Introduction
Successful cryopreservation of mammalian oocytes has numerous practical, economical and ethical benefits, which may affect animal breeding programmes and assisted conception in humans positively (Zhou et al., Reference Zhou, Al Naib, Sun and Lonergan2010). However, one of the greatest challenges to reproductive cryobiologists today is to develop an efficient cryopreservation method for human and domestic animal oocytes (Rojas et al., Reference Rojas, Palomo, Albarracin and Mogas2004) and specifically in porcine, as a model for animal breeding or for human studies, in which oocyte cryopreservation has had limited success in practice (Isachenko et al., Reference Isachenko, Soler, Isachenko, Perez-Sanchez and Grishchenko1998; Dobrinsky, Reference Dobrinsky2002).
Chilling injury is the main obstacle to successful oocyte cryopreservation, and affects morphological and functional components of the cells, such as membrane integrity (Hyttel et al., Reference Hyttel, Vajta and Callesen2000), and leads to cytoskeleton disorganization associated with abnormal microtubules, spindle and chromosome abnormalities (Liu et al., Reference Liu, Sun, Li, Jiao and Wang2003; Rojas et al., Reference Rojas, Palomo, Albarracin and Mogas2004; Vajta & Kuwayama, Reference Vajta and Kuwayama2006; Wu et al., Reference Wu, Rui, Dai, Zhang, Ju and Xie2006; Succu et al., Reference Succu, Leoni, Berlinguer, Madeddu, Bebbere, Mossa, Bogliolo, Ledda and Naitana2007). These abnormalities include premature extrusion of the cortical granules that then leads to an abrupt hardening of the zona pellucida and a decrease in sperm penetrability (Carroll et al., Reference Carroll, Depypere and Matthews1990; Mavrides & Morroll; Reference Mavrides and Morroll2005), damage of key molecules involved in cell cycle (Succu et al., Reference Succu, Leoni, Berlinguer, Madeddu, Bebbere, Mossa, Bogliolo, Ledda and Naitana2007) and altered gene expression (Succu et al., Reference Succu, Bebbere, Bogliolo, Ariu, Fois, Leoni, Berlinguer, Naitana and Ledda2008). Therefore, cryopreservation procedures with extreme cooling rates such as ultra-rapid freezing and vitrification could be alternative procedures to prevent chilling injuries.
Vitrification is the rapid cooling of cells in liquid medium to avoid ice crystal formation and this process was first used in mouse embryos by Rall & Fahy (Reference Rall and Fahy1985). Nevertheless, few advancements have been achieved with oocytes and offspring have been produced using vitrified oocytes only in species such as cow (Vieira et al., Reference Vieira, Mezzalira, Barbieri, Lehmkuhl, Rubin and Vajta2002), horse (Maclellan et al., Reference Maclellan, Carnevale, Coutinhoda Silva, Scoggin, Bruemmer and Squires2002), cat (Gómez et al., Reference Gómez, Kagawa, Pope, Kuwayama, Leibo and Dresser2007), mouse (Bos-Mikich et al., Reference Bos-Mikich, Wood, Candy and Whittingham1995; Aono et al., Reference Aono, Abe, Hara, Sasada, Sato and Yoshida2005), human (Fadini et al., Reference Fadini, Brambillasca, Renzini, Merola, Comi, De Ponti and Dal Canto2009). In porcine, progress has been made but to date there has been no report of offspring obtained from cryopreserved porcine oocytes (Somfai et al., Reference Somfai, Dinnyés, Sage, Marosán, Carnwath, Ozawa, Kikuchi and Niemann2006, Reference Somfai, Noguchi, Kaneko, Nakai, Ozawa, Kashiwazaki, Egerszegi, Rátky, Nagai and Kikuchi2010; Gupta et al., Reference Gupta, Uhm and Lee2007; Shi et al., Reference Shi, Jin, Kim, Mohana Kumar, Balasubramanian and Choe2007; Huang et al., Reference Huang, Li, Zhao, Li, Han, Chen, Xiao, Wu, Jiang, Hu and Liu2008; Pribenszky et al., Reference Pribenszky, Du, Molnár, Harnos and Vajta2008; Fu et al., Reference Fu, Shi, Zhang, Zhao, Yan, Hou, Zhou, Fan, Suo, Wusiman, Wang and Zhu2009; Ogawa et al., Reference Ogawa, Ueno, Nakayama, Matsunari, Nakano, Fujiwara, Ikezawa and Nagashima2010).
Vitrification can only be induced with unsuitably high concentrations of cryoprotectants and/or with an extreme increase in the cooling and warming rates (Vajta & Kuwayama, Reference Vajta and Kuwayama2006). Vitrification with high cooling rates decreases chilling injury, i.e. damage of the intracellular lipid droplets and the cytoskeleton (Fu et al., Reference Fu, Shi, Zhang, Zhao, Yan, Hou, Zhou, Fan, Suo, Wusiman, Wang and Zhu2009), and alleviates the adverse effects that are generally associated with high concentrations of cryoprotectants. Also, the toxicity and the osmotic pressure created by high cryoprotectant concentrations are highly damaging to cells; it is therefore crucial to restrict cryoprotectants to low concentrations (Yavin et al., Reference Yavin, Aroyo, Roth and Arav2009). One characteristic of vitrification is that as the cooling rate chosen increases, the cryoprotectant concentration can be lowered and vice versa (Vajta & Kuwayama, Reference Vajta and Kuwayama2006; Yavin & Arav, Reference Yavin and Arav2007).
The common approaches that attempt to deal with low success rates of cryopreservation for porcine oocytes are: (i) to modify cryopreservation procedures (cryoprotectants and cooling–warming rates); and (ii) to modify the cells themselves to make them more cryopreservable (Zhou & Li, Reference Zhou and Li2009). Most studies have used a ‘trial and error’ approach to examine the effects of various strategy methods (Fujihira et al., Reference Fujihira, Kishida and Fukui2004; Somfai et al., Reference Somfai, Dinnyés, Sage, Marosán, Carnwath, Ozawa, Kikuchi and Niemann2006; Wu et al., Reference Wu, Rui, Dai, Zhang, Ju and Xie2006; Gupta et al., Reference Gupta, Uhm and Lee2007; Shi et al., Reference Shi, Jin, Kim, Mohana Kumar, Balasubramanian and Choe2007; Huang et al., Reference Huang, Li, Zhao, Li, Han, Chen, Xiao, Wu, Jiang, Hu and Liu2008) or to apply cellular modifications such as cytoskeleton stabilizers (Isachenko et al., Reference Isachenko, Soler, Isachenko, Perez-Sanchez and Grishchenko1998; Sun et al., Reference Sun, Lai, Wu, Park, Day, Prather and Schatten2001; Fujihira et al., Reference Fujihira, Nagai and Fukui2005; Shi et al., Reference Shi, Zhu, Zhang, Wang, Tang, Hou and Tian2006; Fu et al., Reference Fu, Shi, Zhang, Zhao, Yan, Hou, Zhou, Fan, Suo, Wusiman, Wang and Zhu2009), lipid content (Hara et al., Reference Hara, Abe, Kumada, Aono, Kobayashi, Matsumoto, Sasada and Sato2005; Park et al., Reference Park, Kwon, Han and Niwa2005; Somfai et al., Reference Somfai, Kashiwazaki, Ozawa, Nakai, Maedomari, Noguchi, Kaneko, Nagai and Kikuchi2008), or high hydrostatic pressure (Du et al., Reference Du, Pribenszky, Molnar, Zhang, Yang, Kuwayama, Pedersen, Villemoes, Bolund and Vajta2008; Pribenszky et al., Reference Pribenszky, Du, Molnár, Harnos and Vajta2008). At the same time, other studies have made use of rigorous mathematical formulations to define and describe the behaviour of oocytes at low temperature, as well as to design cryoprotectants addition and dilution procedures in a calculated manner (Mazur, Reference Mazur1963; Hunter et al., Reference Hunter, Bernard and Fuller1992; McGrath, Reference McGrath1997). One approach has been to reduce the cytotoxic effects of cryoprotectants used for vitrification of oocytes (Takahashi et al., Reference Takahashi, Igarashi, Doshida, Takahashi, Nakahara, Tezuka and Kurachi2004). However, the mechanisms of toxicity of vitrifiable solutions have not been elucidated (Fahy et al., Reference Fahy, Wowk, Wu and Paynter2004). Partly for this reason, it is not possible at present to predict the toxicity of either individual cryoprotective agents or mixtures thereof, and there is a virtually unlimited number of possible mixtures to choose from in composing candidate vitrification solutions. Fahy et al. (Reference Fahy, Wowk, Wu and Paynter2004) showed a compositional variable to rationally account for the toxicity of many complex cryoprotectant mixtures; the concentrations of these mixtures were just sufficient to permit vitrification at slow cooling rates at both ambient and elevated pressures. Based on this calculation, Fahy et al. (Reference Fahy, Wowk, Wu and Paynter2004) were able to predict and successfully test several vitrification solutions with low toxicity. As an example, VM3 solution has been used successfully to vitrify a variety of different living systems such as rat tissues (Pichugin et al., Reference Pichugin, Fahy and Morin2006; de Graaf et al., Reference de Graaf, Draaisma, Schoeman, Fahy, Groothuis and Koster2007) and rabbit tissues (Fahy et al., Reference Fahy, Wowk, Wu and Paynter2004). However, VM3 solution has only been used to vitrify mouse oocytes and the rate of development to blastocysts was 80% of the rate of untreated control oocytes (Fahy et al., Reference Fahy, Wowk, Wu and Paynter2004).
Therefore, the purpose of the present study was to optimize the concentration of VM3 solution needed to vitrify porcine oocytes and to evaluate the effects on viability, chromosomal organization and cortical granule distribution of oocytes at the metaphase II stage.
Materials and methods
Chemicals and supplies
All chemicals were purchased from Sigma–Aldrich Química SA (Madrid, Spain) unless otherwise stated. VM3 solution and ice blockers SuperCool X-1000 and SuperCool Z-1000 were purchased from 21st Century Medicine Inc. (Fontana, CA, USA). Open pulled straws (OPS) were purchased from LEC Instruments (Victoria, Australia).
Collection of oocytes
Porcine ovaries were obtained from a local abattoir and transported to the laboratory at 30–35°C in Dulbecco's phosphate-buffered saline (DPBS) within 2 h of slaughter. Cumulus–oocyte complexes (COCs) were aspirated from 2 to 6 mm follicles using an 18-gauge needle attached to a 5 ml syringe. COCs with three or more layers of cumulus cells and a homogeneous cytoplasm were selected for the experiment.
In vitro maturation
Selected oocytes were washed three times in DPBS supplemented with 36 μg/ml pyruvate, 50 μg/ml gentamycin and 0.5 mg/ml bovine serum albumin (BSA). The basic maturation medium was NCSU37 (Petters & Wells, Reference Petters and Wells1993), supplemented with 10 IU/ml human chorionic gonadotropin (hCG) (Chorulon, Intervet International B.V., Boxmeer, Holland), 10 IU/ml equine chorionic gonadotropin (eCG) (Folligon, Intervet International B.V., Boxmeer, Holland) and 10% (v/v) fetal calf serum (FCS). Groups of 45–50 oocytes were cultured in 500 μl maturation medium at 38.5°C under 5% CO₂ in air. Oocytes were shipped in maturation medium for 20–22 h and then transferred to maturation medium without gonadotropins for an additional 20–22 h.
Vitrification and thawing
Only morphologically high quality oocytes, as determined by uniform granular homogeneously distributed cytoplasm surrounded by expanded layers of cumulus cells, were used for vitrification. The vitrification was carried out in three steps using VM3 solution (Fahy et al., Reference Fahy, Wowk, Wu and Paynter2004) and VM3 optimized solution. Both formulations are described in Experimental design. Groups of 10 oocytes were first pipetted into a 50-μl droplet that contained 9.2% of medium for 3 min. Next, oocytes were rinsed through a 50-μl droplet that contained 26% of medium for 1 min. Finally, oocytes were transfer into a 10-μl droplet that contained 90% of medium for 1 min and loaded into the OPS by negative pressure and then the straws were plunged into liquid nitrogen. The warming solution was TCM199 medium that contained various concentrations of sucrose. Stepwise warming was performed by placing the pulled end of the straws directly into a 1.25 M sucrose solution for 2 min. COCs were then rinsed with other solutions of 0.5, 0.25 and 0.125 M sucrose, each for 30 s and finally rinsed twice in TCM199 medium. Later, oocytes were denuded using hyaluronidase (300 IU/ml) in DPBS for 10 min and repeated pipetting.
Experimental design
Experiment 1. Apparent vitrification of cryoprotectants concentration and cryo-scanning electron microscopy results
This experiment was aimed at examining the minimum concentration of cryoprotectant present in the VM3 solution needed to vitrify using an OPS device. VM3 solution (v/v, 7.6 M) consists of penetrating solutes dimethyl sulfoxide (Me2SO, 22.3% w/v), ethylene glycol (EG, 16.84% w/v), formamide (12.86% w/v) and non-penetrating solutes polyvinyl pyrrolidone K12 (PVP, 7% w/v), SuperCool X-1000 (1% w/v) and SuperCool Z-1000 (1% w/v) (Fahy et al., Reference Fahy, Wowk, Wu and Paynter2004). The vitrification state was determined by loading a 10-μl droplet that contained a decreasing solution concentration from 7.6 M to 5.6 M into the OPS by negative pressure, and then plunging into liquid nitrogen; the appearance of opacity (or visible ice formation) with cooling solutions at –196°C was observed. If there was no observable opacity, it was noted as vitrification.
Cryo-scanning electron microscopy (cryo-SEM) was used to examine the ultrastructure of oocytes in the VM3 solution (7.6 M) with and without ice blockers and in VM3 optimized solution (5.6 M) with ice blockers. Working in liquid nitrogen, the OPS straw was broken manually, the plastic removed, and a cylinder of the vitrified medium mounted in a mechanical grip holder. The grip holder was transferred to an Oxford HT 1500F CryoSystem chamber attached to the microscope (JEOL Scanning Microscope 6320F, Tokyo, Japan). Once the sample was inside the chamber, the sample was fractured to get a fresh clean surface to be examined. The temperature of the sample was raised by heating the holder to −92°C for 5 min in order to sublimate free water in the solid state lakes, followed by a temperature decrease to −130°C to stabilize the sample. The coated sample was then transferred to the microscope chamber where it was analyzed at a temperature range of −125 to −135°C. Digital images were collected at 5 kV.
Experiment 2. Viability, chromosomal alignment and cortical granules distribution
Immediately after in vitro maturation, COCs were divided randomly into three groups: (1) control group: fresh COCs; (2) VM3 solution group: COCs vitrified with VM3 solution; and (3) VM3 optimized solution group: COCs vitrified with the minimum concentration of cryoprotectant present in the VM3 solution needed to vitrify. For the three-step process, equilibrated, vitrified and warmed procedures were performed as described previously in Materials and methods. The viability, correct chromosomal configuration and cortical granules distribution were compared between groups.
Viability of oocytes was evaluated by fluorescent dye Cell Trace Calcein Red-Orange AM (Invitrogen). Oocytes were incubated in calcein red-orange (2 μM) for 10 min in the dark. After this step, cells were washed three times in DPBS and then observed under a stereomicroscope with fluorescence using the DSRed filter (558 nm for excitation and 583 nm for emission). Live oocytes show red fluorescence in their cytoplasm, whereas dead oocytes are non-fluorescent. Propidium iodide (PI) was used to evaluate the nuclear status of oocytes. Before staining, the oocytes were fixed (4% paraformaldehyde) for 45 min under refrigeration (4°C). Then, oocytes were washed with PBS, incubated with PI (1 mg/ml) and kept in the dark for 15 min. Finally, the oocytes were again washed in DPBS and mounted for evaluation under a fluorescence microscope (filters: 400–500 nm for excitation and LP 515 nm for emission). Chromosomal organization was classified in two groups: (1) compact chromosomes arranged at the equator of the structure (normal alignment metaphase-II); or (2) dispersed, decondensed or absence of the chromosomes (misalignment metaphase II). For assessment of cortical granules (CG), the distribution of zonae pellucidae (ZP)-free oocytes was used. The ZP were removed by treating the oocytes with pronase (0.5% w/v) for 2–3 min. Oocytes without ZP were fixed for 45 min at 4°C (4% w/v paraformaldehyde), then washed with permeabilization solution (0.02% v/v Triton X-100), for 30 min at 37°C. After this step, oocytes were incubated with 100 μg/ml lens culinaris fluorescein isothiocyanate (FITC–LCA), for CG staining for 15 min, at 37°C, in the dark. Next, the oocytes were washed with DPBS that contained 7.5% BSA and were mounted on glass slides for evaluation. Distribution of the CGs was observed under a confocal microscope (Leica TCS SL Confocal System). Finally, the oocytes were classified into two groups: (1) monolayer of fluorescence beneath the oolemma; or (2) scattered fluorescence in the cytoplasm and only a little fluorescence in the cytoplasm.
Statistical analysis
The data for viability, chromosomal alignment and distribution patterns of cortical granules were expressed as mean ± standard deviation (SD) and analysed using one-way analysis of variance (ANOVA). Statistical analyses were performed using a commercially available statistics package (Statgraphics Plus, Version 5.1, STSC Inc., Rockville, MD, USA). Differences were considered to be significant at a level of P < 0.05.
Results
Experiment 1
As shown in Table 1, the minimum total cryoprotectant concentration present for VM3 solution for apparent vitrification was 5.6 M (v/v) when it was combined with the OPS method. VM3 optimized solution consisted of 12.63% (w/v) ethylene glycol, 9.65% w/v formamide, 16.7% (w/v) dimethyl sulfoxide, 5.25% (w/v) PVP, and 0.75% (w/v) final concentrations of commercially available SuperCool X-1000 and SuperCool Z-1000 ice blockers.
Table 1 Apparent vitrification matrix of the reduction in total cryoprotectant concentration present in VM3 solution and using open pulled straws
CPA, cryoprotectant. The single plus and minus symbols represent apparent vitrification and appearance of visible ice crystal (opacity) in the whole solution volume. The symbol +/– indicates partial apparent vitrification.
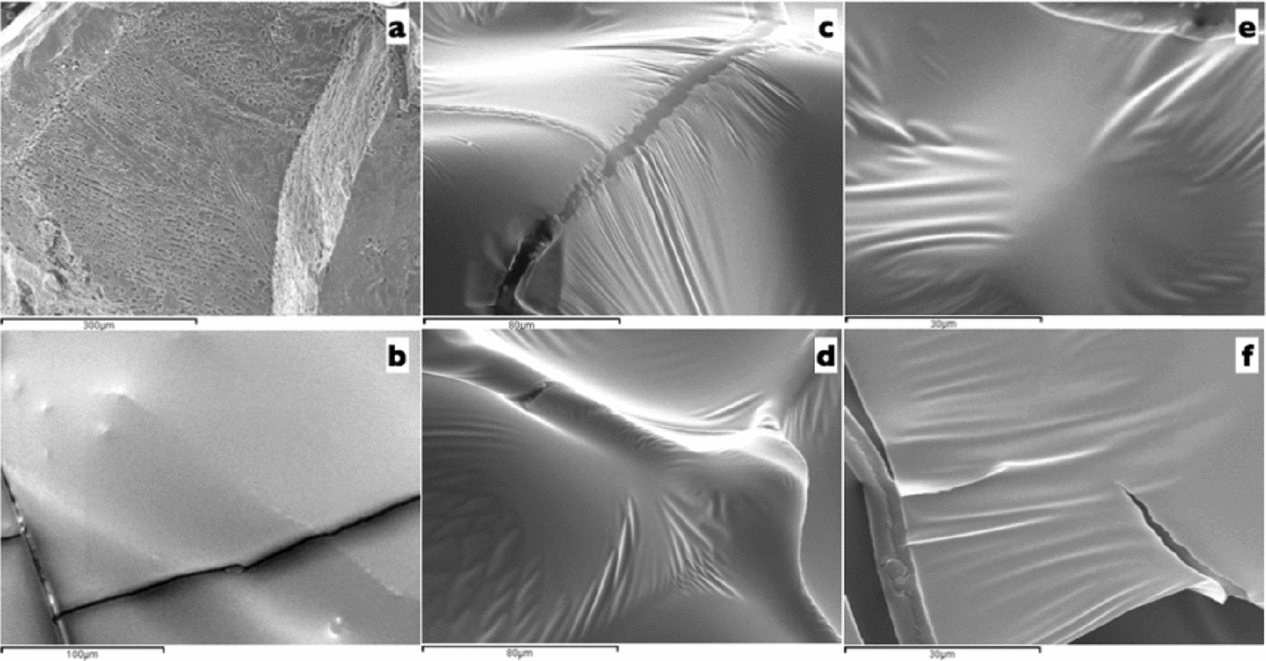
Representative cryo-SEM micrographs are depicted in Fig. 1. After cross-fracturing and deep etching, the fracture formation in a vitrified 7.6 M VM3 solution on OPS had no surface cavities (Fig. 1b). When VM3 and VM3 solutions were compared (7.6 M and 1% (w/v) of SuperCool X-1000 and SuperCool Z-1000 versus 5.6 M and 0.75% (w/v) of SuperCool X-1000 and SuperCool Z-1000, respectively), similar compact structure were observed (Fig. 1c–f). The effect of both ice blockers concentrations was a characteristic plasticized surface.

Figure 1 Cryo-scanning electron microscopy (cryo-SEM) micrographs of oocytes in VM3 solution. (a) Low cryoprotectant concentration (3.1 M, cryo-SEM control). (b) Image of VM3 solution (7.6 M) without ice blockers. (c, e) Image of VM3 solution (7.6 M) with 1% of SuperCool X-1000 and 1% SuperCool Z-1000 ice blockers at ×1000 or ×2000 magnification, respectively. (d, f) Image of VM3 optimized solution (5.6 M) with 0.75% of SuperCool X-1000 and 0.75% SuperCool Z-1000 ice blockers at ×1000 and ×2000 magnification, respectively).

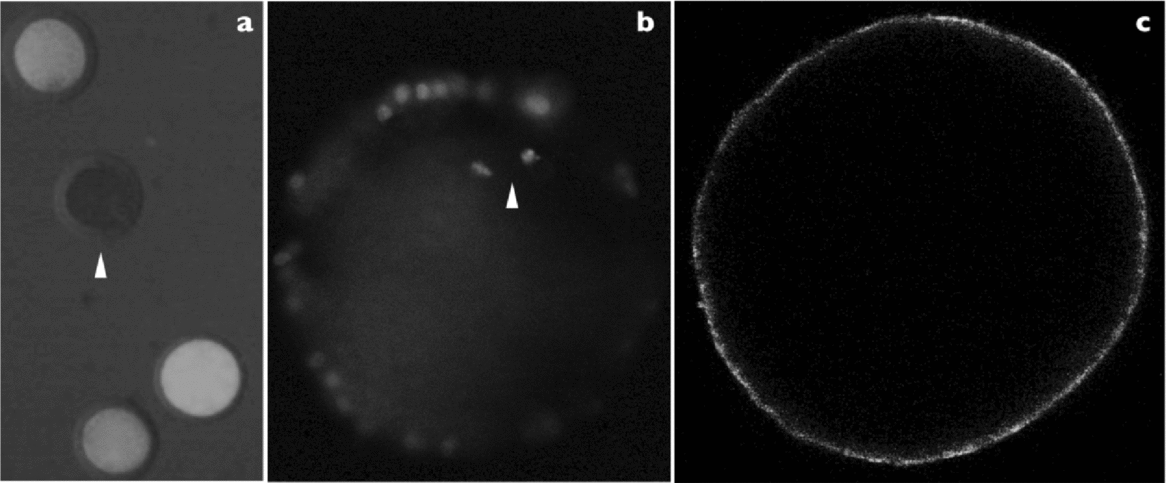
Figure 2 Example of assay in oocytes. (a) Viability based on calcein red-orange ×100 magnification (arrowheads show dead oocytes). (b) Normal chromosomes arranged on a compact metaphase plate at the equator of the structure based on propidium iodide ×400 magnification. (c) Correct peripheral cortical granules distribution established in metaphase II oocytes and based on lens culinaris fluorescein isothiocyanate; ×400 magnification of in vitro matured porcine oocytes.
Experiment 2
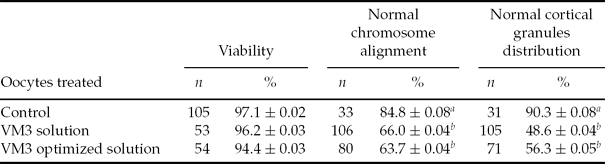
Viability rates of oocytes after vitrification are shown in Table 2. After vitrification the viability rate was similar to that of the fresh oocyte group. Also, as shown in Table 2, most oocytes had normal chromosome alignment (84.8%) and normal peripheral cortical granules distribution (90.3%) in the fresh group. However, when VM3 solution or VM3 optimized solution was used for vitrification, the percentage of oocytes with normal chromosome alignment was significantly lower in the fresh group (84.8% versus 66.0% and 63.7% for fresh versus VM3 and VM3 optimized solutions, respectively). Also, the percentage of oocytes with peripheral cortical granules distribution was significantly lower in the fresh group (90.3% versus 48.6% and 56.3% for fresh versus VM3 and VM3 optimized solutions, respectively). However, the difference between both vitrification solutions was not significant for normal chromosome alignment and peripheral cortical granules distribution.
Table 2 Viability, chromosomal alignment and cortical granules distribution of porcine in vitro matured oocytes, vitrified and warmed and using open pulled straws (six replicates)

Data are expressed as mean ± standard deviation (SD). Differences were considered significant at a level of P < 0.05.
a ,b Values with different superscripts in the same column are significantly different (P < 0.05).
n: Data number.
Discussion
Porcine oocytes are particularly difficult to cryo-preserve due to characteristically larger amounts of cytoplasmic lipids than found in other mammals (Isachenko et al., Reference Isachenko, Isachenko, Michelmann, Alabart, Vazquez, Bezugly and Nawroth2001; Fujihira et al., Reference Fujihira, Kishida and Fukui2004; Genicot et al., Reference Genicot, Leroy, Soom and Donnay2005). Nevertheless, Zhou & Li (Reference Zhou and Li2009) considered vitrification as the method of choice for cryopreservation of porcine oocytes. Cryoprotectant cytotoxicity is of particular concern in vitrification, which, to achieve the vitreous state, requires a much higher cryoprotectant concentration than does freezing (Lawson et al., Reference Lawson, Ahmad and Sambanisa2011). However, the minimal volume together with the high cooling rate enabled a reduction in cryoprotectant concentration without increasing the likelihood of crystallization throughout vitrification and warming (Yavin & Arav, Reference Yavin and Arav2007). Several methods have been developed to minimize the likelihood of ice formation during the vitrification procedure, including evaluation of cryoprotectants with differing composition, the use of antinucleating and antifreeze compounds, minimization of the cryoprotectant volume and evaluation of different cooling strategies (Vajta et al., Reference Vajta, Holm, Kuwayama, Booth, Jacobsen, Greve and Callesen1998; Liebermann et al., Reference Liebermann, Tucker, Graham, Han, Davis and Levy2002; Abe et al., Reference Abe, Hara, Matsumoto, Kobayashi, Sasada, Ekwall, Rodriguez-Martinez and Sato2005; Kuwayama et al., Reference Kuwayama, Vajta, Ieda and Kato2005; Kuwayama, Reference Kuwayama2007). In the current study, we attempted to optimize the process of porcine oocytes vitrification with OPS by use of the minimum cryoprotectant concentration of a low toxicity solution, called VM3 solution (Fahy et al., Reference Fahy, Wowk, Wu and Paynter2004). VM3 solution is a low toxicity cryoprotectant solution and exhibits a low tendency for ice crystal formation upon cooling and warming (high stability; Fahy et al., Reference Fahy, Wowk, Wu and Paynter2004). VM3 solution was designed to vitrify organs; the main problems in vitrification of organs are the requirements to use high concentrations (60–65%) of cryoprotectants, elevated permeability and high volume. In the present study, we have optimized vitrification with an OPS device. Our hypothesis was that a reduction in cryoprotectant concentration may be beneficial to the oocytes as would reduce both toxic and osmotic effects. A 16% reduction in VM3 solution achieved successful vitrification when OPS were used. Our results could address the ideal cryopreservation protocol, which would be one that combines the benefits of conventional slow freezing (i.e. reduced toxicity secondary to low cryoprotective agent concentrations) with the benefits of vitrification (i.e., absence of intracellular ice crystal formation) (Lee et al., Reference Lee, Elmoazzen, Wright, Biggers, Rueda, Heo, Toner and Toth2010). Also, VM3 solution contains antinucleating and ice growth-inhibiting solutes (SuperCool X-1000 and SuperCool Z-1000 ice blockers) to largely preclude ice formation even during rewarming. The presence of SuperCool X-1000 (polyvinyl alcohol and vinyl acetate) and SuperCool Z-1000 (polyglycerol) provides extra stability against ice formation and could, in some applications, allow cryoprotectant concentrations to be reduced to achieve an overall reduction of solution toxicity (Fahy et al., Reference Fahy, Wowk, Wu and Paynter2004). In fact, cryo-scanning electron microscopy imaging in our study for cells using both vitrification solutions (free water) contrasted with results using the freezing solution (3.1 M). We found that VM3 and VM3 optimized solutions present a similar continuous surface with a plastic aspect due to the presence of ice blockers. Future studies will be necessary to elucidate more fully the mechanisms by which ice blockers generate viscoelasticity and plasticity.
One of the most promising approaches for oocyte vitrification to improve survival is to minimize osmotic injury and cryoprotectant toxicity by reduction in cryoprotectant concentration (Kuwayama et al., Reference Kuwayama, Vajta, Ieda and Kato2005). Our comparison for both VM3 cryoprotectant solution molarities resulted in similar viability, normal chromosome alignment and cortical granules distributed as monolayers beneath the oolemma. However, the viability of oocyte assessed by calcein-AM was not affected by both VM3 and VM3 optimized vitrification solutions compared with fresh oocytes. Calcein-AM has been used previously by Santos et al. (Reference Santos, Tharasanit, Figueiredo, van Haeften and van den Hurk2006), Lopes et al. (Reference Lopes, Santos, Celestino, Melo, Chaves, Campello, Silva, Báo, Jewgenow and de Figueiredo2009) and Ebrahimi et al. (Reference Ebrahimi, Valojerdi, Eftekhari-Yazdi, Baharvand and Farrokhi2010) in preantral follicles and sheep oocytes, respectively. In previous porcine vitrification studies using a fluorescein diacetate (FDA) stain, survival rates ranged between 50–75% (Fahy, Reference Fahy1986; Shi et al., Reference Shi, Zhu, Zhang, Wang, Tang, Hou and Tian2006; Gupta et al., Reference Gupta, Uhm and Lee2007; Fu et al., Reference Fu, Shi, Zhang, Zhao, Yan, Hou, Zhou, Fan, Suo, Wusiman, Wang and Zhu2009). Because both staining methods (FDA or calcein-AM) are cleaved by esterase enzymes in the cytoplasm of living cells, we can speculate that the highest viability obtained in this study could be explained by low toxicity of the VM3 solutions.
Vitrified–warmed oocytes have altered meiotic spindle assembly, microtubules, cortical granule distribution and zona pellucida characteristics (Fahy, Reference Fahy1986; Rojas et al., Reference Rojas, Palomo, Albarracin and Mogas2004; Wu et al., Reference Wu, Rui, Dai, Zhang, Ju and Xie2006; Gupta et al., Reference Gupta, Uhm and Lee2007; Shi et al., Reference Shi, Jin, Kim, Mohana Kumar, Balasubramanian and Choe2007; Huang et al., Reference Huang, Li, Zhao, Li, Han, Chen, Xiao, Wu, Jiang, Hu and Liu2008). The absence of the meiotic spindle in oocytes after cryopreservation also has been attributed to the toxic effect of cryoprotectants (Fahy, Reference Fahy1986; Succu et al., Reference Succu, Leoni, Berlinguer, Madeddu, Bebbere, Mossa, Bogliolo, Ledda and Naitana2007). In the present study, our results indicate that low concentrations of cryoprotectants (VM3 optimized solution) did not reduce chromosome misalignment. Previous studies have reported similar proportions of oocytes with normal chromosome alignment (Shi et al., Reference Shi, Zhu, Zhang, Wang, Tang, Hou and Tian2006). In the present study, our results also indicated that low concentrations of cryoprotectants (VM3 optimized solution) did not reduce the premature release of cortical granules or alter cortical granule distribution. Some researchers have reported that cryopreservation of mammalian oocyte leads to premature release of cortical granules (Ghetler et al., Reference Ghetler, Skutelsky, Ben Nun, Ben Dor, Amihai and Shalgi2006; Morato et al., Reference Morato, Izquierdo, Paramio and Mogas2008; Nottola et al., Reference Nottola, Coticchio, Sciajno, Gambardella, Maione, Scaravelli, Bianchi, Macchiarelli and Borini2009; Tan et al., Reference Tan, Song, Liu, You and Wan2009; Coticchio et al., Reference Coticchio, Borini, Distratis, Maione, Scaravelli, Bianchi, Macchiarelli and Nottola2010). To the best of our knowledge, only the Wu et al. (Reference Wu, Rui, Dai, Zhang, Ju and Xie2006) study has shown the effects of vitrification on cortical granule distribution in in vitro matured porcine oocytes. Wu et al. (Reference Wu, Rui, Dai, Zhang, Ju and Xie2006) did not observe a premature release of cortical granules in vitrified oocytes, but only 37.2% of vitrified oocytes showed a normal distribution of actin filaments. Cortical granule migration to the cortex depends on microfilament assembly (Connors et al., Reference Connors, Kanatsu-Shinohara, Schultz and Kopf1998). Our data confirmed these previous results and suggested that vitrification compromised the normal cortical granule distribution in matured oocytes and that this factor might cause reduced cleavage rates as a consequence of their decreased ability to be penetrated by spermatozoa after in vitro fertilization (Somfai et al., Reference Somfai, Ozawa, Noguchi, Kaneko, KurianiKarja and Farhudin2007). This problem can be overcome by intracytoplasmic sperm injection (Porcu et al., Reference Porcu, Fabbri, Damiano, Giunchi, Fratto, Ciotti, Venturoli and Flamigni2000) or by vitrification in a calcium-free system (Larman et al., Reference Larman, Sheehan and Gardner2006). Therefore, we suggest that toxicity of cryoprotectant mixtures is of particular concern in the vitrification procedure, the minimum concentration of cryoprotectants required to obtain glass-like solidification is inevitably toxic, and toxicity of cryoprotectant mixtures could not be completely prevented with the methods currently in use. Although we optimized the VM3 solution to minimize its toxic effects, cryo-damage to chromosomal structure and cortical granules vesicles was obtained in a similar way as described in previous reports. In conclusion, the present results suggest that VM3 solution can be optimized and a reduction in molarity to 5.6 M would help to obtain the vitrification state using OPS. When the VM3 solution was evaluated using cryo-SEM, plastic film formation was observed as a consequence of the presence of ice blockers. Furthermore, the combination of the VM3 optimized solution with an OPS container to vitrified in vitro matured porcine oocytes had no beneficial effect. More extensive studies to modify vitrification methods and to modify cells are necessary in order to advance porcine oocytes cryopreservation.
Acknowledgements
This work was supported by funds from the Generalitat Valenciana research programme (Prometeo 2009/125) and Ajudes per a la realització de projectes precompetitius d'I+D per a equips [Grants for pre-competitive R&D projects for research teams] (GVPRE/2008/206). English text version was revised by the N. Macowan English language service.