Introduction
The practice of semen cryopreservation is a useful tool for male fertility preservation and at present is used routinely in assisted reproductive technology (ART). Nevertheless, the deleterious effects of cryopreservation on sperm structure and function are commonly observed after thawing, including diminished motility, deteriorated viability (O'Connell et al., Reference O'Connell, McClure and Lewis2002; Donnelly et al., Reference Donnelly, McClure and Lewis2001), loss of mitochondrial function (O'Connell et al., Reference O'Connell, McClure and Lewis2002) and DNA damage (Donnelly et al., Reference Donnelly, McClure and Lewis2001; Zribi et al., Reference Zribi, Chakroun, EI Euch, Gargouri, Bahloul and Keskes2010). To date a great deal of effort has been made to prevent cryodamage, but this damage is still at an unsatisfactory level, especially for abnormal semen samples, especially as low motility and poor DNA integrity make asthenozoospermia sperm more susceptible to cryodamage (Colleu et al., Reference Colleu, Lescoat, Boujard and Le Lannou1988).
Most of these deleterious effects are related to an increase in reactive oxygen species (ROS) during freezing–thawing of spermatozoa (Wang et al., Reference Wang, Zhang, Ikemoto, Anderson and Loughlin1997; Anger et al., Reference Agarwal, Saleh and Bedaiwy2003). The plasma membranes of mammalian sperm cells contain a high content of polyunsaturated fatty acids (PUFA) and there is a lack of antioxidant systems to defend against peroxidative damage (Lenzi et al., Reference Lenzi, Picardo, Gandini and Dondero1996). Furthermore, ROS has been shown to be one key factor in the pathogenesis of male infertility (Lanzafame et al., Reference Lanzafame, La Vignera, Vicari and Calogero2009). Recent studies have found that high levels of ROS are associated with increased sperm DNA damage (Tremellen, Reference Tremellen2008) and are negatively correlated with sperm motility (Athayde et al., Reference Athayde, Cocuzza, Agarwal, Krajcir, Lucon, Srougi and Hallak2007) and mitochondria membrane potential (Wang et al., Reference Wang, Sharma, Gupta, George, Thomas, Falcone and Agarwal2003).
In order to prevent the negative effect of ROS in the cryopreservation–thawing process, various antioxidants, including vitamins (Kalthur et al., Reference Kalthur, Raj, Thiyagarajan, Kumar, Kumar and Adiga2011; Li et al., Reference Li, Lin, Liu, Xiao and Liu2010), enzymes (Li et al., Reference Li, Lin, Liu, Xiao and Liu2010; Moubasher et al., Reference Moubasher, El-Din, Ali, El-Sherif and Gaber2013) and some natural compounds (Zribi et al., Reference Zribi, Chakroun, Abdallah, Elleuch, Sellami, Gargouri, Rebai, Fakhfakh and Keskes2012; Meamar et al., Reference Meamar, Zribi, Cambi, Tamburrino, Marchiani, Filimberti, Fino, Biggeri, Menezo, Forti, Baldi and Muratori2012), have been added to freezing media to enhance sperm resistance to oxidative stress. In these experiments, it was shown that supplementation of antioxidants to the sperm freezing extender is useful for the inhibition of ROS generation and for improving the quality of cryo-thawed human spermatozoa.
l-Carnitine (LC) is an amino acid that is distributed widely in animal livers and has been shown over a long period of time to have antioxidant properties in a number of controlled and uncontrolled human and animal studies (Dokmeci, Reference Dokmeci2005). It is known to play a crucial role in sperm metabolism and maturation by providing readily available energy for use by spermatozoa, and positively affects sperm motility, maturation and the spermatogenic process. In cellular systems, LC serves as a facilitator for the transport of activated fatty acids across the inner membrane of the mitochondria, so that they can be broken down through β-oxidation to produce ATP (Agarwal & Said, Reference Agarwal and Said2004; Ng et al., Reference Ng, Blackman, Wang and Swerdloff2004).
Many human clinical trials have shown that LC and acetyl-l-carnitine (ALC) therapy can optimize sperm motion parameters in men with asthenozoospermia or oligoasthenozoospermia (Sigman et al., Reference Sigman, Glass, Campagnone and Pryor2006; Lenzi et al., Reference Lenzi, Sgrò, Salacone, Paoli, Gilio, Lombardo, Santulli, Agarwal and Gandini2004). Moreover, several in vitro studies have documented that carnitines enhance sperm motility (Dokmeci, Reference Dokmeci2005). Besides oxidative stress, an increase in spermatozoa apoptosis is another mechanism responsible for cryopreservation damage. LC was also demonstrated to have antiapoptotic properties (Moretti et al., Reference Moretti, Famularo, Marcellini, Boschini, Santini, Trinchieri, Lucci, Alesse and De Simone2002).
Earlier studies have shown, however, that the addition of ALC before freezing does not help in preventing the freeze–thaw-induced loss of motility and membrane integrity in subfertile semen samples with normal parameters (Duru et al., Reference Duru, Morshedi, Schuffner and Oehninger2000). In a recent study by Banihani et al. (Reference Banihani, Agarwal, Sharma and Bayachou2013), it was reported that the addition of LC improves human sperm motility and vitality, but has no effect on sperm DNA oxidation after cryopreservation.
Previous studies that have examined the effects of LC on sperm cryopreservation were mostly focused on normozoospermic semen samples. In asthenozoospermia patients, the seminal plasma LC concentration is much lower than that of the normozoospermic control (Ng et al., Reference Ng, Blackman, Wang and Swerdloff2004). The effects of LC supplementation during cryopreservation on human spermatozoa with low motility are still unclear. In the present study, we focused on the cryoprotective effect of LC on asthenozoospermic semen samples. Furthermore, we tried to determine the differing protective effects of LC on asthenozoospermic and normozoospermic semen samples.
Materials and methods
Ethic statement
The study was approved by the Institutional Ethics Committee. All the participants were informed of the study and a written consent was obtained from them.
Semen sample collection
Seventy specimens were recruited for the study from 70 infertile men who visited the Reproductive Medicine Center at the First Affiliated Hospital of ZheJiang University, China for semen analysis. Men with azoospermia, severe oligozoospermia or leukocytospermia were excluded from the study. Freshly ejaculated semen samples were obtained by masturbation after 3 to 7 days of sexual abstinence. All samples were collected in sterile containers and liquefied at room temperature.
Sperm cryopreservation and thawing
A commercial glycerol-based cryoprotectant with egg yolk (Quinn's Advantage Sperm Freezing Medium, SAGE BioPharma, USA) was used as the base freezing extender in this study. After assessment of sperm motility, viability (VIA), mitochondrial membrane potential (MMP) and DNA fragmentation index (DFI) of fresh ejaculates, all the semen samples were divided into two aliquots and semen samples were mixed with equal volumes of sperm freezing medium, the first aliquot was cryopreserved with freezing medium only (control) and the second was cryopreserved with freezing medium supplemented with LC. The choice of LC concentration (1.0 g/l) in this study was based on previously published literature (Banihani et al., Reference Banihani, Sharma, Bayachou, Sabanegh and Agarwal2012).
After a 10 min equilibration at room temperature, the aliquots were transferred to screw-top cryovials (Nunc, Denmark), which were first exposed to liquid nitrogen vapours for 10 min and then plunged into liquid nitrogen for storage. After 2 weeks, thawing was performed by transferring the cryovials to a water bath of 37°C for 15 min. The samples were then washed twice with HTF-HEPES buffer and then by centrifugation at 1800 g for 8 min to remove all cryoprotective medium before subsequent analysis.
To minimize variations, all the following analyses of fresh and thawed semen were performed using the same technique and by the same technician.
Motility analysis
Sperm motility values were evaluated using Makler's counting chamber (Sefi Instruments, Israel) and computer-assisted semen analysis (CASA, Hamilton Thorn Research). The criteria proposed by the World Health Organization (WHO, fourth edition guidelines) were employed to estimate motility (World Health Organization, 2001), in which sperm based on their speed were ascribed to one of four categories: (a) sperm velocity > 35 μm/s; (b) 10 μm/s < sperm velocity < 35 μm/s; (c) (sperm velocity < 10 μm/s); or (d) (sperm velocity = 0). Motility of semen samples in this study was expressed as the percentage of sperms in categories a, a + b, or a + b + c, which represented fast forward motility, forward motility and total motility respectively.
Based on the estimated motility, the 70 semen samples were assigned to either the normozoospermic group (n = 33, sperm density ≥ 20 × 106/ml, a ≥ 25% or a + b ≥ 50%) or the asthenozoospermia group (n = 37, sperm density ≥ 20 × 106/ml, a < 25% and a + b ≤ 50%).
VIA analysis
VIA was assessed by eosin-Y staining. Staining was performed by mixing 20 μl of semen with an equal volume of 0.5% eosin Y stain on a glass slide and allowed to dry. Because the plasma membrane integrity of the dead sperm had been compromised, their membrane permeability and therefore their ability to absorb the dye increased. Consequently, live spermatozoa remained white and dead spermatozoa stained red. In total, 200 spermatozoa were counted at ×400 magnification. VIA was expressed as the percentage of live spermatozoa (%).
DFI analysis
In this study, sperm DNA integrity was evaluated by acridine orange (AO), following the protocol described by Tejada et al. (Reference Tejada, Mitchell, Norman, Marik and Friedman1984). Semen samples were washed three times in phosphate-buffered saline (PBS; 0.1 M). After centrifugation, the sperm pellet was resuspended to approximately 5 × 106/ml. An aliquot (20 μl) of washed sperm suspension was smeared on a pre-cleaned glass slide, air-dried smears were then fixed overnight in Carnoy's solution (methanol/acetic acid, 3:1).
After fixation, slides were air dried and stained by freshly prepared AO staining solution (10 ml of 1% AO stock solution in distilled water added to a mixture of 40 ml of 0.1 M citric acid and 2.5 ml of 0.3 M Na2HPO4.7H2O and pH adjusted to 2.5) for 5 min in the dark. Then, slides were gently rinsed with distilled water. To avoid rapidly fading fluorescence, all the slides were evaluated within 5 min using a fluorescence microscope (Olympus CX41, Japan) at ×400 magnification and at the excitation wavelength range of 450–490 nm.
At least 300 sperm cells for each sample were evaluated by the same examiner. Spermatozoa displaying green fluorescence were scored as having normal DNA (double stranded), whereas those displaying red, orange or yellow fluorescence were considered to have denatured or single-stranded DNA. The DFI was expressed as the percentage of the spermatozoa to have damaged DNA.
Analysis of MMP
In this study, the MMP changes in spermatozoa were evaluated using the lipophilic cationic probe 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl carbocyanine iodide (JC-1) (Smiley et al., Reference Smiley, Reers, Mottola-Hartshorn, Lin, Chen, Smith, Steele and Chen1991). JC-1 is able to selectively enter mitochondria. In depolarized mitochondria with low MMP, JC-1 monomers accumulated and emitted green fluorescence. In comparison in mitochondria with high MMP, JC-1 aggregates were formed in the inner mitochondrial membranes and emitted an orange-red fluorescence.
The fluorescence of JC-1 monomers and aggregates was detected in fluorescence channels 1 (FL1, Ex = 488 nm, Em = 525 nm) and 2 (FL2, Ex = 488 nm, Em = 575 nm), respectively. JC-1 (Invitrogen Molecular Probes T-3168, Molecular Probes) was dissolved in dimethyl sulfoxide (DMSO; D8418; Sigma Aldrich, St. Louis, MO, USA) and stored at –20°C until use.
Semen samples were washed and spermatozoa concentrations adjusted to 5 × 106/ml; cells were incubated in phosphate-buffered saline (PBS) containing 1 mg/l JC-1 for 10 min at room temperature in the dark. At the end of the incubation period, spermatozoa samples were analyzed by flow cytometry (Beckman Coulter, EPICS XL-MCL).
Minimally, 10,000 spermatozoa were analyzed for each sample. JC-1 aggregates were examined using flow cytometry at 488 nm excitation and 575 nm emission settings. Spermatozoa that acquired JC-1 staining, exhibiting red fluorescence, were considered to have functional mitochondria. Spermatozoa that exhibited green fluorescence were considered to have non-functional mitochondria. MMP was expressed as the percentage of red fluorescence/green fluorescence spermatozoa.
Statistical analysis
All data were presented as mean ± standard error (SE). A paired-sample T-test was used to compare the adverse effects of cryopreservation on semen parameters. The cryoprotective effects of LC (1.0 g/l) supplementation on different semen samples were analyzed by analysis of variance (ANOVA).
Prior to the ANOVA, the variables were transformed to freezing efficiency (FE) to describe the exact influence of cryopreservation on semen parameters:
In this equation, a is the sperm parameter before freezing and a′ is the corresponding parameter after thawing. All parameters, including a, a + b, a + b + c, VIA, DFI and MMP were transformed this way. The reciprocal of transformed DFI was used to keep other variables consistent. Results were analyzed using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA), and results with a P-value < 0.05 were considered to be significant.
Results
Adverse effects of cryopreservation on the semen parameters
Thirty-seven asthenozoospermic samples and 33 normozoospermic samples were recruited for the experiments. The frozen–thawed semen samples from both asthenozoospermic and normozoospermic men demonstrated a lower percentage of total motility (a + b + c), fast forward motility (a) and forward motility (a + b) compared with the fresh ejaculates of respective groups (P < 0.01) (Table 1). Cryopreservation resulted in a significant increase in DFI and a significant loss of VIA and MMP in the asthenozoospermic as well as in the normozoospermic group (P < 0.01; Table 1).
Table 1 Effects of l-carnitine supplementation (1.0 g/l) to cryoprotective medium on sperm motility, MMP, DFI and VIA in post-thaw semen samples of men with asthenozoospermia or normozoospermia

LC: l-carnitine; a: fast forward motility; a + b: forward motility; a + b + c: total motility; MMP: mitochondrial membrane potential; DFI: DNA fragmentation index; VIA: viability.
aP< 0.01 when comparing parameters evaluated before freezing and after freezing with LC; bP < 0.01 when comparing parameters evaluated before freezing and after routine freezing.
Effect of semen type on cryopreservation
Statistical analysis showed that there were no differences in FEs of forward motility (F = 2.08, df = 1, P > 0.05), MMP (F = 1.56, df = 1, P > 0.05) and total motility (F = 0.36, df = 1, P > 0.05) between normozoospermic and asthenozoospermic semen samples with and without LC. Different semen types did however affect FEs in fast forward motility (F = 46.55, df = 1, P < 0.01), DFI (F = 264.70, df = 1, P < 0.01) and VIA (F = 91.00, df = 1, P < 0.01) (Tables 2 and 3).
Table 2 The freezing efficiency of untreated frozen–thawed semen and frozen–thawed semen treated with l-carnitine in the asthenozoospermia and normozoospermia groups

a: fast forward motility; a + b: forward motility; a + b + c: total motility.
DFI: DNA fragmentation index; FE: freezing efficiency; LC: l-carnitine; MMP: mitochondrial membrane potential; VIA: viability.
Table 3 The ANOVA result of asthenozoospermia and normozoospermia group treated with or without l-carnitine
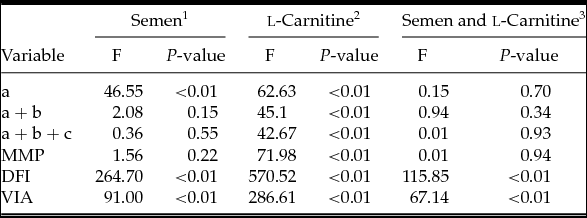
a: fast forward motility; a + b: forward motility; a + b + c: total motility.
DFI: DNA fragmentation index; LC: l-carnitine; MMP: mitochondrial membrane potential; VIA: viability.
1: Comparison between asthenozoospermia and normozoospermia group; 2: Comparison between groups with or without l-carnitine; 3: Interaction effect between LC supplementation and semen type.
Protective effects of LC supplementation on the semen parameters
As for FE, there was a significant increase in motility, including fast forward motility (F = 62.63, df = 1, P < 0.01), forward motility (F = 45.10, df = 1, P < 0.01), total motility (F = 42.67, df = 1, P < 0.01), VIA (F = 286.61, df = 1, P < 0.01) and MMP (F = 71.98, df = 1, P < 0.01) in samples with LC when compared with samples without LC (either asthenozoospermic or normozoospermic). In parallel, addition of LC prior to cryoprotectant significantly reduced the DFI (F = 570.52, df = 1, P < 0.01) compared with the corresponding control groups regardless of the type of semen samples (Tables 2 and 3).
Interaction effect between LC supplementation and semen type
The interaction effect between LC supplementation and semen type was also calculated. There were no statistical differences in the parameters of fast forward motility (F = 0.15, df = 1, P = 0.70), forward motility (F = 0.94, df = 1, P = 0.34), total motility (F = 0.01, df = 1, P = 0.93) and MMP (F = 0.01, df = 1, P = 0.94) between normozoospermic and asthenozoospermic semen samples. Media supplemented with LC showed a better protective effect for asthenozoospermic semen samples in DFI (F = 115.85, df = 1, P < 0.01) and VIA (F = 67.14, df = 1, P < 0.01) than for normozoospermic semen samples (Table 3).
Discussion
In the present study, we observed a statistically significant decrease in sperm motility, viability, and mitochondrial activity in frozen–thawed spermatozoa from both asthenozoospermic and normozoospermic groups when compared with fresh samples. In addition, a significant increase in percentage of DNA damage was also observed in both groups. Losses of motility, viability, and mitochondrial potential as well as sperm DNA damage are the most common cryodamage found in frozen–thawed spermatozoa (Anger et al., Reference Agarwal, Saleh and Bedaiwy2003). Of all the possible mechanisms behind cryodamage to spermatozoa, excessive production of ROS has been suggested as a major contributing factor (Chatterjee & Gagnon, Reference Chatterjee and Gagnon2001). When ROS levels exceed the potential of the antioxidant scavenging activities, peroxidative damage to the spermatozoa occurs. Mammalian sperm are extremely sensitive to ROS because of their high content of PUFA in cellular and intracellular membranes (Lenzi et al., Reference Lenzi, Picardo, Gandini and Dondero1996). Therefore, sperm plasma membrane integrity (viability) and the mitochondria membrane integrity (MMP) would be attacked by excessive production of ROS, which causes a loss of sperm motility (Alvarez & Storey, Reference Alvarez and Storey1992; Saleh & Agarwal, Reference Saleh and Agarwal2002; Agarwal et al., Reference Agarwal, Saleh and Bedaiwy2003). Furthermore, ROS induced DNA damage, altered gene expression and apoptosis (Stohs et al., Reference Stohs, Bagchi, Hassoun and Bagchi2000).
l-Carnitine (LC) and ALC accumulate in the epididymis and play a crucial role in sperm metabolism, maturation, and the spermatogenic process (Agarwal & Said, Reference Agarwal and Said2004). In a variety of human and animal studies (Apak et al., Reference Apak, Güçlü, Ozyürek and Karademir2004; Silva-Adaya et al., Reference Silva-Adaya, Pérez-De La Cruz, Herrera-Mundo, Mendoza-Macedo, Villeda-Hernández, Binienda, Ali and Santamaría2008; Vanella et al., Reference Vanella, Russo, Acquaviva, Campisi, Di Giacomo, Sorrenti and Barcellona2000), the role of LC in reducing the cellular oxidative stress and its antioxidant properties has been proved. Furthermore, LC had been used extensively in vitro as a sperm motility enhancer because the primary function of LC is to act as a carrier for translocation of long-chain fatty acids across the inner membrane of mitochondria for β-oxidation, hence ATP production provides readily available energy for use by spermatozoa (World Health Organization, 2001; Akhondi et al., Reference Akhondi, Chapple and Moore1997; Hinton et al., Reference Hinton, Brooks, Dott and Setchell1981; Tanphaichitr, Reference Tanphaichitr1997). Our results manifested that supplementation of LC to sperm freezing medium significantly improved post-thaw motility, viability, mitochondrial activity and DNA integrity of cryopreserved spermatozoa in both asthenozoospermic and normozoospermic groups.
Reyes-Moreno et al. (Reference Reyes-Moreno, Gagnon, Sullivan and Sirard2000) utilized carnitine-rich epididymal cells as cryoprotectant and found that post-thaw motility was improved during bovine sperm cryopreservation. Another study by Banihani et al. (Reference Banihani, Agarwal, Sharma and Bayachou2013) also reported that addition of LC to human sperm cryoprotectant significantly increased their motility and vitality after thawing. These findings are all in line with our results. Motility is the most institutional and visualized index to evaluate semen quality. The energy required for sperm motility is mainly supplied by the tri-carboxylic acid (TCA; or citric acid or Krebs) cycle that takes place in the mitochondria. When the energy generated by the TCA cycle is conducted along the respiratory chain, protons are pumped out from the matrix inside the mitochondrial inner membrane to give the MMP. As a result, any damage to mitochondrial function, such as increased seminal ROS, sperm apoptosis and higher DNA fragmentation level could impair sperm motility and fertilization (O'Connell et al., Reference O'Connell, McClure and Lewis2002). Moreover, disruption of mitochondrial electron transport flow would, in turn, cause the generation of ROS (Koppers et al., Reference Koppers, De Iuliis, Finnie, McLaughlin and Aitken2008).
It has been reported that carnitine can protect cell membrane against ROS damage and maintain membrane structure by regulating carbohydrate metabolism (Lenzi et al., Reference Lenzi, Lombardo, Sgrò, Salacone, Caponecchia, Dondero and Gandini2003; Gülçin, Reference Gülçin2006). Being a facilitator for activated fatty acid transport into the mitochondrial matrix for β-oxidation, LC decreases the availability of lipids for peroxidation. This action may protect the sperm membrane and the mitochondrial membrane, thereby increasing sperm viability and MMP (Kalaiselvi & Panneerselvam, Reference Kalaiselvi and Panneerselvam1998). In a series of cryobiology studies, supplementation with antioxidants, such as vitamin E (Kalthur et al., Reference Kalthur, Raj, Thiyagarajan, Kumar, Kumar and Adiga2011), ascorbate and catalase (Li et al., Reference Li, Lin, Liu, Xiao and Liu2010), catalase (Moubasher et al., Reference Moubasher, El-Din, Ali, El-Sherif and Gaber2013), and quercetin (Zribi et al., Reference Zribi, Chakroun, Abdallah, Elleuch, Sellami, Gargouri, Rebai, Fakhfakh and Keskes2012), was shown to minimize post-thaw human sperm DNA damage.
In the present study, we found that DNA fragmentation levels were significantly reduced in asthenozoospermic and normozoospermic semen samples when these were cryopreserved with LC. Our finding of the protective effect of LC against DNA damage is in agreement with studies by Haripriya et al. (Reference Haripriya, Sangeetha, Kanchana, Balu and Panneerselvam2005) and Thangasamy et al. (Reference Thangasamy, Jeyakumar, Sittadjody, Joyee and Chinnakannu2009), who reported that LC could reduce DNA damage in lymphocytes and in the brain cerebral cortex of aged rats. Studies have demonstrated that carnitine could act on cell DNA and on membranes, protecting them against damage induced by free oxygen radicals (Arduini, Reference Arduini1992). In a recent study by Abdelrazik et al. (Reference Abdelrazik, Sharma, Mahfouz and Agarwal2009), it was demonstrated that LC could improve in vitro blastocyst development in mice by reducing H2O2-induced DNA damage. Moreover, it was reported that LC has a favorable effect on DNA repair of regenerated germ cells (Agarwal & Said, Reference Agarwal and Said2004). In contrast, in a similar study by Banihani et al. (Reference Banihani, Agarwal, Sharma and Bayachou2013), no significant effect of LC in decreasing DNA oxidative damage in spermatozoa was found. The difference in semen-freezing protocols and different means of DNA fragmentation analysis used by our group may account for the difference between the two studies.
Asthenozoospermia, defined as low sperm motility, is a significant cause of male infertility. Asthenozoospermia is a prevalent condition in the human population at this time, therefore the percentage of frozen–thawed samples that are asthenozoospermic would currently be increased. According to Kalthur et al. (Reference Kalthur, Raj, Thiyagarajan, Kumar, Kumar and Adiga2011), fresh ejaculates from asthenozoospermic men have a significantly higher sperm DFI compared with normozoospermic subjects. Furthermore, former studies have shown that ROS levels in asthenozoospermia semen are statistically higher than those in the normozoospermic group (Agarwal et al., Reference Agarwal, Ikemoto and Loughlin1994). Asthenozoospermic spermatozoa have poorly organized chromatin and a high incidence of DNA damage, which makes them more susceptible to freeze–thaw-induced DNA damage compared with normozoospermic men (Evenson et al., Reference Evenson, Darzynkiewicz and Melamed1980; Colleu et al., Reference Colleu, Lescoat, Boujard and Le Lannou1988; Hammadeh et al., Reference Hammadeh, Askari, Georg, Rosenbaum and Schmidt1999; Erenpreiss et al., Reference Erenpreiss, Elzanaty and Giwercman2008). An effective cryoprotectant for asthenozoospermic spermatozoa is therefore needed. In this study, we showed that the cryoprotective efficiency of LC for asthenozoospermic semen samples was the same as that of normozoospermic samples for motility (fast forward, forward and total motility) and MMP. In addition, for the FEs of DFI and VIA, the cryoprotective efficiency of LC in asthenozoospermic samples was better than that in normozoospermic samples. Our finding provide empirical support for LC as an effective cryoprotective addition to medium used in the freezing–thawing process for asthenozoospermic semen samples.
Base ROS level and LC concentration in semen plasma were not detected in the present experiment. Further work is needed to test whether the protective effect of LC can improve the fertilization capacity of frozen–thawed human spermatozoa in ART settings.
In conclusion, supplementation of LC to cryoprotective medium enhanced post-thaw sperm motility and helped protect sperm from freeze–thaw-induced DNA damage. Supplementation maintained the functional levels of mitochondria, especially in asthenozoospermic ejaculates. LC, therefore, is a promising cryoprotective agent to improve post-thaw sperm quality and may play an important role in conserving fertility of asthenozoospermic patients.
Acknowledgements
The authors wish to thank Dr Hu Xiao for technical support in flow cytometry maintenance and operation.
Funding
This study was supported by the scientific research projects of the Zhejiang Provincial Department of Education, China (grant no. N20130393).