli cells have a key role in maintaining spermatogenesis and sperm life. One effect of SARS-CoV-2 virus on Sertoli cells may be infertility in men possibly due to abnormal or irregular production of sex hormones, followed by dysfunction of the male gonads and of Sertoli cell secretion. The ACE2 receptor, through which this virus enters the host cell, is highly expressed in Sertoli cells. Entry can destroy Sertoli cells and the BTB, spermatogenesis is also disrupted. We suggest that studies on the effects of SARS-CoV-2 on Sertoli cells and their vital role should be a high priority including investigation of its association with infertility in men.
Acknowledgements
This research was supported by the Faculty of Biotechnology of Amol University of Special Modern Technologies, Islamic Republic of Iran and the University of Heidelberg, Institute for Anatomy and Cell Biology III, Department of Neuroanatomy, Germany.
Author contributions
Amirreza Niazi Tabar, Kiana Sojoudi, Hannaneh Henduei, and Hossein Azizi contributed to the design and implementation of the research, to the analysis of the results and to the writing of the manuscript.
Funding
None
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical statement
This study was approved by the ethical committee of Amol University of Special Modern Technologies (Ir.ausmt.rec.1398.03.07).