Introduction
Over the past decades remarkable advances in the techniques and protocols for cryopreservation of germinal tissues have contributed greatly to the establishment and maintenance of germplasm banks. The feasibility to preserve and restore fertility in species of high commercial value and those species at risk of extinction highlight the economical and ecological potential of cryotechnology (Demirci et al., Reference Demirci, Lornage, Salle, Poirel, Guerin and Franck2003; Liu et al., Reference Liu, Xie, Zhang, Xu, Bujard and Jun2008a). In addition, the association of ovarian tissue cryopreservation and assisted reproduction techniques have important clinical relevance as it permits the development of new strategies to restore fertility in women who are at risk of premature ovarian failure (Shea et al., Reference Shea, Woodruff and Lowe2008; West et al., Reference West, Zelinski, Kondapalli, Gracia, Chang, Coutifaris, Critser, Stouffer, Shea and Woodruff2009, Smitz et al., Reference Smitz, Dolmans, Donnez, Fortune, Hovatta, Jewgenow, Picton, Plancha, Shea, Stouffer, Telfer, Woodruff and Zelinski2010).
The main alternatives for fertility preservation used in the clinical routine are limited to the protection of the ovaries against radiation (oophoropexy) or oocyte retrieval for in vitro fertilization (IVF) with subsequent cryopreservation of oocytes and embryos (Sonmezer & Oktay, Reference Sonmezer and Oktay2004). Although oophoropexy may offer some protection to germ cells, this technique can reduce greatly the chances for successful future pregnancies (Wallace et al., Reference Wallace, Anderson and Irvine2005). There are also serious limitations in the use of IVF in cancer patients. This factor is due mainly to the hormonal stimulation protocol required to obtain mature eggs that may delay the beginning of cancer treatment and therefore is not recommended for prepubertal patients (Sonmezer & Oktay, Reference Sonmezer and Oktay2004). Damage to the ovarian germline cells caused by radiotherapy and/or chemotherapy can be prevented by the removal and cryopreservation of ovarian biopsies before the initiation of therapies, by which immature tissue can be restored further for fertility therapy proposals (Shea et al., Reference Shea, Woodruff and Lowe2008).
Ovarian tissue transplantation and in vitro follicle culture are emerging as promising approaches to restore fertility, specifically in women who are undergoing cancer treatments and who may not have time or are not good candidates for the IVF option (Woodruff & Snyder, Reference Woodruff and Snyder2008). Although ovarian tissue transplantation has successfully yielded viable offspring in humans (Donnez et al., Reference Donnez, Dolmans, Demylle, Jadoul, Pirard, Squifflet, Martinez-Madrid and Van Langendonckt2004; Meirow et al., Reference Meirow, Levron, Eldar-Geva, Hardan, Fridman, Zalel, Schiff and Dor2005), the in vitro ovarian follicle culture approach has been considered more desirable because it eliminates the possibility of reintroduction of cancer cells into the patient (Shea et al., Reference Shea, Woodruff and Lowe2008). Even though the meiotic competence and developmental capacity of human oocytes grown from pre-antral stages in vitro have not yet been reported, animal studies indicate that in vitro follicle culture is a valid prospect for humans.
Given the importance of safeguarding female fertility, in the present work we review the progress of ovarian cryopreservation techniques followed by tissue transplantation or in vitro ovarian follicle culture experimentally applied in programmes of female fertility restoration. We also present an overview of the most relevant outcomes obtained to date, in different animal models as well as the future direction of this field.
Ovarian tissue cryopreservation
The first cell survival tests completed with freezing and thawing were carried out in the 1930s using sperm. Their success was achieved largely by prior cellular dehydration in hypertonic solution (Luyet & Hodapp, Reference Luyet and Hodapp1938). Years later, Polge et al. (Reference Polge, Smith and Parkes1949) and Smith (Reference Smith1950) observed sperm and erythrocyte survival, respectively, using glycerol as a cryoprotectant (CPA). Lovelock (Reference Lovelock1953) found that blood cells were damaged after freezing without CPA, however remained preserved when frozen with glycerol. Based on this finding, the author hypothesized that this protection was related to the colligative properties of glycerol. The following year, this same author was responsible for discovering that the protective action of glycerol can be shared with a number of other neutral solutes of low molecular weight. Later, Lovelock verified that cells such as erythrocytes, particularly cattle erythrocytes, are not preserved when using just glycerol as a CPA. As glycerol has low penetration in this cell type it suggested that a smaller molecule, such as dimethyl sulphoxide (DMSO), would be an excellent CPA. This suggestion was confirmed by Lovelock (Reference Lovelock1954) in subsequent years.
The first studies on ovarian tissue cryopreservation were performed in rodents more than half a century ago (Parkes & Smith, Reference Parkes and Smith1953; Parkes, Reference Parkes1957). Since the emergence of this technique, encouraging results have been reported. These results include the maintenance of morphology (Parkes, Reference Parkes1955) and viability of the ovarian follicle structure (Parkes, Reference Parkes1956). Results have also shown the restoration of endocrine function (Parkes, Reference Parkes1955) after cryopreservation and transplantation of ovarian tissue. Several studies were conducted, years later, in the 1990s, with different cryoprotectants such as DMSO (Hovatta et al., Reference Hovatta, Silye, Krausz, Abir, Margara, Trew, Lass and Winston1996; Newton et al., Reference Newton, Aubard, Rutherford, Sharma and Gosden1996; Candy et al., Reference Candy, Wood and Whittingham1997; Newton & Illingworth, Reference Newton and Illingworth2001), ethylene glycol (Newton et al., Reference Newton, Aubard, Rutherford, Sharma and Gosden1996; Candy et al., Reference Candy, Wood and Whittingham1997), propylene glycol (Hovatta et al., Reference Hovatta, Silye, Krausz, Abir, Margara, Trew, Lass and Winston1996; Newton et al., Reference Newton, Aubard, Rutherford, Sharma and Gosden1996; Candy et al. Reference Candy, Wood and Whittingham1997; Newton & Illingworth, Reference Newton and Illingworth2001) and even glycerol (Newton et al., Reference Newton, Aubard, Rutherford, Sharma and Gosden1996, Candy et al., Reference Candy, Wood and Whittingham1997, Newton & Illingworth, Reference Newton and Illingworth2001) in order to improve ovarian cryopreservation protocols. Several of these studies (Newton et al., Reference Newton, Aubard, Rutherford, Sharma and Gosden1996, Candy et al., Reference Candy, Wood and Whittingham1997, Newton & Illingworth, Reference Newton and Illingworth2001) showed that DMSO is one of the CPAs that stands out for its cryoprotective effect in female gonads.
The cryopreservation of ovarian tissue has also been investigated extensively in animal production. In goats, for example, the first studies carried out were based on the effect of different CPAs (DMSO or PROH, Rodrigues et al. (Reference Rodrigues, Amorim, Costa, Matos, Santos, Lucci, Bao, Ohashi and Figueiredo2004a); and EG or GLY, Rodrigues et al. (Reference Rodrigues, Amorim, Costa, Matos, Santos, Lucci, Bao, Ohashi and Figueiredo2004b)) on follicular morphology. The effect of these CPAs was investigated to ascertain the viability of ovarian follicles in the early stages of folliculogenesis (primordial follicles) as described by Amorim et al. (Reference Amorim, Rondina, Rodrigues, Goncalves, de Figueiredo and Giorgetti2004). Recent studies in cattle reported the follicle survival in different stages of development (primordial, primary and secondary) after ultralow temperatures (Celestino et al., Reference Celestino, dos Santos, Lopes, Martins, Matos, Melo, Bao, Rodrigues, Silva and de Figueiredo2008). Currently, other studies have been conducted in order to define the best protocol for cryopreservation of ovarian tissue collected from goats (Luz et al., Reference Luz, Santos, Pinto, Soares, Celestino, Mafezoli, Campello, Figueiredo and Rodrigues2009; Carvalho et al., Reference Carvalho, Faustino, Silva, Castro, Luz, Rossetto, Lopes, Campello, Figueiredo, Rodrigues and Costa2011; Castro et al., Reference Castro, Carvalho, da Silva, Faustino, de Figueiredo and Rodrigues2011), sheep (Faustino et al., Reference Faustino, Santos, Silva, Pinto, Celestino, Campello, Figueiredo and Rodrigues2010) and sow (Borges et al., Reference Borges, Silva, Futino, Rocha, Amorim, Bao and Lucci2009) using the methods of slow freezing or vitrification.
Slow freezing is considered to be the classic method of cryopreservation. After a period of exposure to low concentrations of CPA, which can vary from 5 to 60 min, the biological material is kept in a programmable freezer stable between 0°C (Donnez et al., Reference Donnez, Dolmans, Demylle, Jadoul, Pirard, Squifflet, Martinez-Madrid and Van Langendonckt2004) and 20°C (Rodrigues et al., Reference Rodrigues, Amorim, Costa, Matos, Santos, Lucci, Bao, Ohashi and Figueiredo2004a,Reference Rodrigues, Amorim, Costa, Matos, Santos, Lucci, Bao, Ohashi and Figueiredob). The temperature is then gradually (about 2°C/min) reduced to –5 to –9°C (Rodrigues et al., Reference Rodrigues, Amorim, Costa, Matos, Santos, Lucci, Bao, Ohashi and Figueiredo2004a,Reference Rodrigues, Amorim, Costa, Matos, Santos, Lucci, Bao, Ohashi and Figueiredob; Andersen et al., Reference Andersen, Rosendahl, Byskov, Loft, Ottosen, Dueholm, Schmidt, Andersen and Ernst2008). At this temperature, the seeding procedure is performed, causing the induction of extracellular formation of ice crystals. This procedure aims to guide the beginning of the freezing solution to keep the biological sample from disordered freezing and super cooling (Zhang et al., Reference Zhang, Sheng, Cao, Wang and Chen2011). This practice is designed to dehydrate the biological material before cryopreservation, thus reducing the chances of ice forming inside the cell (Zhang et al., Reference Zhang, Sheng, Cao, Wang and Chen2011). The vitrification method is characterized by an ultrafast cooling rate, which uses high concentrations of cryoprotectants in order to increase the viscosity of the extender solution. This method also promotes the transition from liquid to the amorphous glassy state, thus avoiding the formation intracellular ice crystals (Vajta et al., Reference Vajta, Holm, Kuwayama, Booth, Jacobsen, Greve and Callesen1998).
Ovarian tissue cryopreservation and fertility restoration
The cryopreservation of a biological material is intended to keep it viable for a long time under low temperature. This material can later be used with minimal damage to its function. The ovarian tissue, for example, after cryopreservation can be used with great potential for assisted reproduction, whether in procedures involving transplantation or in vitro culture.
Transplantation of ovarian tissue
After an indeterminate period of cryopreservation, ovarian tissue can be removed from liquid nitrogen and transplanted for the purpose of recovering its activity. According to the graft recipient or transplanted tissue, the transplant can be classified as xenotransplantation (when performed between different species), allotransplantation (when performed within the same species) or autotransplantation (if performed in the same animal). The transplant may also be identified according to the site of implantation. Orthotropic implantation is defined as tissue transplanted to its place of origin. The other possibility is heterotopic implantation, in which tissue is transplanted to a different region (Sonmezer & Oktay, Reference Sonmezer and Oktay2010).
The transplantation of cryopreserved ovarian tissue is already used in human clinical assisted reproduction (Oktay et al., Reference Oktay, Turkcuoglu and Rodriguez-Wallberg2011). Several studies have shown that women (even before reproductive age) who are affected by cancer, before undergoing chemotherapy treatments and/or radiotherapy, could have the ovarian cortex removed and preserved. This material can be cryopreserved indefinitely and, after medical therapy, can be used as grafts in transplantation techniques for the re-establishment of the endocrine and reproductive functions (Andersen et al., Reference Andersen, Rosendahl, Byskov, Loft, Ottosen, Dueholm, Schmidt, Andersen and Ernst2008; Oktay et al., Reference Oktay, Turkcuoglu and Rodriguez-Wallberg2011). The association between ovarian cryopreservation and ovarian transplantation has been performed successfully in some species. Several studies have shown that ovarian function can be restored, including follicular growth and development (Demeestere et al., Reference Demeestere, Simon, Emiliani, Delbaere and Englert2006) and steroidal hormone production (Andersen et al., Reference Andersen, Rosendahl, Byskov, Loft, Ottosen, Dueholm, Schmidt, Andersen and Ernst2008), as well as term pregnancies (Salle et al., Reference Salle, Demirci, Franck, Berthollet and Lornage2003) and the live birth of both animals (Gosden et al., Reference Gosden, Baird, Wade and Webb1994) and humans (Donnez et al., Reference Donnez, Dolmans, Demylle, Jadoul, Pirard, Squifflet, Martinez-Madrid and Van Langendonckt2004).
However, the clinical applicability to human transplantation is not always a viable option. For patients in whom cancer has metastasized, there is a risk of reintroducing cancer cells by transplanting previously harvested tissue, even after the cancer is cured (Aubard et al., Reference Aubard, Piver, Pech, Galinat and Teissier2001). As an alternative to transplantation, the ovarian tissue can be grown in vitro in order to obtain mature oocytes suitable for fertilization and in vitro production of embryos. This principle has already been demonstrated using fresh follicles in mice (Wang et al., Reference Wang, Catt, Pangestu and Temple-Smith2011) and goats (Magalhães et al., Reference Magalhães, Duarte, Araujo, Brito, Soares, Lima, Lopes, Campello, Rodrigues and Figueiredo2011), respectively.
Live births from transplanted frozen ovarian tissue
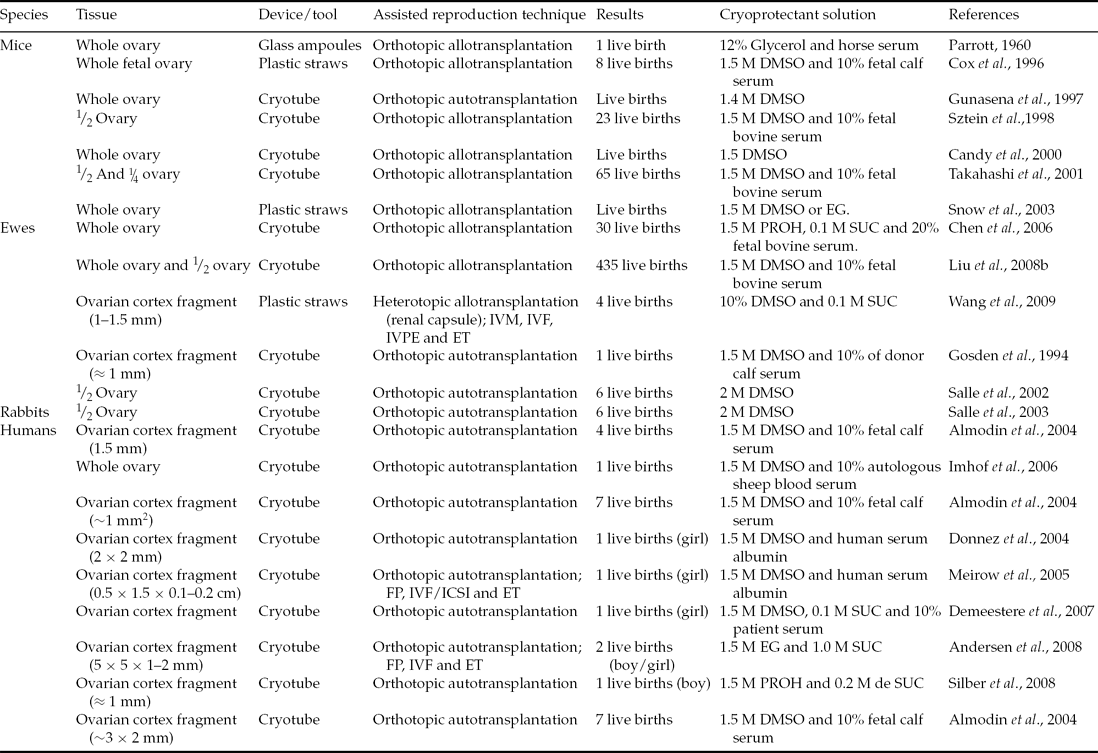
The ovarian tissue can be transplanted by either heterotopic or orthotropic procedures after freezing/thawing. After the first type the patient can have a spontaneous pregnancy but heterotopic transplantation requires the use of supplementary techniques. Techniques such as in vitro culture of ovarian follicles followed by in vitro maturation and fertilization of oocytes in order to produce and transfer embryos and obtain offspring may be necessary. The use of freezing/thawing of ovarian tissue has resulted in births in mice, hares, sheep and humans (Table 1). The first births from frozen/thawed ovarian tissue were possible after orthotopic transplantation. This site provides optimal conditions for the deployment of folliculogenesis, as well as adequate blood supply and temperature for normal development and functioning of the organ (Nisolle et al., Reference Nisolle, Casanas-Roux, Qu, Motta and Donnez2000; Oktay et al., Reference Oktay, Buyuk, Veeck, Zaninovic, Xu, Takeuchi, Opsahl and Rosenwaks2004).
Table 1 Live births from transplanted frozen ovarian tissue

DMSO: dimethylsulphoxide; EG: ethylene glycol; ET: embryo transfer; FP: follicular puncture; ICSI, intracytoplasmic sperm injection; IVF: in vitro fertilization; IVM: in vitro maturation; IVPE: in vitro production of embryos; PROH: propylene glycol; SUC: sucrose.
After whole ovary orthotopic transplantation previously frozen and thawed, Parrott (Reference Parrott1960) described the first birth, a single offspring in mice. Using similar protocols in the same species, Cox et al. (Reference Cox, Shaw and Jenkin1996) and Gunasena et al. (Reference Gunasena, Villines, Critser and Critser1997) obtained a pregnancy rate of 33% and 73%, respectively, and about three and four births per litter. Other researchers have also obtained promising results (23 births) after orthotopic transplantation of frozen/thawed half ovaries (Sztein et al., Reference Sztein, Sweet, Farley and Mobraaten1998). Takahashi et al. (Reference Takahashi, Miyoshi and Nagasu2001) proposed that ovarian tissue after the death of an animal could be perfectly viable after a period of storage at low temperatures. These researchers aimed to preserve animals of high genetic value, such as founders to a transgene of interest, in case of death before the establishment of a germplasm bank. For this process, the researchers froze the ovaries from transgenic mice (lineage α1A6.3-lacZ-15 that carried a 6.3 kb fragment in the 5′ upstream gene cacna1a) 2 h after the death of animals. Subsequently, the ovaries were allografted to non-transgenic ovariectomized recipients. These animals were also mated with non-transgenic males, resulting in 10 offspring, all carriers of the gene cacna1a.
Encouraging results were obtained in farm animals by Gosden et al. (Reference Gosden, Baird, Wade and Webb1994). These authors reported the resumption of cyclic activity and pregnancy, achieving a birth after orthotopic autotransplantation of contralateral fragments of a frozen/thawed ovarian cortex in sheep. Eight years after these first births, Salle et al. (Reference Salle, Demirci, Franck, Rudigoz, Guerin and Lornage2002) also documented the birth of healthy offspring. In this study, ovaries from six animals were frozen/thawed and after autologous transplantation, resulting in four pregnancies and the birth of six lambs. The sheep that received the ovarian graft after delivery continued to be monitored for 2 years after transplantation, in which time other lambs were conceived (Salle et al., Reference Salle, Demirci, Franck, Berthollet and Lornage2003). Almodin et al. (Reference Almodin, Minguetti-Camara, Meister, Ceschin, Kriger and Ferreira2004a) using 18G hypodermic needles filled with fragments of ovarian tissue – frozen–thawed ovary and contralateral irradiated ovary – were grafted into two ewes. Six months after grafting the two sheep were pregnant. The lambs were conceived and were healthy, showing that the females’ fertility even after ovarian failure caused by radiation could be restored. Similar results were also reported in rabbits (Almodin et al., Reference Almodin, Minguetti-Camara, Meister, Ferreira, Franco, Cavalcante, Radaelli, Bahls, Moron and Murta2004b). Demonstrating the feasibility of contralateral whole freezing/thawing ovary autotransplantation, with microvascular anastomoses of the ovarian pedicle in nine sheep, Imhof et al. (Reference Imhof, Bergmeister, Lipovac, Rudas, Hofstetter and Huber2006) reported the birth of a healthy lamb. In this study it was shown that microvascular anastomosis of whole ovaries and orthotopic transplantation after cryopreservation is technically feasible.
The above studies revealed that cases of young women who needed immediate chemotherapy and who wished to preserve fertility, could rely only on ovarian cryopreservation. Once treatment is completed and the patient is free of disease it is possible to transplant ovarian tissue in hopes of pregnancy and birth. To date, 15 births have occurred after orthotropic transplantation of frozen ovarian tissue (Table 1). In 2004, Donnez and colleagues published a landmark study in human reproductive medicine, which brought hope to thousands of women who have suffered from and overcome cancer (Donnez et al., Reference Donnez, Dolmans, Demylle, Jadoul, Pirard, Squifflet, Martinez-Madrid and Van Langendonckt2004). In this study, one patient had their endocrine and gametogenic function restored and reported a pregnancy after 11 months post orthotopic autotransplantation of ovarian fragments. This procedure showed that after treatment and cure of cancer other women could become pregnant and bear healthy children without the use of assisted reproduction techniques such as IVF (Silber et al., Reference Silber, DeRosa, Pineda, Lenahan, Grenia, Gorman and Gosden2008, Reference Silber, Kagawa, Kuwayama and Gosden2010; Demeestere et al., Reference Demeestere, Simon, Emiliani, Delbaere and Englert2007). A recent study by Ernst et al. (Reference Ernst, Bergholdt, Jorgensen and Andersen2010) also showed that it is possible to keep implantation endocrine and gametogenic activities for an extended period. One patient in this study underwent both cancer treatment and ovarian tissue transplantation, similar to cases described previously. However, this individual was the first to give birth to two children at different times (the first birth in 2008 and second in 2010) from two pregnancies and only one graft. Similarly, orthotopic autotransplantation of ovarian cortex fragments cannot only be applied to reverse infertility in patients after cancer treatment, but also in women with health problems such as sickle cell anemia. This disease is sometimes treated with curative allogeneic bone marrow, which is generally preceded by chemotherapy and the use of the drug cyclophosphamide, which can damage oocytes and granulosa cells (Meirow et al., Reference Meirow, Lewis, Nugent and Epstein1999). Donnez & Dolmans (Reference Donnez and Dolmans2010) and Roux et al. (Reference Roux, Amiot, Agnani, Aubard, Rohrlich and Piver2010) reported cases in which patients were treated for sickle cell anemia, thereby posing premature loss of ovarian function. These women received orthotopic autologous transplantations of ovarian tissue and after a few months showed follicular growth and ovulation, which later allowed term pregnancies and births.
Although the orthotopic implantation spot provides optimal folliculogenesis conditions, possibly due to good conditions such as temperature, adequate blood supply and increased angiogenic capacity (Nisolle et al., Reference Nisolle, Casanas-Roux, Qu, Motta and Donnez2000; Oktay et al., Reference Oktay, Buyuk, Veeck, Zaninovic, Xu, Takeuchi, Opsahl and Rosenwaks2004), heterotopic autotransplantation allows the retrieval of oocytes and IVF to improve the chance of pregnancy. Assisted reproductive techniques can be applied subsequently to orthotopic autologous transplantation. In this type of procedure, the graft can be stimulated with hormones and can accelerate follicular growth, reducing the time between transplantation and the first subsequent pregnancy (Meirow et al., Reference Meirow, Levron, Eldar-Geva, Hardan, Fridman, Zalel, Schiff and Dor2005; Andersen et al., Reference Andersen, Rosendahl, Byskov, Loft, Ottosen, Dueholm, Schmidt, Andersen and Ernst2008). Meirow et al. (Reference Meirow, Levron, Eldar-Geva, Hardan, Fridman, Zalel, Schiff and Dor2005) described, in woman, treatment and cure from lymphoma and the restoration of fertility after receiving an autograft. The tissue, 9 months after implantation, showed spontaneous growth of the ovarian follicle, by 15 mm. From this observation they performed ovarian stimulation with the gonadotropins (GnRH antagonist) and human chorionic gonadotrophin (hCG). After 34 to 36 h of hormonal stimulation, a single oocyte was recovered, fertilized by intracytoplasmic sperm injection (ICSI), and developed into a 4-cell embryo. This embryo was transferred and resulted in both a term pregnancy and birth.
Live births from transplanted vitrified ovarian tissue
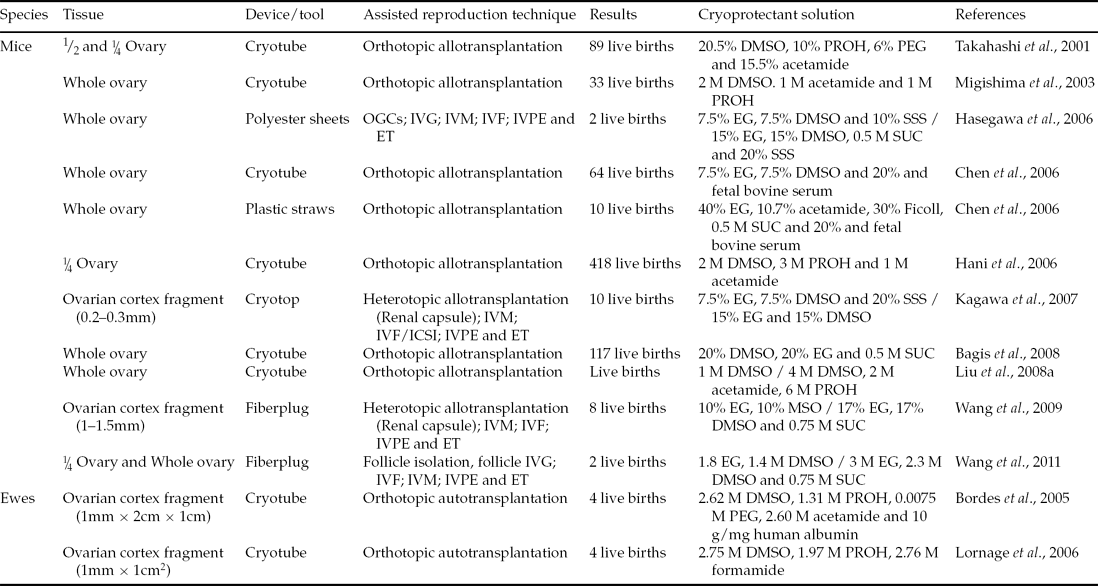
While slow freezing has been used successfully for several years, this technique has some disadvantages in relation to vitrification. Among the disadvantages, the key lies in the fact that the slow freezing process can cause intracellular ice formation (IIF), which then causes irreversible damage to cellular structures. In this context, vitrification is advantageous, as it uses fast cooling, which results in solidification without crystallization, thus avoiding damage resulting from the IIF (Bagchi et al., Reference Bagchi, Woods and Critser2008). To date, there have been investigations in vitrification of ovarian tissue in a variety of species with different protocols and different sizes of tissue, such as fragments (Bordes et al., Reference Bordes, Lornage, Demirci, Franck, Courbiere, Guerin and Salle2005; Lornage et al., Reference Lornage, Courbière, Mazoyer, Odagescu, Baudot, Bordes, Poirel, Franck and Salle2006; Kagawa et al., Reference Kagawa, Kuwayama, Nakata, Vajta, Silber, Manabe and Kato2007; Wang et al. Reference Wang, Catt, Pangestu and Temple-Smith2009), halves (Takahashi et al., Reference Takahashi, Miyoshi and Nagasu2001) or even whole ovaries (Migishima et al., Reference Migishima, Suzuki-Migishima, Song, Kuramochi, Azuma, Nishijima and Yokoyama2003; Chen et al., Reference Chen, Chien, Wu, Chen, Lai, Lin and Yang2006; Hasegawa et al., Reference Hasegawa, Mochida, Ogasawara and Koyama2006; Bagis et al., Reference Bagis, Akkoq, Tas and Aktoprakligil2008; Liu et al., Reference Liu, Xie, Zhang, Xu, Bujard and Jun2008a). Some of these studies reported births, which are summarized in Table 2, and protocols are described below.
Table 2 Live births from transplanted or in vitro cultured vitrified ovarian tissue

DMSO: dimethylsulphoxide; EG: ethylene glycol; ET: embryo transfer; IVF: in vitro fertilization; IVG: in vitro growth; IVM: in vitro maturation; IVPE: in vitro production of embryos; OGCs: Oocyte–granulosa cell complexes; PEG: polyethylene glycol; PROH: propylene glycol; SSS: serum substitute supplement; SUC: sucrose.
Migishima et al. (Reference Migishima, Suzuki-Migishima, Song, Kuramochi, Azuma, Nishijima and Yokoyama2003) vitrified whole ovaries of female mice and found that the ovary is capable of restoring their physiological condition and maintaining a pregnancy. In this study, ovarian fragments of transgenic female mice for green fluorescent protein (GFP) were transplanted into ovariectomized non-transgenic mice. After this procedure 33 pups were born, of which 48.5% were able to express the gene for GFP and thus the authenticity of the progeny. Years later, Chen et al. (Reference Chen, Chien, Wu, Chen, Lai, Lin and Yang2006) compared conventional vitrification, in a straw, with direct coverage vitrification (DCV) in a cryotube, getting 10 and 64 pups, respectively. Liu et al. (Reference Liu, Xie, Zhang, Xu, Bujard and Jun2008a) found that the recipients of vitrified whole ovaries have their reproductive activity regained after transplantation. In addition, pups had their origin proven by the polymerase chain reaction (PCR) technique. That same year, and in order to improve mice ovarian tissue cryotolerance, Bagis et al. (Reference Bagis, Akkoq, Tas and Aktoprakligil2008) investigated the effect of antifreeze protein (AFP) type III during vitrification of whole ovaries of female mice. Antifreeze protein was found originally in marine organisms that inhabit regions overwhelmed by conditions of extreme cold, and is characterized by giving natural freeze tolerance. After vitrification, the ovaries were transplanted into non-transgenic recipients who give birth to live pups.
In sheep there are two successful reports, both after orthotopic autotransplantation of ovarian tissue (Bordes et al., Reference Bordes, Lornage, Demirci, Franck, Courbiere, Guerin and Salle2005; Lornage et al., Reference Lornage, Courbière, Mazoyer, Odagescu, Baudot, Bordes, Poirel, Franck and Salle2006). The first study utilized six animals whose hemi-ovaries had the medulla removed and cortical fragments were vitrified and transplanted. Four months after transplantation, resumption of endocrine function in all animals was detected. Also three of these sheep gave birth to four lambs after natural mating (Bordes et al., Reference Bordes, Lornage, Demirci, Franck, Courbiere, Guerin and Salle2005). The following year, Lornage et al. (Reference Lornage, Courbière, Mazoyer, Odagescu, Baudot, Bordes, Poirel, Franck and Salle2006) studied whole and fragment ovary vitrification, as well as the physical properties involved in the formation of ice crystals, and found that vitrification of whole ovaries using solution with DMSO, propanediol and formamide, is important for follicular morphology preservation. Paradoxically, this team has obtained three pregnancies from which four lambs were born after transplantation of ovarian cortex fragments.
Kagawa et al. (Reference Kagawa, Kuwayama, Nakata, Vajta, Silber, Manabe and Kato2007) vitrified ovarian fragments by the cryotop technique and completed allogeneic transplantation to mice kidney capsules. The recipients were stimulated, after 9 days of transplantation, with 7.5 IU of equine chorionic gonadotropin (eCG). After 48 h, the tissue was removed and antral follicles were grown in vivo and punctured. The recovered oocytes were matured and underwent ICSI. In total, 57 embryos were transferred to six recipients who gave birth to 10 pups. Similarly, Wang et al. (Reference Wang, Catt, Pangestu and Temple-Smith2009) vitrified ovaries of adult mice using the solid surface technique. Subsequently, the ovaries were transplanted to the kidney capsules of recipient mice. After 10 days of transplantation cumulus–oocyte complexes (COCs) were recovered, underwent IVF and were cultured to the blastocyst stage. Eight pups were born after the transfer of 32 blastocysts.
In situations in which a transplant is not feasible for the restoration of fertility, the alternatives of in vitro culture of ovarian tissue or artificial ovary can be considered. Over the past year progress in this area has been seen and it is now possible to grow pre-antral follicles in several species (mice: O'Brien et al., Reference O'Brien, Pendola and Eppig2003; humans: Telfer et al., Reference Telfer, McLaughlin, Ding and Thong2008; sheep: Arunakumari et al., Reference Arunakumari, Shanmugasundaram and Rao2010). This practice allows the recovery of oocytes from these follicles, which can also be matured and fertilized in vitro to generate embryos (Magalhães et al., Reference Magalhães, Duarte, Araujo, Brito, Soares, Lima, Lopes, Campello, Rodrigues and Figueiredo2011).
The association between vitrification and in vitro culture resulted in the birth of mice (Hasegawa et al., Reference Hasegawa, Mochida, Ogasawara and Koyama2006, Wang et al., Reference Wang, Catt, Pangestu and Temple-Smith2011). In the study by Hasegawa et al. (Reference Hasegawa, Mochida, Ogasawara and Koyama2006), 36 embryos were produced in vitro and transferred to two recipients, resulting in the pregnancy and births of two offspring in one of the recipients. Wang et al. (Reference Wang, Catt, Pangestu and Temple-Smith2011) obtained 16 blastocysts, which were transferred and resulted in pregnancy and two births.
In vitro culture of ovarian pre-antral follicles
The association between ovarian tissue cryopreservation and ovarian pre-antral follicle culture might offer a value alternative to preserve and restore fertility by reducing the chances of reintroduction of cancer cells derived from the transplanted tissue to the patient (Shea et al., Reference Shea, Woodruff and Lowe2008; Woodruff & Snyder, Reference Woodruff and Snyder2008). Furthermore, this method may also ensure a greater number of mature oocytes for subsequent fertilization (West et al., Reference West, Zelinski, Kondapalli, Gracia, Chang, Coutifaris, Critser, Stouffer, Shea and Woodruff2009). So far, this technology is available for human follicles; however, in vitro maturation of oocytes derived from cultured pre-antral follicles does not show significant results. Although the use of a traditional in vitro (bidimensional) systems has supported the full growth and differentiation of immature mice follicles, as well as meiotic competent oocytes (Eppig & O'Brien, Reference Eppig and O'Brien1996), similar results have not yet been reported for other species of mammals, including bovine (Gutierrez et al., Reference Gutierrez, Ralph, Telfer, Wilmut and Webb2000), sheep (Tambe & Nandedkar, Reference Tambe and Nandedkar1993) and humans (Abir et al., Reference Abir, Franks, Mobberley, Moore, Margara and Winston1997). This fact is due mainly to the difficulty in maintainance of the architectural structure of the follicle in large mammals, a problem similar to that found in vivo (Picton et al., Reference Picton, Danfour, Harris, Chambers and Huntriss2002). In vivo and in vitro studies have shown the importance of maintainance of follicular architecture to the process of growth and differentiation of the oocytes (Matzuk et al., Reference Matzuk, Burns, Vivieros and Eppig2002), which reaffirms the structural dependency component to the success of follicular cultivation.
Unlike the in vitro bidimensional culture system, in which somatic cells disconnected from the oocyte and spread through the area of culture, the architecture of the follicle is kept intact when performed on a three-dimensional in vitro culture system (Smitz et al., Reference Smitz, Dolmans, Donnez, Fortune, Hovatta, Jewgenow, Picton, Plancha, Shea, Stouffer, Telfer, Woodruff and Zelinski2010). In this system, individual immature follicles are encapsulated within an extracellular matrix of hydrogels of sodium alginate (West et al., Reference West, Xu, Woodruff and Shea2007). Divalent cations, such as calcium, bind cooperatively between the blocks of adjacent alginate chains, creating ionic inter-chain bridges. This action causes gelling of aqueous alginate solutions while maintaining cellular viability without causing adsorption of non-specific proteins and cellular adhesion (Rowley et al., Reference Rowley, Madlambayan and Mooney1999). In addition to mechanical array support, the alginate is highly porous, thereby facilitating diffusion of soluble hormones and factors between the culture medium and the follicle, a process necessary for the survival and growth of follicles (West et al., Reference West, Xu, Woodruff and Shea2007). In fact, this system was able to guarantee the growth and complete differentiation of immature mouse follicles, furthermore allowing the oocyte maturation and development of healthy progeny (Xu et al., Reference Xu, Kreeger, Shea and Woodruff2006). The use of this technique for non-human primate follicular culture has generated promising results. The alginate hydrogel matrix was also able to maintain the three-dimensional structure of secondary follicles in monkeys, from fresh and frozen ovarian tissue, and allowed follicular growth and steroidogenesis by more than 30 days in vitro (Xu et al., Reference Xu, West-Farrell, Stouffer, Shea, Woodruff and Zelinski2009a; Ting et al., Reference Ting, Yeoman, Lawson and Zelinski2011).
Other studies from this same research group have examined the role of endocrine and paracrine factors on in vitro follicular growth, as well as the influence of age on the viability of follicular tissue (Xu et al., Reference Xu, Bernuci, Lawson, Yeoman, Fisher, Zelinski and Stouffer2010; Xu et al., Reference Xu, Lawson, Yeoman, Pau, Barrett, Zelinski and Stouffer2011). So far, only two studies have tested the feasibility of the alginate matrix in the cultivation of human immature follicles. The results of these two studies are still inconclusive and insufficient for the standardization of the in vitro culture of follicles to produce eggs that are able to be fertilized (Amorim et al., Reference Amorim, Van Langendonckt, David, Dolmans and Donnez2009; Xu et al., Reference Xu, Barrett, West-Farrell, Kondapalli, Kiesewetter, Shea and Woodruff2009b). However, in vitro cultivation can be considered as a possibility for use in cryopreserved pre-antral follicles, especially when the transplantation of ovarian tissue is not the most appropriate alternative.
Conclusions
The reviewed data presented here reinforce the feasibility of ovarian tissue cryopreservation to safeguard fertility, specifically for cancer patients who want to protect their germ line cells from the destructive effects of chemotherapy and/or radiotherapy. The combination of cryopreservation, tissue transplantation, and in vitro ovarian follicle culture emerged as an alternative option to restore female fertility. Although ovarian tissue transplants have successfully yielded viable offspring in different species, the data obtained so far using the in vitro ovarian follicle culture approach are still preliminary and more efforts are needed to generate an adequate milieu for complete in vitro development of the immature ovarian follicles in large species, especially human.
Disclosure statement
The authors have nothing to disclose.
Acknowledgements
This research was supported by grants from the National Council for Scientific and Technological Development (CNPq), Brazil. F.O. Lunardi is supported by a grant from Cearense Foundation for Research Support (FUNCAP). J.R. Figueiredo and A.P.R Rodrigues are supported by a grant from CNPq.