Introduction
In cattle, dry matter intake is reduced during the last weeks of pregnancy and the early postpartum period due to the metabolic stress suffered by dairy cows. Therefore, cows drive into a negative energy balance (NEB) state as a result of energy loss by milk production that cannot be compensated by energy intake (Bell, Reference Bell1995). During NEB, many metabolic hormones and their receptors alter their concentrations; in particular, plasma acylated ghrelin concentration reaches its maximum (Wertz-Lutz et al., Reference Wertz-Lutz, Knight, Pritchard, Daniel, Clapper and Smart2006). It has been demonstrated that dairy cows undergo a process called ‘nutrient partitioning’, emphasizing milk production over reproductive activity throughout the first stage of lactation (Lucy, Reference Lucy2003). Previous reports suggest that NEB induces deleterious effects on cow fertility (Britt, Reference Britt1992; Butler, Reference Butler2003; van Knegsel et al., Reference van Knegsel, van den Brand, Dijkstra, Tamminga and Kemp2005).
Nutritional status is an important factor for regulating endogenous plasma acylated ghrelin concentration (Wertz-Lutz et al., Reference Wertz-Lutz, Knight, Pritchard, Daniel, Clapper and Smart2006) and reproductive physiology. It is known that metabolic hormones and nutritional signals have a direct effect on the ovary (Tena-Sempere et al., Reference Tena-Sempere, Barreiro, Gonzalez, Gaytan, Zhang, Caminos, Pinilla, Casanueva, Dieguez and Aguilar2002; Dupont et al., Reference Dupont, Maillard, Coyral-Castel, Rame and Froment2010). Plasma acylated ghrelin concentration is associated with energy balance (Bradford & Allen, Reference Bradford and Allen2008) and may act as a regulatory signal that links energy balance and reproductive function (Fernandez-Fernandez et al., Reference Fernandez-Fernandez, Martini, Navarro, Castellano, Dieguez, Aguilar, Pinilla and Tena-Sempere2006); it is a gastrointestinal peptide mainly synthesized in bovine abomasum (Hayashida et al., Reference Hayashida, Murakami, Mogi, Nishihara, Nakazato, Mondal, Horii, Kojima, Kangawa and Murakami2001), whose expression and actions have been described in various reproductive organs (Gualillo et al., Reference Gualillo, Caminos and Blanco2001; Gaytan et al., Reference Gaytan, Barreiro, Chopin, Herington, Morales, Pinilla, Casanueva, Aguilar, Dieguez and Tena-Sempere2003; Tena-Sempere, Reference Tena-Sempere2008). Indeed, the ghrelin gene is expressed in multiple tissues exerting endocrine and paracrine effects (van der Lely et al., Reference van der Lely, Tschöp, Heiman and Ghigo2004).
Studies performed in different species have indicated that the addition of acylated ghrelin to mouse embryo culture medium inhibits the development of 2-cell embryos to the hatching blastocyst stage (Kawamura et al., Reference Kawamura, Sato, Fukuda, Kodama, Kumegai, Tanikawa, Nakamura, Honda, Sato and Tanaka2003). Also, high acylated ghrelin concentrations (50–500 ng/ml) added to porcine oocyte in vitro maturation (IVM) medium affect the density and distribution of cytoplasmic microtubules (Suzuki et al., Reference Suzuki, Sasaki, Shimizu, Matsuzaki, Hashizume and Kuwayama2010). In ovine, a high acylated ghrelin concentration (250 ng/ml) added during IVM decreased subsequent embryo development to the blastocyst stage (Wang et al., Reference Wang, Lin and Yu2013). Dovolou and colleagues (Reference Dovolou, Messinis, Periquesta, Dafopoulos, Gutierrez-Adan and Amiridis2014) demonstrated that acylated ghrelin accelerates the oocyte maturational process. However, the understanding of ghrelin action on the bovine cumulus–oocyte complex (COC) is still limited.
The aim of this study was to determine whether acylated ghrelin influences bovine COC metabolism and oocyte maturation. For this purpose, we evaluated the effect of increasing acylated ghrelin concentrations added to IVM medium on bovine cumulus expansion and cumulus cell (CC) viability, apoptosis and DNA damage. Furthermore, we evaluated oocyte embryo developmental capacity and embryo quality of COC matured in the presence of increased acylated ghrelin concentrations.
Materials and methods
Reagents and medium
All reagents were purchased from Sigma Chemical Co. (St. Louis, MO, USA) unless otherwise indicated. The maturation medium was bicarbonate-buffered TCM-199 supplemented with 10% (v/v) FCS, 0.2 mM sodium pyruvate, 1 mM glutamine, 1 µg/ml FSH, 1 µg/ml 17β-estradiol and 50 µg/ml kanamycin. The fertilization medium consisted of TALP (Parrish et al., Reference Parrish, Susko-Parrish, Leibfried-Rutledge, Critser, Eyestone and First1986) supplemented with 6 mg/ml fatty acid free BSA, 20 mM penicillamine, 10 mM hypotaurine and 10 µg/ml heparin sulfate. The culture medium for embryo development consisted of modified synthetic oviduct fluid (SOFm), composed of SOF (Tervit et al., Reference Tervit, Whittingham and Rowson1972) supplemented with 1 mM glutamine, 2% (v/v) BME-essential amino acids, 1% (v/v) MEM–non-essential amino acids and 4 mg/ml fatty acid free bovine serum albumin (BSA; 274–276 mOsm/kg) (Gardner et al., Reference Gardner, Lane, Spitzer and Batt1994). Acylated ghrelin was purchased from Phoenix Pharmaceuticals Inc., USA (cat. no. 031–52). An annexin-V–FLUOS Staining Kit (cat. no. 11-858-777-001) was purchased from Roche Diagnostics GmbH, Roche Applied Science (Penzberg, Germany). Normal and low melting agaroses were purchased form Carlsbad (Carlsbad, CA, USA).
Oocytes
Bovine ovaries were obtained from an abattoir and transported to the laboratory in sterile NaCl solution (9 g/l) with streptomycin and penicillin at 37°C within 3 h of slaughter. The ovaries were pooled regardless of the stage of the estrous cycle of the donor. COC were aspirated from 3- to 8-mm follicles using an 18-G needle connected to a sterile test tube and to a vacuum line (50 mmHg). Only intact COC with evenly granulated cytoplasm were selected, using a low-power (×20–30 magnification) stereomicroscope (Nikon SMZ 800, Tokyo, Japan) for in vitro maturation (IVM).
In vitro maturation
COC were washed twice in TCM-199 buffered with 15 mM HEPES containing 10% (v/v) FCS, and twice in IVM medium. COC were then transferred into 50 μl IVM medium pre-equilibrated in a CO2 incubator, either individually (for expansion evaluation) or in groups of 10 COC (for the other assays). Incubations were carried out under mineral oil (Squibb) at 39°C in an atmosphere of 5% CO2 in air with saturated humidity for 24 h. Oocytes were matured in IVM medium supplemented with acylated ghrelin at a final concentration of 0 (Control), 20, 40, and 60 pM. The criteria for these doses was based on obtaining a medium acylated ghrelin concentration similar to the doses of cows in NEB (Bradford and Allen, Reference Bradford and Allen2008).
Cumulus expansion area
After IVM, cumulus expansion area was measured in each COC using a computerized image-digitizing system with Image ProPlus® 3.1 which allows the measurement of irregular areas. The system units were transformed to μm2 by calibration with a Maklert chamber. For comparison, each COC area was measured before IVM. For this purpose, 159 COC were matured in three replicates (a separate batch of ovaries for each day).
CC number in COC
COC, either compact (before IVM) or expanded (after IVM), were dispersed by pipetting the cells up and down several times under a stereomicroscope. The number of cells were estimated by counting in a haemocytometer chamber. In total, 164 COC were used in three replicates.
Oocyte nuclear maturation
After IVM, oocytes were stripped of surrounding CC and fixed in 4% formaldehyde. They were washed three times in phosphate-buffered saline (PBS) supplemented with 1% polyvinylpyrrolidone (PVP). Oocytes were placed in 1% Triton X-100 overnight, stained with Hoechst 33342, mounted on slides and covered with a coverslip. Germinal vesicle (GV), metaphase I (MI), metaphase II and the first polar body (MII + PB), and degenerate (D) oocytes were determined under an Olympus BX40 epifluorescence microscope with a ×40 magnification objective (Nikon, Tokyo, Japan) equipped with a 365-nm excitation filter, a 400-nm barrier filter, and a 400-nm emission filter (Furnus et al., Reference Furnus, de Matos, Picco, GarcÍa, Inda, Mattioli and Errecalde2008). In total, 454 COC were matured in three replicates.
CC viability
After maturation, CC viability was evaluated. Cumulus cells were incubated for 10 min at 37°C in PBS medium with 2.5 μg/l fluorescein diacetate fluorochrome and 2.5 g/l trypan blue stain. Then, cells were washed in PBS medium and observed under an Olympus BX40 epifluorescence microscope with a 409 fluor objective equipped with a 330–490 nm excitation filter and a 420–520 nm emission filter at ×200 magnification. Live CC are visible in green fluorescence, whereas dead ones show a characteristic blue staining under white light (Hoppe & Bavister, Reference Hoppe and Bavister1984; Fig. 1). In total, 512 COC were matured in three replicates and at least 400 CC per treatment were analyzed in each replicate.

Figure 1 Cumulus cells viability evaluated by trypan blue/fluorescein diacetate assay. (A) Dead cumulus cell (*) shows a characteristic blue staining under white light, whereas a live cell (arrow) is visible in green fluorescence (B).
CC apoptosis by annexin-V staining assay
Annexin-V is a calcium-dependent phospholipid-binding protein with high affinity for phosphatidyl serine (PS) (van Engeland et al., Reference van Engeland, Nieland, Ramaekers, Schutte and Reutelingsperger1998). Early apoptosis was evaluated by membrane redistribution of PS with the annexin-V–FLUOS Staining Kit. The assay involves simultaneous staining with both annexin-V–FLUOS (green) and the DNA stain propidium iodide (PI, red). Intact cells exclude PI and annexin-V–FLUOS. CC were classified following the criteria reported by Pläsier et al. (Reference Pläsier, Lloyd, Paul, Thomas and Al-Rubeai1999) as live (annexin-V negative/PI negative), early apoptotic (annexin-V positive/PI negative), late apoptotic (annexin-V positive/PI positive), and necrotic (annexin-V negative/PI positive) cells (Fig. 2). Briefly, at the end of IVM, CC were washed twice with PBS and centrifuged at 200 g for 5 min. Then, the pellet was resuspended in 100 µl of annexin-V–FLUOS labelling solution (annexin-V + fluorescein, HEPES buffer and PI) and incubated in the dark for 10–15 min at room temperature. In total, 360 COC were matured in three replicates. At least 200 CC per treatment were analyzed in each replicate. Scoring was made at ×400 magnification using an epifluorescence microscope (Olympus BX40) equipped with a 515–560-nm excitation filter.

Figure 2 Apoptosis in cumulus cells (CC) stained with annexin-V–FITC and propidium iodide. This assay involves simultaneous staining with both annexin-V–FLUOS (green) and the DNA stain propidium iodide (PI, red). CC were classified as live (annexin-V negative/PI negative), early apoptotic (annexin-V positive/PI negative), late apoptotic (annexin-V positive/PI positive), and necrotic cells (annexin-V negative/PI positive). The cells were analyzed using phase contrast and epifluorescence microscopy: (A) live cells and early apoptotic cell (arrow); (B) late apoptotic cell; and (C) necrotic cells.
Comet assay
At the end of IVM, all oocytes from each treatment were stripped of surrounding CC by repeated pipetting with a narrow-bore glass pipette in TCM 199 buffered with HEPES. CC were washed three times in calcium- and magnesium-free PBS containing 1 mg/ml PVP, and complete cell disruption was achieved by repeated aspiration using a narrow-bore pipette. The comet assay was performed using the alkaline version described by Singh et al. (Reference Singh, McCoy, Tice and Schneider1988) with modifications (Tice & Strauss, Reference Tice and Strauss1995). Briefly, slides were covered with a layer of 180 µl 0.5% normal agarose. Then, 75 µl of 0.5% low melting point agarose was mixed with cells and layered onto the slides, which were immediately covered with cover slips. After agarose solidification at 4°C for 10 min, cover slips were removed and slides were immersed overnight in fresh lysis solution at 4°C. Then, the slides were equilibrated in alkaline solution for 20 min. Electrophoresis was performed for 20 min at 25 V and 300 mA (1.25 V/cm). Thereafter, slides were neutralized by washing (5 min each) three times with TRIS buffer (pH 7.5) and then with distilled water. Slides were stained with 1/1000 SYBR Green I solution (Molecular Probes; Eugene, OR, USA) (Olive, Reference Olive1999). Scoring was made at ×40 magnification using an epifluorescence microscope (Olympus BX40) equipped with a 515–560-nm excitation filter. Based on the extent of strand breakage, cells were classified according to their tail length into five classes, ranging from Class 0 (no visible tail), Class 1 (comets with a tiny tail), Class 2 (comets with a dim tail), Class 3 (comets with a clear tail), and Class 4 (comets with a clear decrease in the diameter of the head and a clear tail); (Fig. 3). DNA damage was quantified according to Collins and expressed in arbitrary units (Collins, Reference Collins2004). Genetic damage index (GDI) was calculated using the formula GDI = [(I) + 2(II) + 3(III) + 4(IV)]/N (0–IV), in which 0–IV represent the nucleoid type, and N0–NIV represent the total number of nucleoid count (Pitarque et al., Reference Pitarque, Vaglenov, Nosko, Hirvonen, Norppa and Creus1999). Visual scoring (arbitrary units) is rapid as well as simple, and there is a very close agreement between this method and computer image analysis (percentage DNA in tail) (Collins, Reference Collins2004). For this purpose, 240 COC were matured in three replicates.

Figure 3 DNA damage at individual cumulus cell level. Class 0 (no visible tail), Class 1 (comets with tiny tail), Class 2 (comets with a dim tail), Class 3 (comets with a clear tail) and Class 4 (comets with a clear decrease in the diameter of the head and a clear tail).
In vitro fertilization (IVF)
The expanded COC were washed twice in HEPES-TALP supplemented with 3 mg/ml BSA-FAF and placed into 50 μl drops of IVF medium under mineral oil. In all experiments, frozen semen from the same bull was used. Two straws containing 4 × 107 spermatozoa were thawed in a 37°C water bath. Spermatozoa were washed in a discontinuous Percoll gradient prepared by depositing 2 ml of 90% Percoll under 2 ml of 45% Percoll in a 15-ml centrifuge tube. Semen samples were deposited on the top of the Percoll gradient and centrifuged at 500 g for 20 min. The pellet was removed and resuspended in 300 μl HEPES-TALP solution and centrifuged at 300 g for 10 min. After removal of the supernatant, spermatozoa were resuspended in IVF medium, counted in a haemocytometer chamber and further diluted in IVF medium to 1 × 107 spermatozoa/ml. Final sperm concentration in IVF was 2 × 106 spermatozoa/ml. Incubations were carried out at 39°C in 5% CO2 in air with saturated humidity for 24 h.
In vitro embryo culture (IVC)
After IVF, the presumptive zygotes were washed twice in HEPES-SOFm and twice in SOFm, cultured without glucose during the first 24 h and then cultured for another 7 days with 1.5 mM glucose (Furnus et al., Reference Furnus, de Matos, Martínez and Matkovic1997). Embryo culture was carried out in 40 μl drops (10 presumptive zygotes/drop) of medium under mineral oil. The embryos were cultured at 39°C in an atmosphere composed of 7% O2, 5% CO2, 88% N2 with saturated humidity. The medium was changed every 48 h. At the end of incubations, the embryos were evaluated for the morphological stages of development with an inverted microscope (Diaphot; Nikon, Tokyo, Japan).
Differential staining of blastocysts
Embryo quality (Fukui et al., Reference Fukui, McGowan, James, Pugh and Tervit1991; O'Hara et al., Reference O'Hara, Forde, Kelly and Lonergan2014) was evaluated by total cell number (TCN), inner cell mass (ICM) cell number, and trophectoderm (TE) cell number. For this purpose, TCN, ICM and TE cell numbers were determined in day-8 blastocysts as described by Thouas et al. (Reference Thouas, Korfiatis, French, Jones and Trounson2001). Briefly, before the fixation and staining steps, embryos were exposed to 1 µg/ml RNase for 1 h at 38.5°C. After incubation, blastocysts were washed in 0.01 M PBS containing 1 mg/ml PVP, stained with 100 µg/ml PI in PBS-PVP with 0.2% (v/v) Triton X-100 for 30 s, repeatedly washed in PBS-PVP, and then fixed in PBS-PVP containing 4% (w/v) paraformaldehyde and 1 µg/ml Hoechst 33342 for 15 min. After repeated washing in PBS-PVP, blastocysts were placed on microscope slides with cover slips containing a small drop of glycerol. ICM and TE cell numbers were visualized in an Olympus BX40 epifluorescence microscope with a fluor objective equipped with a 330–490 nm excitation filter and a 420–520 nm emission filter to distinguish TE cell nuclei (red) from ICM (blue), (Fig. 4). In total, 49 day-8 blastocysts were used in three replicates.

Figure 4 Differential staining of blastocysts. ICM (blue) and TE (red) cells were stained with Hoechst 33342 and propidium iodide respectively.
Statistical analyses
A completely randomized block design was used. Statistical models included the random effect of block (n = 3 or 6 depending on the experiment) and the fixed effect of treatment (0 versus 20 verus 40 versus 60 pM ghrelin). CC number, cumulus area and GDI were analyzed with a mixed model (SAS Institute, Cary, NC, USA). Cumulus area before IVM (T0) was used as covariate in the analysis of cumulus expansion. Oocyte nuclear maturation, oocyte viability, DNA damage and the rates of apoptosis, cleavage, blastocysts and hatching were analyzed by logistic regression using the GENMOD procedure (SAS Institute). TCN per embryo, ICM and TE cell numbers were analyzed by Poisson regression using the GENMOD procedure (SAS Institute) with Poisson distribution and log link. CC number, cumulus area, TCN per blastocyst, ICM, TE and ICM/TE cell ratio are expressed as mean ± standard error of the mean (SEM). Oocyte nuclear maturation, oocyte viability, DNA damage and the rates of apoptosis, cleavage, blastocyst and hatching are expressed as percentage. Statistical significance was set at P < 0.05.
Results
Effect of ghrelin on oocyte nuclear maturation
In Experiment 1, nuclear maturation was not significantly different in oocytes matured with 0, 20, 40 and 60 pM acylated ghrelin concentrations (Table 1; P ≥ 0.05).
Table 1 Effect of ghrelin on meiotic maturation of bovine oocytes in vitro
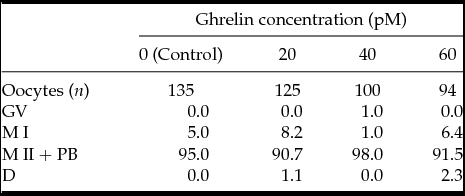
Differences among treatments within each category were not significant (P > 0.05).
Bovine COC were incubated in IVM medium with 20, 40 and 60 pM ghrelin during 24 h. Data are expressed as percentage. MI, metaphase I; M II + PB, metaphase II and the first polar body; GV, germinal vesicle; D, degenerate.
Effect of ghrelin on cumulus expansion and CC number in COC
In Experiment 2, differences in cumulus expansion area after IVM were not significant in COC matured with 0, 20, 40 or 60 pM acylated ghrelin concentrations [523,541 ± 40,748 µm² (n = 38); 448,626 ± 39,196 µm² (n = 48) 462,383 ± 40,748 µm² (n = 38); 461,749 ± 42,443 µm² (n = 35), respectively]. CC number per COC either before (8341.60 ± 1497.87; n = 37) or after IVM were similar at any acylated ghrelin concentration tested [9409.50 ± 1497.87 (n = 32); 8395.8 ± 1497.87 (n = 30); 9434.3 ± 1497.87 (n = 33); 11.215 ± 1497.87 (n = 32) for 0, 20, 40 or 60 pM acylated ghrelin, respectively; P ≥ 0.05].
Effect of ghrelin on CC viability
In Experiment 3, CC viability was significantly higher in COC matured without acylated ghrelin (Control 0 pM acylated ghrelin; P < 0.05) as compared with treated ones [76.75% (307/400); 70.50% (282/400); 65.25% (261/400) and 44.75% (179/400) for Control, 20, 40 and 60 pM acylated ghrelin, respectively; P < 0.05] (Fig. 5). Statistical differences were found between Control and 20 pM, Control and 40 pM, Control and 60, 20 and 60 pM, and 40 and 60 pM. No differences were found between 20 and 40 pM acylated ghrelin (Fig. 5).

Figure 5 Effect of ghrelin on cumulus cells (CC) viability after IVM. Cumulus cells viability was evaluated with FDA/trypan blue assay. COCs were treated during IVM with different ghrelin concentrations, and harvested after 24 h of treatment. Results are expressed as percentage of cell viability from three independent experiments. a – c Columns without a common superscript differed (P < 0.05).
Effect of ghrelin on CC apoptosis
Data of apoptosis in CC exposed to acylated ghrelin concentrations for 24 h are presented in Table 2 (Experiment 4). The frequency of live cells diminished when acylated ghrelin was added to the medium. However, differences were not statistically significant among acylated ghrelin treatments. Results showed an enhancement of apoptotic cells when CC were treated with 20, 40 and 60 pM acylated ghrelin (P < 0.05). Moreover, addition of such acylated ghrelin concentrations increased the frequency of necrotic cells in a nearly dose-dependent manner (P < 0.05).
Table 2 Analysis of apoptosis in cumulus cells (CC) exposed to different ghrelin concentrations measured by annexin-V–FITC/PI
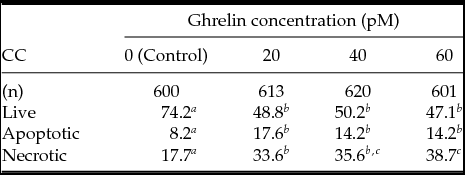
a-c Lines without a common superscript differed (P < 0.05).
COC were exposed to 20, 40, and 60 pM ghrelin during IVM.
Apoptotic early + late apoptotic CC.
Data are presented as percentage.
Effect of ghrelin on CC DNA integrity
Data of comet assay obtained in CC exposed to different ghrelin concentrations for 24 h (Experiment 5), the proportion of damaged nucleoids and GDI data are presented in Table 3. The frequencies of damaged CC were higher in the presence of 20, 40 and 60 pM acylated ghrelin than in the Control (P < 0.05). However, the frequency of DNA damage significantly increased when CC were exposed to 60 pM as compared with 20 and 40 pM acylated ghrelin. GDI increased in the presence of 20 and 60 pM ghrelin with respect to the Control (P < 0.05). No significant differences were found in CC treated with 40 pM acylated ghrelin versus the Control.
Table 3 DNA damage in cumulus cells exposed to ghrelin during 24 h measured by the comet assay
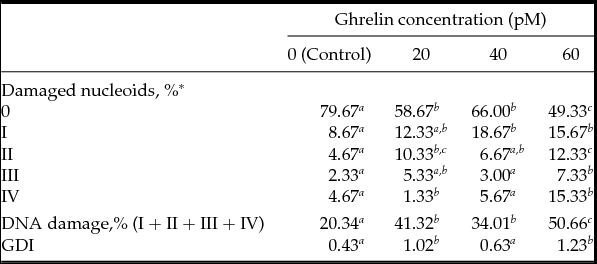
a-c Lines without a common superscript differed (P < 0.05). *0–IV indicate grades of DNA damage as percentage of pooled data from three independent experiment.
**Genetic damage index (GDI) = (1 × I + 2 × II + 3 × III + 4 × IV)/(0 + I + II + III + IV).
COC were matured during 24 h (three replicates, 80 COC per replicate, 20 COC per treatment) and at least 200 cells were analyzed per treatment from three replicates performed on different days.
Effect of acylated ghrelin concentrations during IVM on subsequent embryo development
In Experiment 6, 1553 oocytes in six replicates were matured and fertilized in vitro (Table 4). There were not significant differences in cleavage rate when acylated ghrelin was added to IVM medium at any concentration. However, there was a diminution in the percentages of blastocyst yield (P ˂ 0.05) in oocytes matured with 40 or 60 pM acylated ghrelin. No differences were found between the Control and 20 pM acylated ghrelin. Blastocyst yields with 20, 40 or 60 pM acylated ghrelin were not statistically different. Hatching rates decreased when oocytes were matured with 20, 40 or 60 pM ghrelin (P ˂ 0.05) (Table 4).
Table 4 Developmental capacity of cattle oocytes matured in vitro with different ghrelin concentration
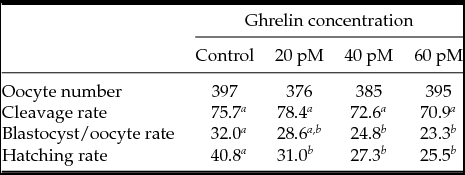
a–c Values with different letters within each line differed (P < 0.05).
Bovine COC were incubated in IVM medium alone (0 pM ghrelin), or 20, 40 or 60 pM ghrelin. Cleavage rates were recorded 48 h after insemination. Data reported for the development to the blastocyst stage included those embryos that progressed to the expanded or hatched blastocyst stages after 8 days in culture. All values for cleavage, development and hatching rates are expressed as percentage (1265 COC in six replicates on different days).
Effect of acylated ghrelin concentrations on embryo quality during IVM
TCN per blastocyst (Experiment 7) was higher in the Control compared with any of the acylated ghrelin concentrations tested (P < 0.01; Table 5). Addition of 20, 40 or 60 pM acylated ghrelin decreased TCN per blastocyst in a dose-dependent manner. Moreover, both ICM cell number and ICM/TE cell ratio were significantly higher when COC were matured without ghrelin in the IVM medium (P < 0.01; Table 5).
Table 5 Effect of different ghrelin concentrations during in vitro maturation on cell number per blastocyst
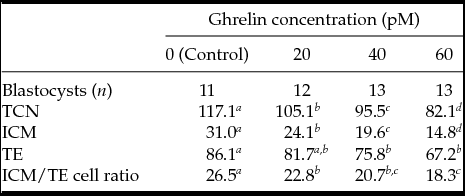
a–c Values with different letters within each column differed (P < 0.05).
TCN, total cell number; TE, trophectoderm; ICM, inner cell mass of day 8 blastocysts developed from oocytes matured in IVM medium alone (0 pM ghrelin) or with 20, 40 and 60 pM ghrelin.
Discussion
In the present study, we evaluated the effect of acylated ghrelin supplementation at physiological doses on bovine COC during IVM. The effect of 20, 40 or 60 pM acylated ghrelin concentrations was tested in IVM of bovine oocytes using oocyte nuclear maturation rate, cumulus expansion area, viability, apoptosis, and DNA damage of CC as quality biomarkers. Additionally, embryo development was analyzed. The results demonstrated that although acylated ghrelin did not affect oocyte nuclear maturation and cumulus expansion area, it induced cell death, apoptosis and DNA damage in CC. For all assays, the damage increased as a function of the concentration employed. The greatest damage was observed in CC exposed to 60 pM acylated ghrelin. Furthermore, the results demonstrated that while the hormone was not able to affect cleavage rates, the percentage of blastocyst yield, hatching and embryo quality decreased with all acylated ghrelin concentrations in the IVM medium.
Ghrelin is synthesized predominantly in the stomach of non-ruminant animals (Kojima et al., Reference Kojima, Hosoda, Date, Nakazato, Matsuo and Kangawa1999) and in abomasum of ruminants (Hayashida et al., Reference Hayashida, Murakami, Mogi, Nishihara, Nakazato, Mondal, Horii, Kojima, Kangawa and Murakami2001; Thouas et al., Reference Thouas, Korfiatis, French, Jones and Trounson2001). This hormone is a 28-amino acid peptide and was initially characterized as an endogenous ligand for growth hormone secretagogue receptor (GHS-R). Apart from having a wide range of physiological roles in many species, including the regulation of food intake and the stimulation of growth hormone secretion (Kojima et al., Reference Kojima, Hosoda, Date, Nakazato, Matsuo and Kangawa1999; Kojima & Kangawa, Reference Kojima and Kangawa2005), it has been postulated that acylated ghrelin participates in hypothalamic-pituitary-gonadal axis regulation (Barreiro & Tena-Sempere, Reference Barreiro and Tena-Sempere2004; Zhang et al., Reference Zhang, Lei, Su and Chen2008). Ghrelin gene is expressed in hypothalamus (Kojima et al., Reference Kojima, Hosoda, Date, Nakazato, Matsuo and Kangawa1999; Cowley et al., Reference Cowley, Smith, Diano, Tschöp, Pronchuk, Grove, Strasburger, Bidlingmaier, Esterman, Heiman, García-Segura, Nillni, Mendez, Low, Sotonyi, Friedman, Liu, Pinto, Colmers, Cone and Horvath2003), pituitary (Korbonits et al., Reference Korbonits, Bustin, Kojima, Jordan, Adams, Lowe, Kangawa and Grossman2001), immune cells (Hattori et al., Reference Hattori, Saito, Yagyu, Jiang, Kitagawa and Inagaki2001), lung (Volante et al., Reference Volante, Fulcheri, Allia, Cerrato, Pucci and Papotti2002) and kidney (Mori et al., Reference Mori, Yoshimoto, Takaya, Hosoda, Ariyasu, Yahata, Mukoyama, Sugawara, Hosoda, Kojima, Kangawa and Nakao2000). Also, the expression of ghrelin and its receptor has been reported in several reproductive tissues including placenta (Gualillo et al., Reference Gualillo, Caminos and Blanco2001), testis (Barreiro et al., Reference Barreiro, Gaytan, Caminos, Pinilla, Casanueva, Aguilar, Dieguez and Tena-Sempere2002; Tena-Sempere et al., Reference Tena-Sempere, Barreiro, Gonzalez, Gaytan, Zhang, Caminos, Pinilla, Casanueva, Dieguez and Aguilar2002) and ovary (Caminos et al., Reference Caminos, Tena-Sempere, Gaytan, Sanchez-Criado, Barreiro, Nogueiras, Casanueva, Aguilar and Dieguez2003; Gaytan et al., Reference Gaytan, Barreiro, Chopin, Herington, Morales, Pinilla, Casanueva, Aguilar, Dieguez and Tena-Sempere2003) of chicken, rat, pig, sheep, cattle and human (Caminos et al., Reference Caminos, Tena-Sempere, Gaytan, Sanchez-Criado, Barreiro, Nogueiras, Casanueva, Aguilar and Dieguez2003; Sirotkin et al., Reference Sirotkin, Grossmann, María-Peon, Roa, Tena-Sempere and Klein2006; Du et al., Reference Du, Xilingaowa, Wang, Li, Zhao and Siqingaowa2009; Rak et al., Reference Rak, Szczepankiewicz and Gregoraszczuk2009; Deaver et al., Reference Deaver, Hoyer, Dial, Field, Collier and Rhoads2013). Despite that, information about the effect of acylated ghrelin on bovine oocyte maturation and early embryo development is scarce and contradictory.
CC play a critical role in the oocyte maturation process (Reference KrisherKrisher, 2004). The oocyte is surrounded and connected by gap junctions with CC, establishing a structural and functional unit called COC. The COC plays an important role in the regulation of nuclear and cytoplasmic oocyte maturation (Tanghe et al., Reference Tanghe, Van Soom, Nauwynck, Coryn and de Kruif2002). CC integrity is considered a good predictor of the ability for further oocyte development (Yuan et al., Reference Yuan, Van Soom, Leroy, Dewulf, Van Zeveren, de Kruif and Peelman2005). Cumulus cell–oocyte communication allows the correct performance of both cell types. Because of this interdependence, any process of cell damage suffered by CC impacts directly into the germ cell itself. Several studies suggest a correlation between CC apoptosis and cleavage rate (Lee et al., Reference Lee, Joo, Na, Yoon, Choi and Kim2001). Moreover, Corn and colleagues (Reference Corn, Hauser-Kronberger, Moser, Tews and Ebner2005) showed that a high percentage of CC apoptosis deteriorates the quality of oocytes, resulting in a decreased ability for later embryonic development to the blastocyst stage. Apoptosis occurs when cell damage, including damage to genetic materials, exceeds the capacity of cell repair. DNA damage may be the end product of apoptosis, or responsible for its development (Wang, Reference Wang2001). Our results are in agreement with this suggestion. It is interesting to note that the presence of 60 pM ghrelin during IVM had a detrimental effect on bovine CC integrity, increasing DNA damage and apoptosis rate and decreasing CC viability. Our findings are consistent with that described by several authors, who observed that high ghrelin concentrations in the IVM medium deteriorate pig and sheep oocytes (Suzuki et al., Reference Suzuki, Sasaki, Shimizu, Matsuzaki, Hashizume and Kuwayama2010; Wang et al., Reference Wang, Lin and Yu2013). Something worth note is that the working concentrations in the present study were at a physiological concentration, trying to achieve a concentration similar to cow at different energy balance (Bradford & Allen, Reference Bradford and Allen2008), and previous studies (Suzuki et al., Reference Suzuki, Sasaki, Shimizu, Matsuzaki, Hashizume and Kuwayama2010; Wang et al., Reference Wang, Lin and Yu2013) were with doses at a pharmacological concentration. Previous reports suggest that ghrelin could play an important role in programmed cell death. In this sense, Kheradmand and colleagues (Arash et al., Reference Arash, Omid and Masoud2014) indicate that ghrelin is involved in the control of gonadal functions, apoptosis and proliferation in the rat ovary. Furthermore, Rui-Xia Bai and colleagues (Reference Rui-Xia, Peng and Gui-fang2013) have suggested that ghrelin could have a potential regulatory role in apoptotic function in sheep oocytes.
Oocyte maturation is a critical phenomenon which depends on the ability of subsequent embryonic development (Krisher et al., 2004). Matured oocytes can be fertilized, develop to the blastocyst preimplantation stage and give viable fetuses (Moor & Trounson, Reference Moor and Trounson1977). Maturation is the result of a complex interaction among the oocyte, CC and the environment that surrounds them (Moor et al., Reference Moor, Dai, Lee and Fulka1998). Although the presence of 20, 40 or 60 pM acylated ghrelin in the microenvironment surrounding COC did not affect nuclear maturation, it caused CC damage and affected the capacity of the oocyte to undergo embryo development, as evidenced by decreasing blastocyst rates. Results of a study performed in pig oocytes showed that high ghrelin concentrations during IVM inhibited oocyte nuclear maturation and affected oocyte cytoskeletal distribution and density (Suzuki et al., Reference Suzuki, Sasaki, Shimizu, Matsuzaki, Hashizume and Kuwayama2010). Moreover, Seino and colleagues (Seino et al., Reference Seino, Saito, Kaneko, Takahashi, Kawachiya and Kurachi2002) demonstrated that increased CC DNA damage during human oocyte maturation reduced fertility rate and blastocyst quality assessed as fragmentation of the embryo mass. In the present study, ghrelin supplementation during bovine oocyte IVM decreased embryo quality assessed as hatching rates and cell number/blastocyst. Cell numbers in TE and ICM, or in both cell populations of blastocysts, are indicators of embryo growth and viability (Van Soom et al., Reference Van Soom, Ysebaert and de Kruif1997; Kawamura et al., Reference Kawamura, Sato, Fukuda, Kodama, Kumegai, Tanikawa, Nakamura, Honda, Sato and Tanaka2003). In our study, ghrelin decreased TCN of blastocysts as a result of a reduction of both ICM and TE cell numbers. Similar results were observed when ghrelin was added to mouse embryo culture medium. Kawamura and colleagues (Reference Kawamura, Sato, Fukuda, Kodama, Kumegai, Tanikawa, Nakamura, Honda, Sato and Tanaka2003) found that ghrelin inhibited mouse preimplantation embryo development from two-cell stage embryo to the blastocyst, fully expanded blastocyst, and hatched blastocyst in vitro in a dose-dependent manner. Dovolou and colleagues (Reference Dovolou, Messinis, Periquesta, Dafopoulos, Gutierrez-Adan and Amiridis2014) suggested that incubation of bovine COC with 237.38 pM ghrelin for 24 h caused oocyte overmaturation, but oocytes matured for 18 h under the same conditions reached MII, the cumulus layer expanded more that the Control, and differences in the expression of various genes with respect to the controls were detected. Dovolou et al. (Reference Dovolou, Messinis, Periquesta, Dafopoulos, Gutierrez-Adan and Amiridis2014), however, observed that the formed blastocysts had a higher hatching rate compared with the Control. It should be noted that we found a negative ghrelin-induced effect during IVM with ghrelin concentrations 11.8 lower than those used by Dovolou et al., (Reference Dovolou, Messinis, Periquesta, Dafopoulos, Gutierrez-Adan and Amiridis2014), suggesting a high sensitivity of the in vitro system to even lower ghrelin concentrations than the physiological ones measured in animals.
Our results are in agreement with the points made by others author, who suggest that certain metabolic hormones and neuropeptides could act as responsible nutritional signals regulating reproductive function (Evans & Anderson, Reference Evans and Anderson2012; Lorenzi et al., Reference Lorenzi, Meli, Marzioni, Morroni, Baragli, Castellucci, Gualillo and Muccioli2009; Tena-Sempere, Reference Tena-Sempere2008). Our study also highlights the importance of acylated ghrelin on bovine reproduction. We thus suggest that this metabolic hormone could function as a signal that prevents the progress to reproductive processes in which energy nutrients into milk production and survival of breeding are prioritized.
In conclusion, the presence of ghrelin in IVM medium induces CC DNA damage and apoptosis during bovine oocyte maturation in vitro, and impairs subsequent early embryo development. Consequently, the results suggest that the action of low ghrelin concentrations during oocyte maturation may have a negative influence on cytoplasmic maturation affecting negatively early embryonic development.
Acknowledgements
We are grateful to the staff of Frigorífico Gorina S.A. for providing bovine ovaries, and to Centro de Inseminación Artificial La Elisa S.A. (CIALE) for providing bovine frozen semen. We also thank A. Di Maggio for manuscript correction.
Funding
This work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica de la República Argentina (PICT BID 1972–2013), Ministerio de Ciencia, Tecnología e Innovación Productiva de la Nación Argentina.












