Introduction
The ability of oocytes to attain functional maturation under post-release from ovarian follicles illustrates their potential to fertilize and undergo embryonic development, eventually forming a viable zygote. This event dictates the prospective developmental competence of an oocyte and is the most crucial phase in determining the successful production of embryo during in vitro embryo production (IVEP; Brackett & Zuelke, Reference Brackett and Zuelke1993; Brevini-Gandolfi & Gandolfi, Reference Brevini-Gandolfi and Gandolfi2001). The milestone of events related to nuclear and cytoplasmic maturation has been identified as the critical events for oocytes competence (Eppig et al., Reference Eppig, Schultz, O’Brien and Chesnel1994). Presently, the identification of oocytes with high developmental competence is a fundamental necessity for successful production of embryos under IVEP. Many intricate signalling pathways are responsible for the resumption of meiotic maturation from the arrested GV stage (Westergaard et al., Reference Westergaard, Byskov, Andersen, Grinsted and McNatty1984; Chian et al., Reference Chian, Buckett and Tan2004). Of the several pathways, the AKT signalling pathway is the major controller of the GVBD event and CDC25B is one of the substrates of AKT that dephosphorylates CDK1, rendering it active to trigger GVBD (Kalous et al., Reference Kalous, Solc, Baran, Kubelka, Schultz and Motlik2006; Oh et al., Reference Oh, Han and Conti2010). The cytoplasmic localization of CDC25B depends upon cyclin B (Karlsson et al., Reference Karlsson, Burman and Akerlund1999). Therefore, altogether AKT is the initial activator, CDC25B is a critical intermediate, while CCNB is the terminal effector molecule along with CDK1 in GVBD event. The dynamics of GBVD-associated genes could eventually reveal the developmental progress for growing oocytes in a time-dependent manner.
A large pool of buffalo oocytes of abattoir origin is available for IVEP production in the Indian subcontinent. To utilize this pool of abattoir-derived cumulus – oocytes complexes (COCs) of heterogeneous quality via IVEP, screening and identification of the optimal developmental capacity of oocytes have become the vital factors for successful development of embryos (Lonergan et al., Reference Lonergan, Monaghan, Rizos, Boland and Gordon1994; Pavlok et al., Reference Pavlok, Lucas‐Hahn and Niemann1992). This process demands a comprehensive understanding of the developmental competence of COCs. Despite the fact that most researchers have reported that they attained an over 90% in vitro maturation rate for buffalo oocytes, the blastocyst rate merely reached ~15–20% (Moor et al., Reference Moor, Dai, Lee and Fulka1998; Presicce Reference Presicce2007; Manjunatha et al., Reference Manjunatha, Ravindra, Gupta, Devaraj and Nandi2009). The lower rate of blastocyst conversion is one of the major bottlenecks in adopting IVEP for buffaloes on a large scale. In fact, BCB staining has provided an initial basis for ensuring the developmental competence of heterogeneous oocytes originating from the slaughter house. However, further strategy needs to be generated for the precise assessment of the developmental ability of growing oocytes. Some previous studies were restricted to identifying oocyte quality based on BCB staining of mouse oocytes (Wu et al., Reference Wu, Liu, Zhou, Lan, Han, Miao and Tan2007), sheep oocytes (Catala et al., Reference Catala, Izquierdo, Uzbekova, Morato, Roura, Romaguera, Papillier and Paramio2011), bovine oocytes (Torner et al., Reference Torner, Ghanem, Ambros, Holker, Tomek, Phatsara, Alm, Sirard, Kanitz, Schellander and Tesfaye2007) and buffalo oocytes (Manjunatha et al., Reference Manjunatha, Gupta, Devaraj, Ravindra and Nandi2007). Here we assessed the chronology of GVBD along with the gene expression profile of differentially matured oocytes to understand their translational ability to develop into prospective embryos.
Materials and methods
All media and chemicals were procured from Sigma-Aldrich, St. Louis, MO, USA, unless otherwise indicated. Disposable plasticware was purchased from Falcon NJ, USA and Nunc, Denmark. Fetal bovine serum (FBS) used was obtained from HyClone, Canada.
Aspiration of oocytes, BCB staining and in vitro maturation
Slaughter house-derived buffalo ovaries were collected under aseptic conditions and brought to laboratory in pre-warmed (37°C) physiological saline supplemented with antibiotics (streptomycin sulphate 100 µg/ml with 0.9% NaCl) within 2–3 h of slaughtering. Before aspiration, ovaries were washed several times in normal saline. Cumulus – oocyte complexes (COCs) were aspirated from visible ovarian follicles (3–6 mm) in HEPES-buffered hamster embryo culture (HH) medium. COCs were picked with the aid of a stereozoom microscope (Leica Microsystems, Germany) and washed three times in HH medium. Only Grade A COCs (those with five compact layers of cumulus cell with a homogenous ooplasm) were selected for BCB staining. BCB staining was carried out based on the protocol of Alm et al. (Reference Alm, Torner, Loehrke, Viergutz, Ghoneim and Kanitz2005) and Bhojwani et al. (Reference Bhojwani, Alm, Torner, Kanitz and Poehland2007). In brief, selected COCs were subjected to 26 µM BCB stain prepared in mDPBS (B-5388, Sigma-Aldrich, Taufenkirchen, Germany) for 90 min at 38.5°C in humidified air and re-washed in mDPBS to remove the excess stain. The oocytes were examined under a stereozoom microscope and classified as BCB+ and BCB− groups according to the colour of their cytoplasm. Subsequently, BCB screened oocytes were washed in washing medium (HECM−HEPES) followed by three washes in maturation medium [modified TCM-199 HEPES supplemented with 10% (v/v) FBS, 0.005% (w/v) streptomycin, 0.01% (w/v) sodium pyruvate, and 0.005% (w/v) glutamine supplemented with 5.0 µg/ml pFSH, 10 µg/ml LH, 1 µg/ml oestradiol 17-β, 50 ng/ml epidermal growth factor (EGF) and 64 µg/ml cysteamine] and cultured in maturation medium. Drops of 100 µl maturation medium containing oocytes were prepared in 65 mm culture dishes overlaid with mouse embryo-tested mineral oil and incubated for at least 22 h at 38.5°C in atmosphere of 5% CO2 in air in an incubator (InnovaC-170, Germany).
Assessment of GVBD by aceto-orcein staining
Cumulus cells were removed from COCs of BCB+ and BCB− groups by vortexing, followed by washing in phosphate-buffered saline (PBS). Oocytes were washed 12 times and then were placed singly on a clean grease-free microscopic glass slide that had rails of wax along its sides. Excess PBS was removed and a coverslip was placed gently on the slide. These slides were then dipped overnight in a Coplin jar containing fixative (methanol:acetic acid in the ratio of 3:1) solution. The entire process from cumulus removal to fixation was done within 20 min. After overnight fixation, the slides were stained with 1% aceto-orcein in 45% glacial acetic acid for 30 min followed by washing with destaining solution. The slides were observed under an Olympus BX51 microscope fitted with DP71 camera. In total, 576 BCB+ and 560 BCB− oocytes from different hours of maturation were subjected to aceto-orcein staining.
In vitro embryo production
Oocytes collection, BCB screening and their IVM maturation were carried out in the same manner as discussed in the previous section. Following IVM, 15 COCs were placed per IVM droplet. In vitro fertilization (IVF) was performed in 100-μl droplets of BO medium supplemented with 1% BSA (fatty acid free), 1.9 mg/ml caffeine sodium benzoate, 0.14 mg/ml sodium pyruvate and 0.01 mg/ml heparin. Before transfer in fertilization drops, matured COCs were washed three times in BO medium. The frozen – thawed buffalo semen were processed for in vitro capacitation and 50 μl of sperm suspension (at a final concentration of 1 × 106/ml) was added to each fertilization drops containing 15 COCs and incubated at 38.5°C with 5% CO2 in air for 14 h. Presumptive zygotes were removed from the fertilization drops after 12 h of insemination (HPI), adhered cumulus cells were mechanically removed by vortexing and washed five times in mCR2aa medium. After washing, 15–20 presumptive zygotes were cultured in vitro (IVC) in 100 μl drops of IVC medium (mCR2aa supplemented with 0.8% BSA, 1 mM glucose, 0.33 mM pyruvate, 1 mM glutamine, 1× MEM essential amino acid, non-essential amino acid and 50 μg/ml gentamycin) and maintained for 8 days at 38.5°C in the presence of 5% CO2 with replacement of medium after every 48 h. The experiments were repeated 10 times for each BCB+ and BCB− COCs.
RNA isolation and cDNA preparation
Ten COCs each from different maturation hours (4, 6, 8, 10, 12, 14 and 16 h) were collected from BCB+ and BCB− groups. The pool of oocytes was processed for isolation of total RNA using the RNAqueous Micro Kit (Ambion, USA) according to the manufacturer’s instructions. Isolated total RNA was treated with RNase-free DNase I (Ambion, USA) and reverse-transcribed using the Revert-Aid cDNA synthesis kit following the manufacturer’s instructions (Fermentas, USA). cDNA samples were diluted three times with nuclease-free water and stored at −20°C until further use.
Quantitative RT-PCR
To attain a correlation between maturation events at the molecular level, genes relevant to GVBD (CCNB, AKT and CDC25B) were quantified at different time points during IVM. Primers for this purpose were designed using Primer-BLAST software (Table 1). Real-time polymerase chain reaction (PCR) was carried out using Mx3005P (Stratagene, Santa Clara, CA) and SYBR Green Master mix (Fermentas, UAB, Lithuania). Normalization was performed using two housekeeping genes i.e. RPS-18 and ACTB (β-actin). The reaction mixture for quantitative PCR consisted of 5 µl SYBR Green qPCR mix, 2 µl cDNA template, optimized primer quantities and nuclease-free water to make a total reaction volume of 10 µl. RT-PCR reactions were performed in duplicate for each sample, following conventional cycling conditions: initial denaturation for 10 min at 95°C, followed by 45 PCR cycles of denaturation at 95°C for 30 s, annealing of primers at 59°C for 30 s, and extension at 72°C for 30 s. After each reaction, a dissociation curve was run to check the specificity of the PCR product. The normalized target values were divided by the calibrator normalized target values to generate the relative expression levels and fold change values (x) were calculated using the following formula: x = 2-ΔΔCt (Livak and Schmittgen, Reference Livak and Schmittgen2001).
Table 1 Primer sequences and corresponding product sizes for genes related to GVBD event

Statistical analysis
Statistical calculation was performed using Graph Pad Prism software (GraphPad, San Diego, CA). All experiments were repeated at least three times independently. Embryo development rate data were subjected to arc – sine transformation. One-way analysis of variance (ANOVA) was performed following Bonferroni post hoc tests to compare the significance of difference between the groups at a 99% and 99.9% confidence interval (P<0.01 and P<0.001).
Results
Assessment of nuclear maturation in BCB screened oocytes
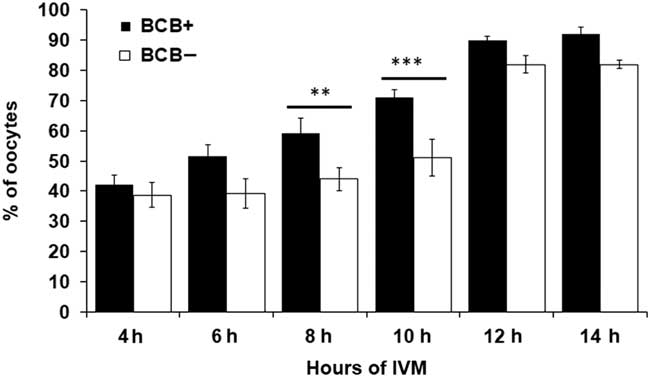
BCB screened oocytes were subjected to aceto-orcein staining at different time intervals during IVM to assess the status of their nuclear maturation (Fig. 1). Figure 2 represents the number of oocytes that underwent GVBD at different maturation stages. As can be seen from the Fig. 2, GVBD rate substantially differed following 8 h and 10 h of IVM between BCB+ and BCB− groups. However, no difference was observed in GVBD rate before 6 h and after 12 h of IVM for both the groups.
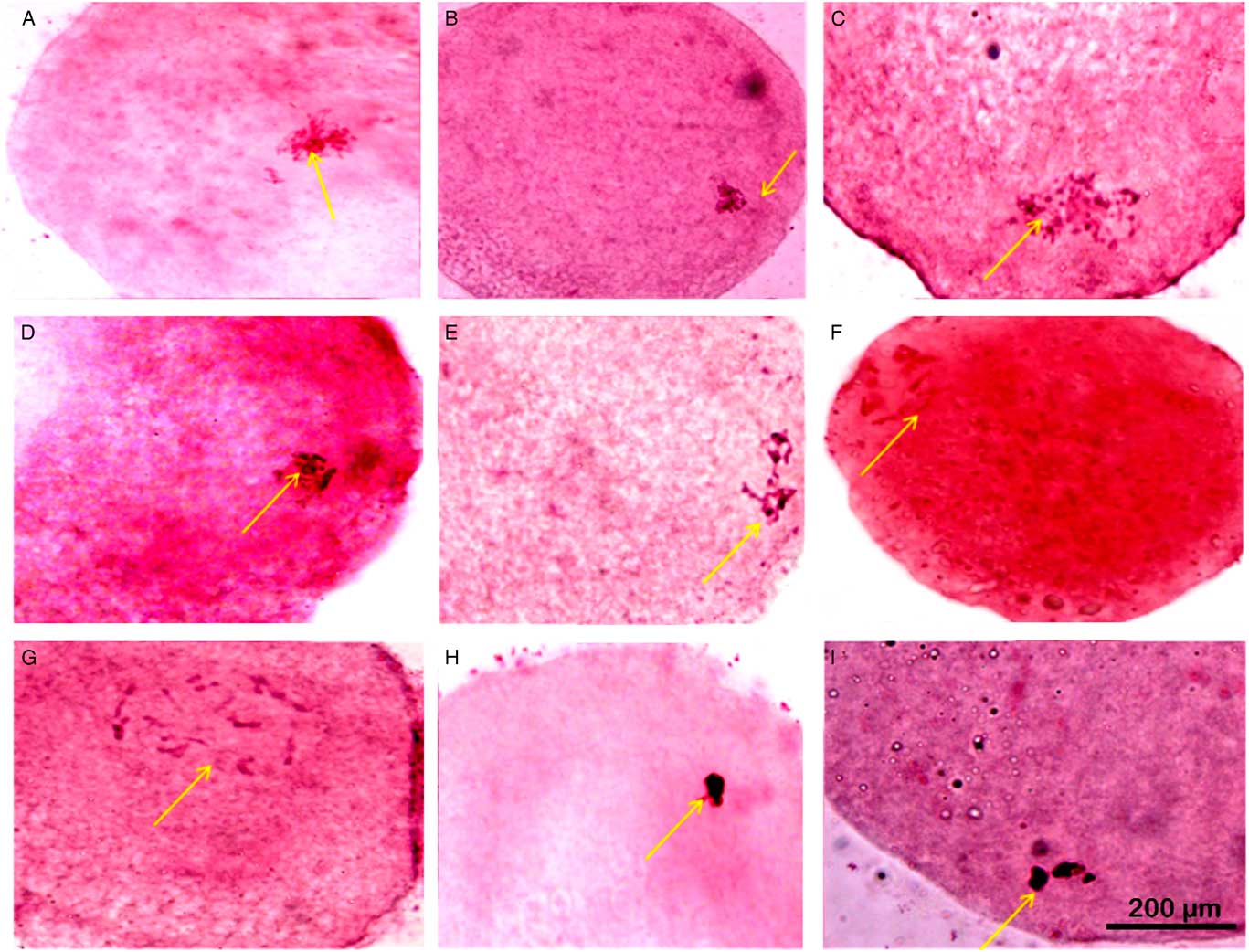
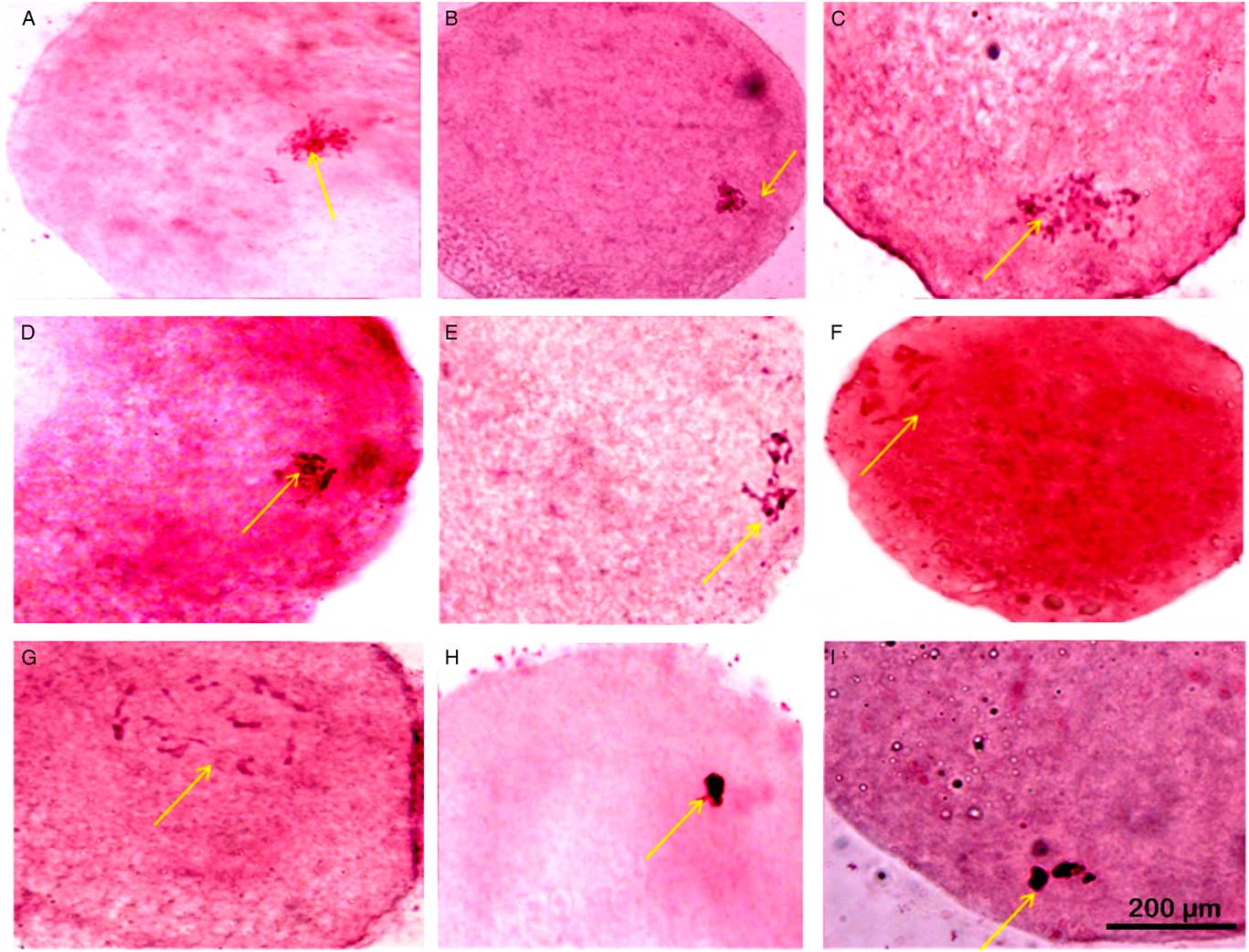
Figure 1 Nuclear maturation observed using aceto-orcein staining at different time points of in vitro maturation (IVM). (A, D) GV stage oocytes with nucleolus and filamentous chromatin (nuclear membrane partially distinguishable). (B) GV stage oocytes with filamentous chromatin with distinct nuclear membrane, no nucleolus. (C) GV stage oocytes with filamentous chromatin. (E) GVBD oocytes with condensed chromatin in form of bivalents. (F, G) GVBD oocytes with condensed chromatin. (H, I) GVBD stage with highly condensed chromatin. Arrows indicate status of nucleus, nucleolus and chromatin that has been described for respective images in the figure legend. Scale: 200 µM.
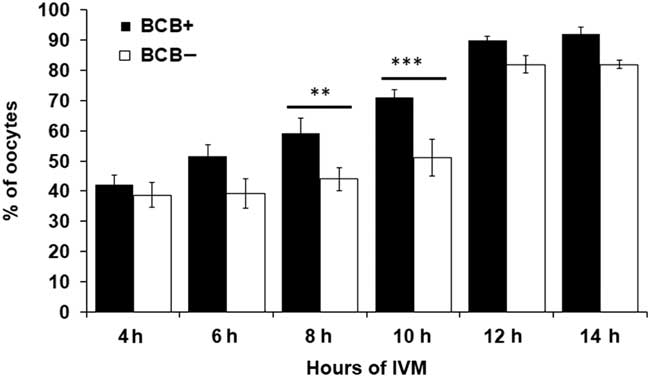
Figure 2 Confirmation of GVBD using aceto-orcein staining at different time intervals during in vitro maturation (IVM) in BCB+ and BCB− oocytes. Bars with different superscripts indicate significant difference at respective hours of IVM. **P<0.01, ***P<0.001.
Differential expression profiles of CCNB, CDC25B and AKT in BCB screened oocytes
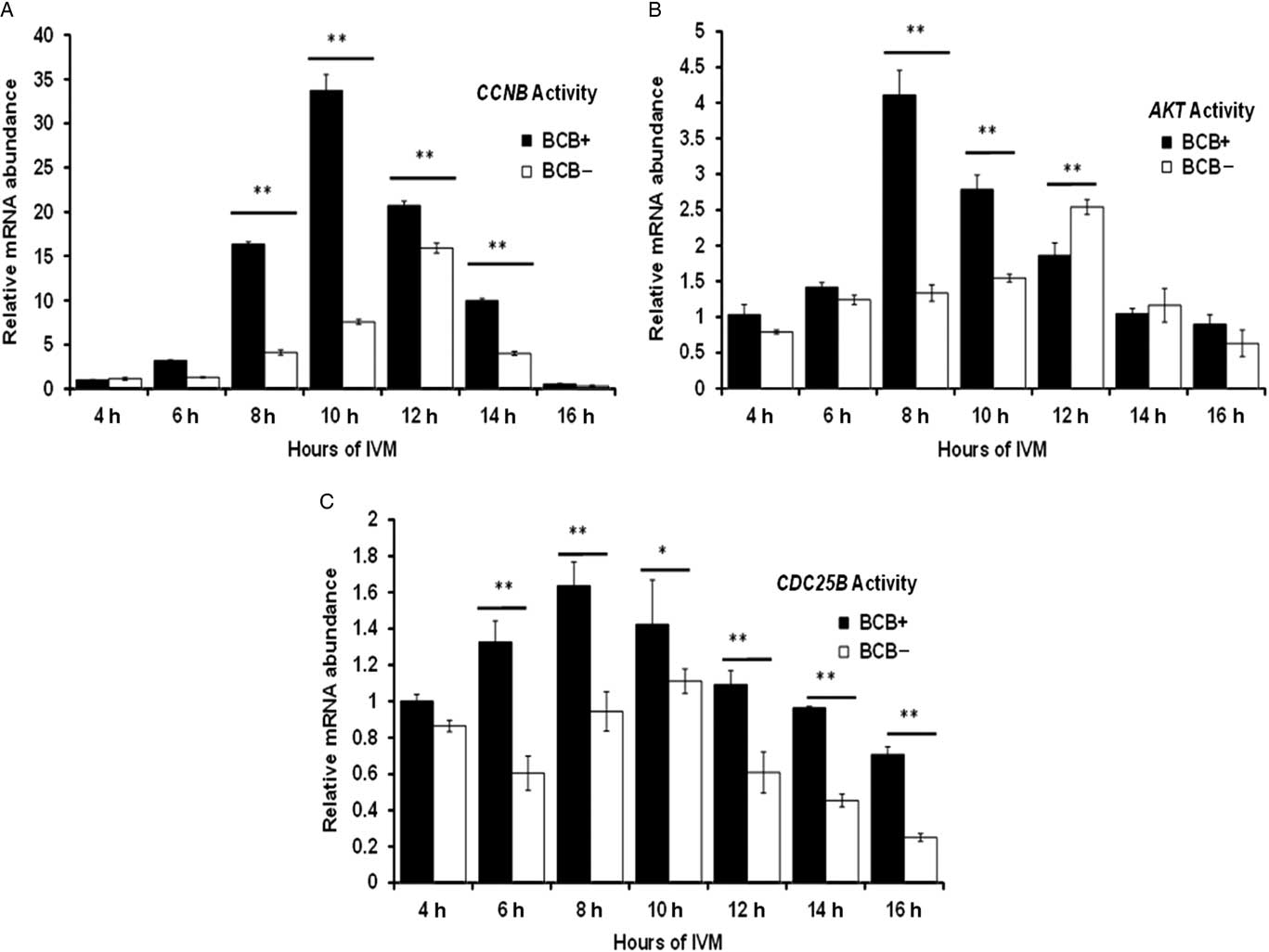
The expression pattern of CCNB, CDC25B and AKT genes was investigated at different time intervals during IVM. A 35-fold increase in CCNB expression in BCB+ oocytes was found at 10 h of IVM and subsequently decline in expression was observed at 14 h and 16 h (Fig. 3A). However, CCNB was significantly less expressed in BCB− oocytes and its expression peaked at around 12 h of IVM. The expression level of the AKT gene was significantly higher at 8 h of IVM in BCB+ oocytes than that in BCB−. Furthermore, AKT expression peaked at 12 h of maturation in the BCB− group. For the CDC25B gene, expression levels were increased from 4 to 8 h of in vitro maturation in BCB+ oocytes and the peak was attained at 8 h of maturation. Contrary to this finding, CDC25B showed a significantly lower expression level in BCB− oocytes and maximum expression level was attained at a later stage (10 h) of IVM (Fig. 3C).
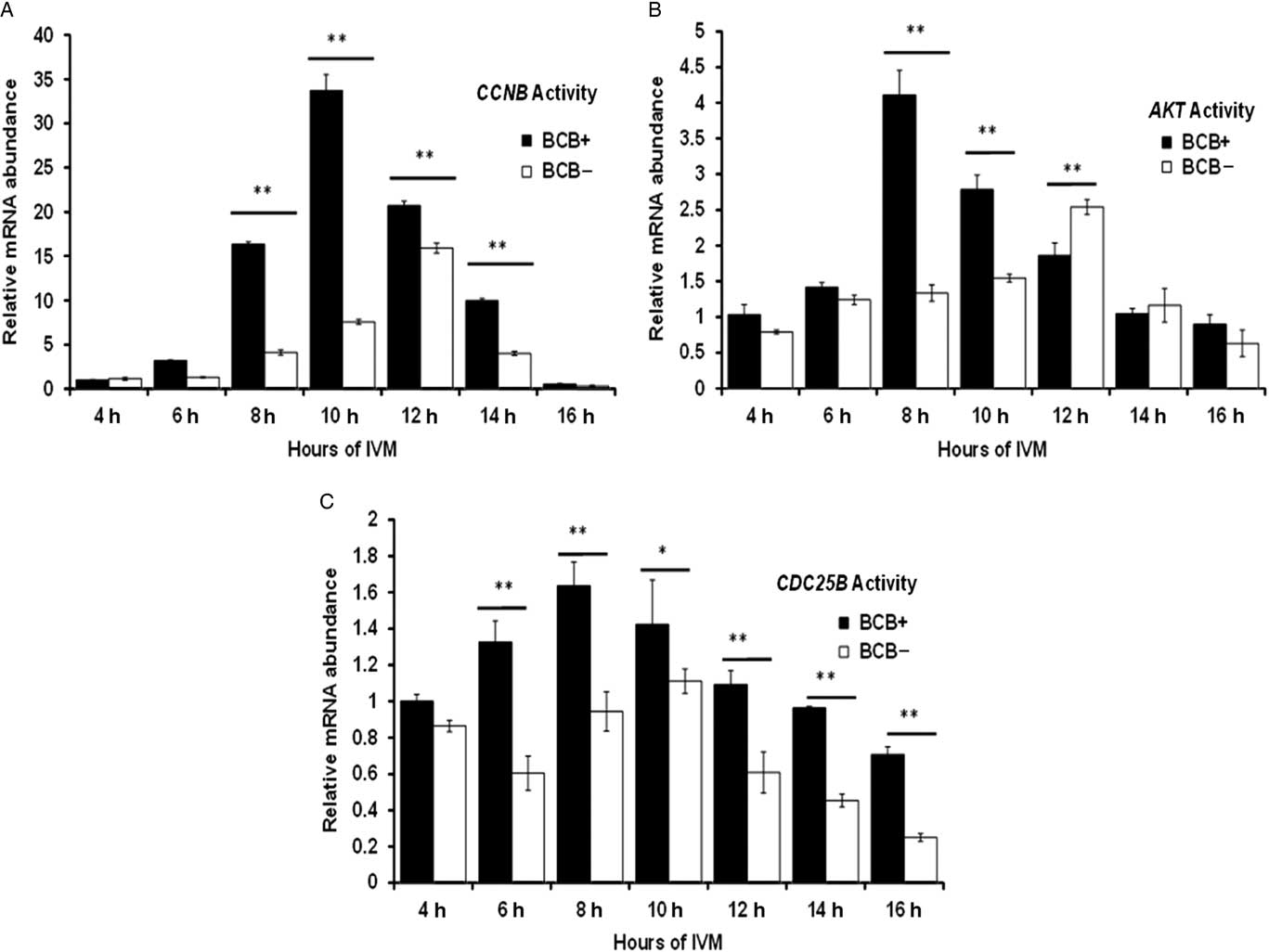
Figure 3 Differential expression by relative mRNA abundance of (A) CCNB, (B) AKT and (C) CDC25B genes in BCB+ and BCB− oocytes at several time points during in vitro maturation. Bars with different superscript indicate significant difference at respective hours of IVM. *P<0.05, **P<0.01.
Developmental ability of IVF-produced embryos from BCB+ and BCB− screened oocytes
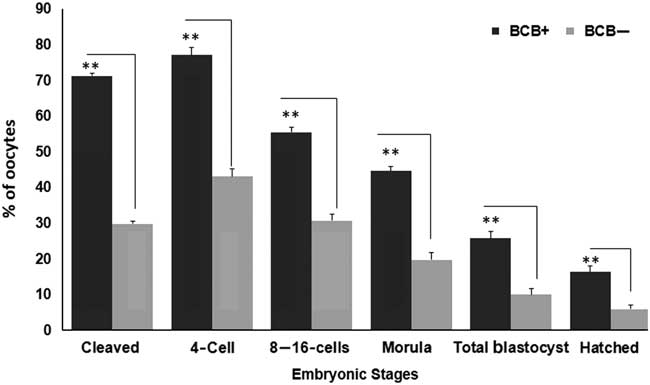
Figure 4 represents the developmental rate of BCB screened oocytes into presumptive embryos. The developmental rate was found to be significantly dissimilar between BCB+ and BCB− oocytes groups (P<0.05). The percentage of cleavage rate differed from 71.23±0.78 in BCB+ to 29.86±0.77 in BCB− group. Of the total cleaved, 77.08±2.10, 55.52±1.32 and 44.62±1.38 reached to 4-cell stage, 8–16-cell and morula stages, respectively in BCB+ oocytes. However, nearly 43.11±2.19, 30.69±1.76 and 19.79±1.95 percentage of BCB− oocytes reached to 4-cell, 8–16-cell and morula stages, respectively. The percentage of blastocysts was significantly different (P<0.05) between BCB+ (25.79±1.88) group and BCB− groups (10.01±1.79). Similarly, hatched blastocysts were found to be significantly different for BCB+ (16.32±1.66) and BCB− (5.99±1.14) groups.
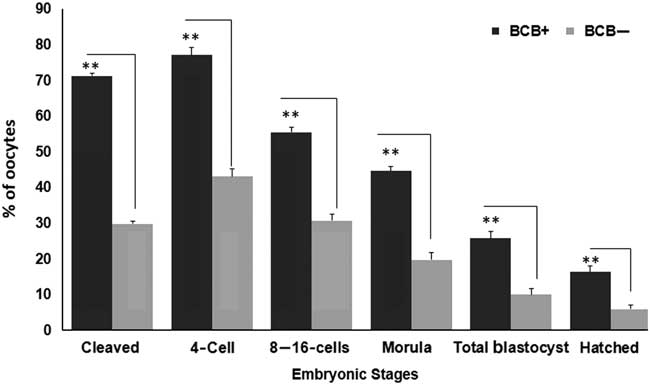
Figure 4 Cleavage rates of embryos derived from BCB screened oocytes. Developmental stages observed include 2-cell, 4-cell, 8–16 cell, morula, blastocyst and hatched blastocyst stages obtained for BCB+ and BCB− groups. Bars with different superscript indicate significant difference for respective embryonic stages. **P<0.01.
Discussion
The selection of oocytes via a BCB screening approach was established as a reliable and consistent approach to achieve a high success rate for conversion of oocyte to embryo during IVEP. Although, it has been known for some time that both BCB+ and BCB− oocytes differ in their developmental competence, apparently there is limited substantial evidence to support this variation. In this study we tried to delineate the molecular signal associated with GVBD of BCB screened buffalo oocytes to get a deeper insight into oocyte developmental capacity during in vitro maturation. Data obtained from the study have revealed that there was a narrow but distinct time gap in BCB+ and BCB− oocytes during which GVBD occurred. Importantly, BCB− showed a tendency of lag behind until ~10 h of IVM, but surprisingly caught up soon afterwards with the maturation rate of BCB+ oocytes. The possible reason for this time gap could be due to BCB− oocytes remaining in their preparatory phase of gaining capability and could be a deficient of essential transcripts needed for GVBD. The rate of GVBD prior to 6 h and post 12 h of IVM did not vary substantially for BCB− and BCB+ oocytes. Interestingly, the developmental competence of BCB screened groups via GVBD varied significantly within the narrow and specific period (8–10 h) of IVM. However, BCB+ oocytes GVBD showed a linear trend with a consistent rise in the number of oocytes attaining GVBD, of which ~70% oocytes completed GVBD by 10 h. This situation suggests that these oocytes must have accomplished their nuclear maturation phase at a faster rate compared with BCB− oocytes.
The mechanisms of GVBD and meiotic resumption are complex and require many endocrine and paracrine signals, and various associated molecules to accomplish the oocyte developmental milestone. So far, the mechanism associated with GVBD and meiotic resumption in buffalo oocytes remains to be fully elucidated. Our findings indicated that the timing of GVBD was intricately connected with the expression levels of CCNB, AKT and CDC25B genes at different progression intervals during IVM. The most remarkable result to emerge from this study was the higher expression levels of CCNB, AKT and CDC25B for BCB+ than those of the BCB− group. Furthermore, results supported that GVBD not only occurred differentially in BCB+ and BCB− oocytes, but was also substantially delayed in the BCB− group. This fact can be seen from the CCNB expression data, which showed a peak at around 10 h of IVM for BCB+ oocytes, whereas maximum expression levels for AKT and CDC25B were found at around 8 h of IVM. Surprisingly, transcript abundance was substantially curtailed beyond 10 h IVM in BCB+ oocytes. Similarly, a time-dependent protein turnover and degradation study of the CCNB gene in mice has been reported that described translation rate, accumulation and degradation as the major determinants of oocyte maturation (Gui and Homer, Reference Gui and Homer2013). In porcine oocytes, the role of either cyclin B1 or cyclin B2 is necessary for induction of GVBD (Kuroda et al., Reference Kuroda, NaitoK Sugiura, Yamashita, Takakura and Tojo2004). These reports and the present results prompted us to conclude that GVBD depends on the availability of CCNB, AKT and CDC25B gene transcripts and the increased levels of expression of these genes during the narrow period of IVM were primarily responsible for GVBD. Considering the fact that molecular signals eventually regulate the oocyte’s ability to achieve the developmental milestone of GVBD, this study convincingly suggested that the delayed and low abundance of molecular signal seem to be responsible for the compromised development competence of BCB− oocytes. This finding confirmed the previous observation in bovine oocytes, in which oocytes remained at the GV stage, had consistent mRNA transcripts and did not proceed beyond the GV stage if there was a deficient in CCNB (Levesque and Sirard, Reference Levesque and Sirard1996). Similarly, BCB+ oocytes have greater stores of cell cycle, transcription and protein biosynthesis transcripts that could be used for resuming meiosis at the earliest time during in vitro culture, making them more competent, and the competent oocytes developed early cleaving embryos (2-cell and 4-cell) with a better chance of developing into blastocysts (Holm et al., Reference Holm, Shukri, Vajta, Booth, Bendixen and Callesen1998). Taken together, we demonstrated a separate but narrow time window for GVBD in BCB+ and BCB− oocytes, supposedly based on the diverse level of transcript signals responsible for GVBD. This finding could eventually lead us to propose a suitable time-gap model for GVBD in BCB+ and BCB− oocytes (Fig. 5).
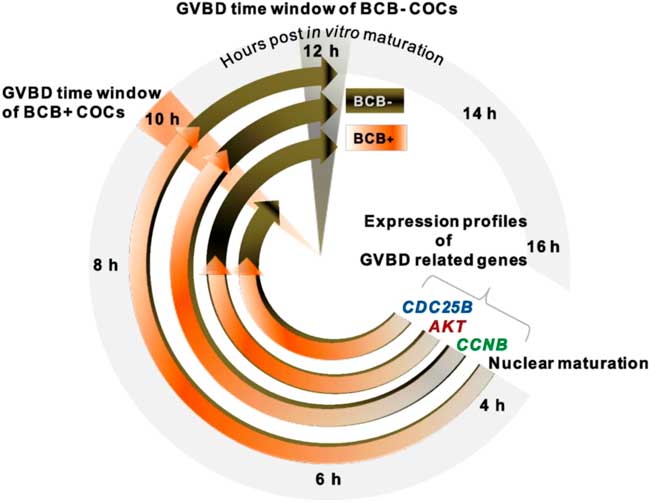
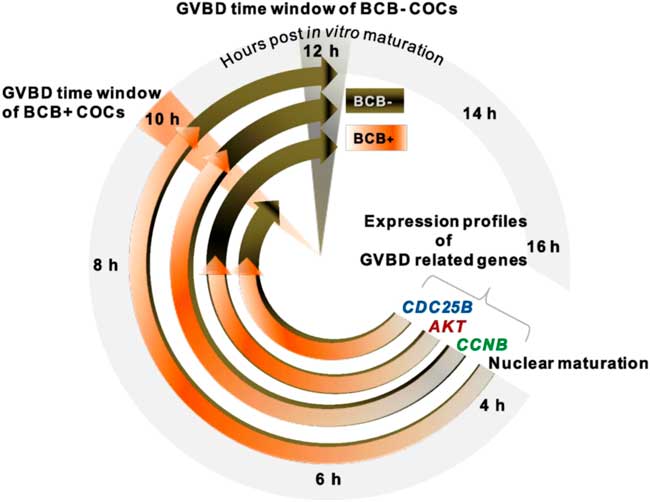
Figure 5 GVBD clock showing differential developmental ability of BCB+ and BCB− COCs from Bubalus bubalis. Resumption of meiosis was evaluated based on the timing of germinal vesicle breakdown (GVBD) in BCB+ and BCB− groups. While BCB+ COCs attained nuclear maturation in 10 h post IVM, BCB− COCs attained this stage at 12 h. Expression profiles of genes (namely CCNB, AKT and CDC25B) crucial to GVBD showed their highest expression levels earlier in the BCB+ group compared with the BCB− group.
The most interesting finding to emerge from this study was the confirmation of distinct embryo development rates for BCB− and BCB+ oocytes. This finding can be reaffirmed from our current data that GVBD was reasonably delayed in BCB− oocytes and that, post 12 h of IVM stage, GVBD rate did not vary significantly between both groups. Surprisingly, increase in GVBD after 12 h of IVM in the BCB− group did not augment the developmental capacity of oocytes, which can be seen in terms of a poor oocyte to blastocyst rate. This situation probably indicated that GVBD beyond 12 h of IVM did not contribute significantly in improvement of oocyte competence. Considering the above results, it may be postulated that poor developmental ability of the BCB− is implied due to the delayed propensity of GVBD for BCB− oocytes. Although, a BCB staining-based approach to assess the development competence of buffalo oocytes was explained by Manjunatha et al. (Reference Manjunatha, Gupta, Devaraj, Ravindra and Nandi2007), their study did not elaborate further on molecular signals associated with GVBD. The ability of oocytes to produce viable blastocysts largely depends upon the initial growth stages during which maternal mRNA and proteins accumulate before the resumption of meiosis (Telford et al., Reference Telford, Watson and Schultz1990). Based on our data, care needs to be taken when deciding oocyte development competence that merely utilizes BCB staining as GVBD by aceto-orcin approaches could be misnomer. This factor probably underlines the incorporation of a combination of diverse approaches to judge the developmental ability of oocytes more efficiently. These findings suggest that, along with some of the earlier known factors responsible for oocytes competence, delayed propensity can be established as an alternative mechanism to assess the ability of buffalo oocytes to develop. Taken together we provide a robust mechanism to delineate the quality of buffalo oocytes, predicting their developmental competence under IVEP regime. Nevertheless, the developmental ability of BCB− oocytes remained largely compromised with a lower blastocyst conversion rate. This aspect remains a matter of concern for buffalo IVEP practice when the oocyte pool has a sizable population of BCB− oocytes. This situation must be seen as an opportunity to increase the ability of poor quality buffalo oocytes to develop.
In conclusion, this work has led us to conclude that BCB screened buffalo oocytes undergo GBVD at differential pace and that the difference was most conspicuous at the narrow and specific time window of IVM. Evidence from this study suggested that the variation was largely dependent on abundance of gene transcripts present in BCB+ and BCB− oocytes. The slower pace of GVBD in BCB− oocytes was primarily thought to be due to the poorer translational ability of the oocytes to their presumptive embryos. Altogether, these results suggested that the propensity of gene transcripts along with BCB screening could offer the ideal assessment criteria to evaluate the developmental status of buffalo oocytes in a heterogeneous group. Finally, a synchronization step for poorly graded oocytes may be thought of as an external stimulus to provide the required impetus to undergo GVBD, however this aspect certainly demands further investigation.
Financial support
Funding received from the NASF fund no. 2049/3040 at the ICAR is gratefully acknowledged.
Conflicts of interest
There are no conflicts of interest.