Introduction
In some species, in vitro embryo production (IVP) efficiency is still low, mainly due to inadequate techniques (Mondéjar et al., Reference Mondéjar, Avilés and Coy2013; Avilés et al., Reference Avilés, Coy and Rizos2015). Two hypotheses can be considered to explain the differences between in vivo and in vitro efficiency of fertilization: (i) difference in physiologically ovulated oocyte quality compared with oocytes collected from growing follicles and used in IVP procedures (El Hajj and Haaf, Reference El Hajj and Haaf2013; Avilés et al., Reference Avilés, Coy and Rizos2015); and/or (ii) events in the oviduct are not fundamental but, as they do not occur during in vitro procedures, only embryos of the highest quality can survive (Avilés et al., Reference Avilés, Coy and Rizos2015). These could be the reasons for the reduced percentage of success in IVP compared with the in vivo situation.
The oviductal environment is responsible for the control of final maturation of spermatozoa, capture of ovulated oocyte and transport of gametes, fertilization, and early embryo development. The oviductal fluid (OF), which is formed by the mixture of plasma exudate, epithelial cell secretion, and follicular fluid released during ovulation (Li and Winuthayanon, Reference Li and Winuthayanon2017), provides a favourable microenvironment for these transformations. In addition to the ionic composition, the OF has a specific profile of extracellular vesicles (EV) and proteins that establish a cross-talk between the oviduct and the gametes and embryos. The term EV encompasses different types of vesicles, released by somatic cells, which are present in body fluids, and contain bioactive molecules such as mRNAs, small ncRNAs, proteins, carbohydrates and lipids (Raposo and Stoorvogel, Reference Raposo and Stoorvogel2013), which play a fundamental role in the regulation of physiological and pathological processes (Thery, Reference Thery2011). These EVs can mediate key processes, such as sperm–oocyte binding and fertilization (Pérez-Cerezales et al., Reference Pérez-Cerezales, Ramos-Ibeas, Acuña, Avilés, Coy, Rizos and Gutiérrez-Adán2018).
Still in relation to fertilization, during the transit in the oviduct several proteins in the OF can bind to the zona pellucida (ZP), modifying its protein and carbohydrate composition, therefore modulating the interaction between spermatozoa and oocyte. Oviduct-specific glycoprotein (OVGP1), osteopontin, lipocalin-type prostaglandin D synthase, and lactoferrin were demonstrated to associate with the ZP of different species (Gonçalves et al., Reference Gonçalves, Staros and Killian2008). OVGP1 is the most studied ZP-associated protein, and its role in pre-fertilization ZP hardening that reduces polyspermy in the pig has been demonstrated (Coy et al., Reference Coy, Cánovas, Mondéjar, Saavedra, Romar, Grullón, Matás and Avilés2008).
Additionally, it has also been reported that OF has a positive effect on sperm viability, allowing sperm survival for 1–2 days for cows and sows (Killian, Reference Killian2011). Reactive oxygen species (ROS) scavenging enzymes (superoxide dismutase, catalase and peroxiredoxins) are especially relevant for sperm as they are easily damaged when exposed to oxidation that modifies the plasma membrane (protein and lipid peroxidation), and which can lead to DNA breaks (Aitken and De Iuliis, Reference Aitken and De Iuliis2010).
In vivo, some OF proteins also bind to the sperm membrane and certainly have a beneficial effect on human (Yao et al., Reference Yao, Ho and Yeung2000), bovine (Bergqvist et al., Reference Bergqvist, Ballester, Johannisson, Hernández, Lundeheim and Rodríguez-Martínez2006) and stallion (Ellington et al., Reference Ellington, Ball and Yang1993) sperm function by capacitating the spermatozoa and inducing their hyperactivation. Proteoglycans such as heparin are present in the OF and have been shown to modulate the activity of oviduct-secreted proteins for the regulation of spermatozoa parameters (Coy et al., Reference Coy, Cánovas, Mondéjar, Saavedra, Romar, Grullón, Matás and Avilés2008; Batista et al., Reference Batista, Moro, Corbin, Alminana, Souza-Fabjan, Freitas and Mermillod2016). In this regard, Coy et al. (Reference Coy, Cánovas, Mondéjar, Saavedra, Romar, Grullón, Matás and Avilés2008) and Batista et al. (Reference Batista, Moro, Corbin, Alminana, Souza-Fabjan, Freitas and Mermillod2016) demonstrated a beneficial effect of the combination of heparin and OF in the modulation of the polyspermy incidence during in vitro fertilization (IVF). Oviduct fluid and cultured oviductal epithelial cells (OEC) can be used to investigate gametes–oviduct interactions in vitro and to recreate the appropriate conditions for a normal fertilization during IVF. In this area, several authors have shown that OECs prolong sperm survival, enhance sperm viability and motility, induce capacitation, and modify the frequency of tail beat in several species: humans (Kervancioglu et al., Reference Kervancioglu, Djahanbakhch and Aitken1994; Morales et al., Reference Morales, Palma, Salgado and Villalón1996; Zhu et al., Reference Zhu, Zhong and Zhang2001), sheep (Gutiérrez et al., Reference Gutiérrez, Garde, García-Artiga and Vázquez1993), dogs (Kawakami et al., Reference Kawakami, Kashiwagi, Hori and Tsutsui2001), rats (Cortés et al., Reference Cortés, Orihuela, Zúñiga, Velásquez and Croxatto2004), cattle (Gualteri and Talevi, Reference Gualteri and Talevi2003), horses (Dobrinski et al., Reference Dobrinski, Suárez and Ball1996) and pig (Yeste et al., Reference Yeste, Lloyd, Badia, Briza, Bonet and Holt2009). However, the co-culture of porcine sperm and oocytes with porcine OECs during IVF increases the incidence of polyspermy, depending on sperm concentration (Romar et al., Reference Romar, Coy, Campos, Gadea, Matás and Ruiz2001; Yeste et al., Reference Yeste, Lloyd, Badia, Briza, Bonet and Holt2009). Conversely, preincubation of oocyte in OF for 30 min before fertilization increased the production of monospermic zygotes, but reduced the global efficiency of the IVF (Coy et al., Reference Coy, Cánovas, Mondéjar, Saavedra, Romar, Grullón, Matás and Avilés2008; Batista et al., Reference Batista, Moro, Corbin, Alminana, Souza-Fabjan, Freitas and Mermillod2016). Therefore, the present study aimed to assess the effect of association of the oviductal fluid flush (OFF) and OEC on monospermic zygotes production during porcine IVF.
Materials and methods
Chemicals were purchased from the Sigma Chemical Co. (Saint Louis, MO, USA) unless otherwise indicated.
Oviducts classification and OFF recovery
Genital tracts from gilts were obtained at a local slaughterhouse and transported to the laboratory on ice. The estrous cycle stage of the females was assessed in the laboratory, on the basis of ovarian morphology on both ovaries from each female. Oviducts were classified as early follicular, late follicular, early luteal or late luteal phase, based on the criteria defined by Hafez and Hafez (Reference Hafez, Hafez, Hafez and Hafez2000). Both oviducts coming from the same genital tract, and classified as late follicular phase were used. Females with ovaries not clearly matching these criteria were discarded. After classification, oviducts were washed once quickly with 70% ethanol solution followed by two washes with Dulbecco’s PBS, then transferred to Petri dishes on ice, and dissected free of surrounding tissues. Each oviduct containing an average of 50 μl of pure OF was perfused with 500 μl PBS inserted in the tip of the ampoule to wash the lumen. The liquid was recovered by gentle massage of the ampoule to the isthmus. This procedure was repeated in 10 oviducts, with the fluid that was recovered in the first oviduct reused to wash the next one, therefore providing a 1:1 dilution of OF:PBS (OFF). The OFF was centrifuged at 7000 g for 10 min at 4°C to remove cell debris. Then, the supernatant was immediately stored at −20°C until use.
Bovine oviduct epithelial cell culture
Genital tracts from cows were obtained at a local slaughterhouse and selected as described. The complete oviduct was rinsed with HEPES-buffered tissue culture medium-199 (TCM-199), dissected free from its surrounding tissues and washed briefly in 70% ethanol. The mucosa was expelled mechanically from the oviduct by gentle scraping with a sterile glass slide. Epithelial cells were then washed four times and recovered after passive sedimentation in TCM-199 supplemented with 10% heat-treated fetal calf serum (FCS) and 80-μg/ml gentamycin. The cell suspension was diluted 100-fold in the same medium, seeded in four-well culture plates (500 μl/well) (NUNC, Roskilde, Denmark) and cultured in a humidified atmosphere of 5% CO2 in air at 38.8°C. The medium was completely renewed after 48 h. Subsequently, half of the medium was replaced every 48 h until the day preceding IVF. OEC monolayers reached confluency within 7 days and were then used for sperm–oocyte co-culture. At 24 h before introducing the oocyte and sperm, all medium was replaced by Tris-buffered medium (TBM).
In vitro maturation (IVM)
Ovaries were collected from slaughtered peripubertal gilts and transported to the laboratory within 2 h in 0.9% NaCl at 30ºC. Cumulus–oocyte complexes (COC) were aspirated from antral follicles (3–6 mm in diameter) with an 18-gauge short bevelled needle connected to a Falcon tube and under a controlled vacuum (30 mmHg). Then, COC were selected under a stereomicroscope. Immature COC with a compact cumulus cell mass were washed three times in 25 mM HEPES-buffered TCM-199 with Earle’s salts supplemented with 4 μg/ml gentamicin (G1272) and 1 mg/ml BSA (A9647) and washed once in maturation medium. Groups of up to 50 COC were transferred into four-well plates (Nunc Roskilde, Denmark). Each well contained 500 μl maturation medium, at 38.8ºC in an atmosphere of 5% CO2 in air with maximum humidity. The maturation medium consisted of TCM-199 with Earle’s salts (M4530) supplemented with 10 ng/ml epidermal growth factor (EGF; E4127), 400 ng/ml follicle-stimulating hormone (FSH; PRIMUFOL, Rhône Mérieux, Lyon, France), 570 μM cysteamine (M9768) and 10% FCS (F2442) (Marchal et al., Reference Marchal, Tomanek, Terqui and Mermillod2001).
Preparation of spermatozoa
Straws of frozen semen were prepared from a pool of ejaculates from three Large White boars (Bussiere et al., Reference Bussiere, Bertaud and Guillouet2000). After thawing in a water bath at 37°C for 30 s, sperm from one straw were washed in Beltsville Thawing Solution (BTS; Landata, France) by centrifugation at 100 g for 10 min at room temperature. Motile spermatozoa were obtained by centrifugation of the pellet on a Percoll discontinuous gradient (2 ml at 45% over 2 ml at 90%; Pharmacia, Uppsala, Sweden) for 30 min at 700 g. Cells collected at the bottom of the 90% fraction were washed in fertilization medium by centrifugation at 100 g for 10 min. The sperm pellet was then resuspended to give a concentration of 2 × 108 cells/ml.
In vitro fertilization (IVF)
After the maturation period, oocytes were denuded by vortexing 2 min in 2 ml TCM-199 HEPES, then washed three times in the same medium and once in fertilization medium before being transferred in groups of 50 oocytes into four-well plates. Each well contained 250 μl fertilization medium. The fertilization medium consisted of modified Tris-buffered medium (mTBM, with 113.1 mM NaCI, 3 mM KCI, 10 mM CaCl2, 20 mM Tris, 11 mM glucose, 5 mM sodium pyruvate, 1 mM caffeine and 0.1% BSA EFAF; Marchal et al., Reference Marchal, Tomanek, Terqui and Mermillod2001). An aliquot of sperm suspension was added to each fertilization well to obtain their final concentrations: 0.5, 1.5, or 4.5 × 105 cells/ml. Fertilization was performed at 38.8°C, 100% humidity in an atmosphere of 5% CO2 in air.
In vitro development (IVD)
Embryo development took place in droplets of modified synthetic oviduct fluid (SOF) medium under mineral oil in a humidified atmosphere of 5% CO2, 5% O2, 90% N2 at 38.8°C (Carolan et al., Reference Carolan, Lonergan, van Langendonckt and Mermillod1995). The SOF medium contained 107.7 mM NaCI, 7.16 mM KCI, 1.19 mM KH2PO4, 1.71 mM CaC12, 0.49 mM MgCl2, 25.07 mM NaHCO3, 3.3 mM Na lactate, 0.3 mM Na pyruvate, 1 mM glutamine, essential and non-essential amino acids, and 0.3% BSA fraction V (Marchal et al., Reference Marchal, Tomanek, Terqui and Mermillod2001). After 20 h of fertilization, sperm were removed by gentle vortexing of the putative zygotes. They were then washed three times in HEPES TCM-199 and once in SOF medium before being transferred in groups of 20 into oil overlaid with 25 μl droplets of SOF medium, supplemented with 10% FCS. On day 8, all blastocysts were washed to remove the mineral oil, fixed in ethanol and stained with Hoechst 33342 (10 µg/ml) to count their total cell number. Cell counting was conducted under an epifluorescence microscope.
Assessment of fertilization
After fertilization, five putative zygotes from each group in each replicate were randomly collected and placed on a slide, air-dried, and fixed in absolute ethanol for 24 h. They then were stained with Hoechst 33342 (10 μg/ml in 2.3% sodium citrate) and visualized with an epifluorescence microscope for analysis of total number of spermatozoa bound to the ZP. Another group of 15 presumptive zygotes per replicate were fixed (alcohol:chloroform:acetic acid, 80:10:10; v/v), stained with 1% (w/v) lacmoid, and examined at ×400 magnification to evaluate sperm penetration and pronuclear formation under a phase contrast microscope. In the second experiment, 15 structures were used to count sperm attached to the ZP and the other ones were used to analyze sperm penetration and pronuclear formation.
Experimental design
In Experiment 1 (Figure 1), the effects of oocyte incubation with OFF or OEC during IVF, on IVF and IVD results were investigated. After IVM, COC were denuded and randomly allocated to one of three groups: (i) Control – IVF in TBM; (ii) OFF – IVF in TBM supplemented with 10% OFF; and (iii) OEC – IVF in TBM medium in the presence of OEC. For each replicate, approximately 450 oocytes were equally allocated into one of three treatments. For IVF, oocytes from each experimental group were allocated into three wells (around 50 oocytes/well) and co-cultured with either 0.5, 1.5 or 4.5 × 105 sperm cells/ml (total of nine groups) for a period of 20 h. After IVF, samples of 20 presumptive zygotes from each group were fixed and stained for analysis of the number of spermatozoa bound to ZP, number of pronuclei per oocyte and IVF efficiency (percentage of monospermic from total inseminated). The remaining presumptive zygotes were transferred to IVD. Embryo cleavage and blastocyst formation rates were evaluated on days 2 and 7 after fertilization, respectively. On day 8, all blastocysts were fixed to count their total cell number. Five replicates were performed.
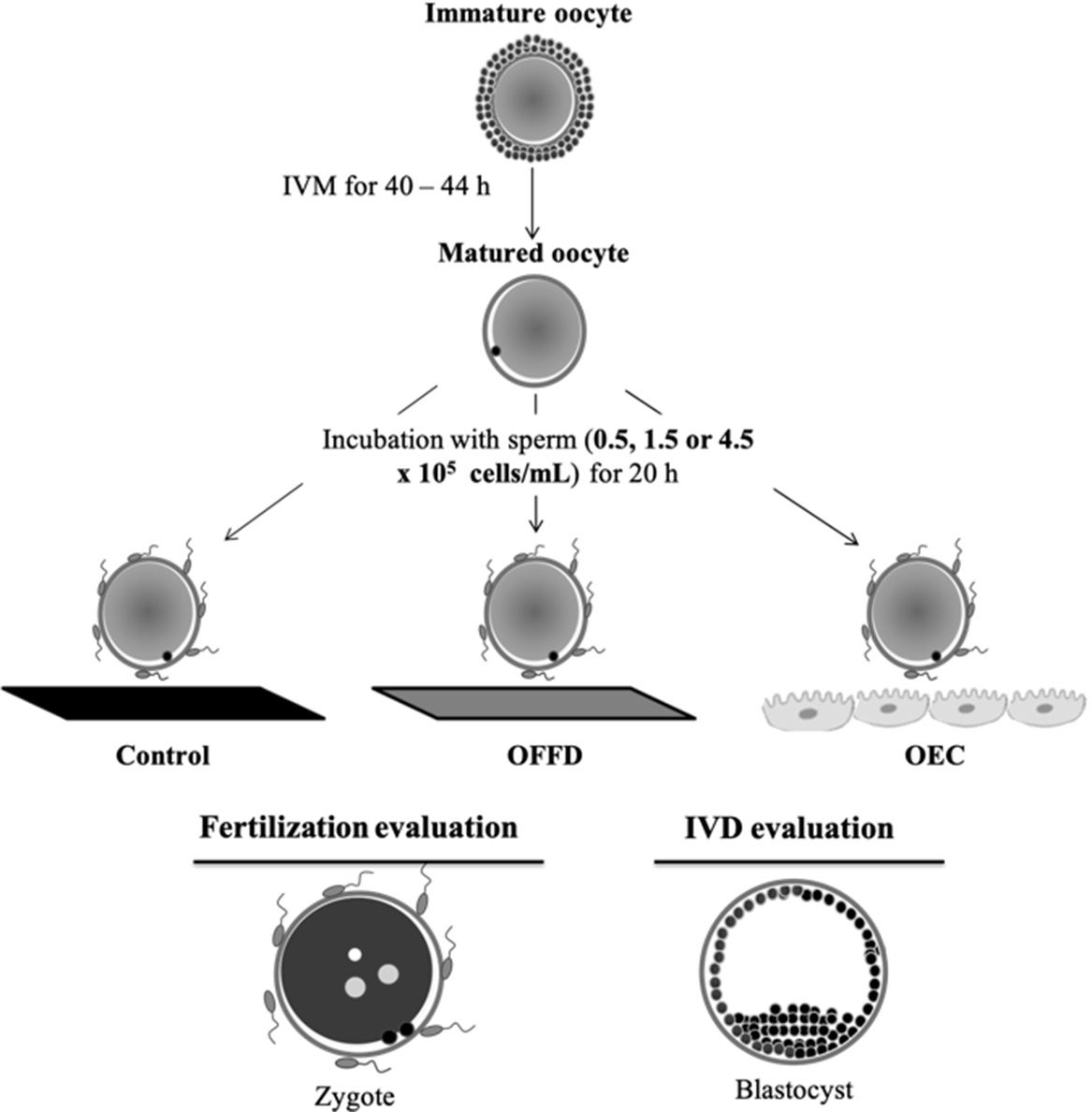
Figure 1. Experimental design of Experiment 1. Effect of oviductal fluid flush (OFF) and bovine oviductal epithelial cells (OEC) during IVF on the incidence of fertilization, polyspermy and development rate of porcine oocytes. IVD, in vitro development.
In Experiment 2 (Figure 2), the effects on IVF outcome of the combination of treatment of oocytes by OFF before IVF and by OEC monolayer during IVF were tested in a fertilization medium consisting of TBM supplemented with 10 ng/ml of heparin (Calbiochem 375 095). After IVM, COC were denuded and randomly allocated for IVF to one of four treatments: (i) Control – IVF in medium alone; (ii) OEC – IVF in the presence of OEC; (iii) OFF 30’ – oocyte incubation in OFF for 30 min, followed by three washes in TBM medium, then transferred to fertilization well; and (iv) OFF 30’ + OEC – oocyte incubation in the OF for 30 min, followed by three washes in TBM medium, and then transferred to fertilization wells containing OEC. Co-culture with spermatozoa (4.5 × 105 cell/ml) was performed for 20 h. Presumptive zygotes from each group were fixed and stained to count the number of sperms bound to the ZP and the number of pronuclei in each presumptive zygote. Five replicates were performed.
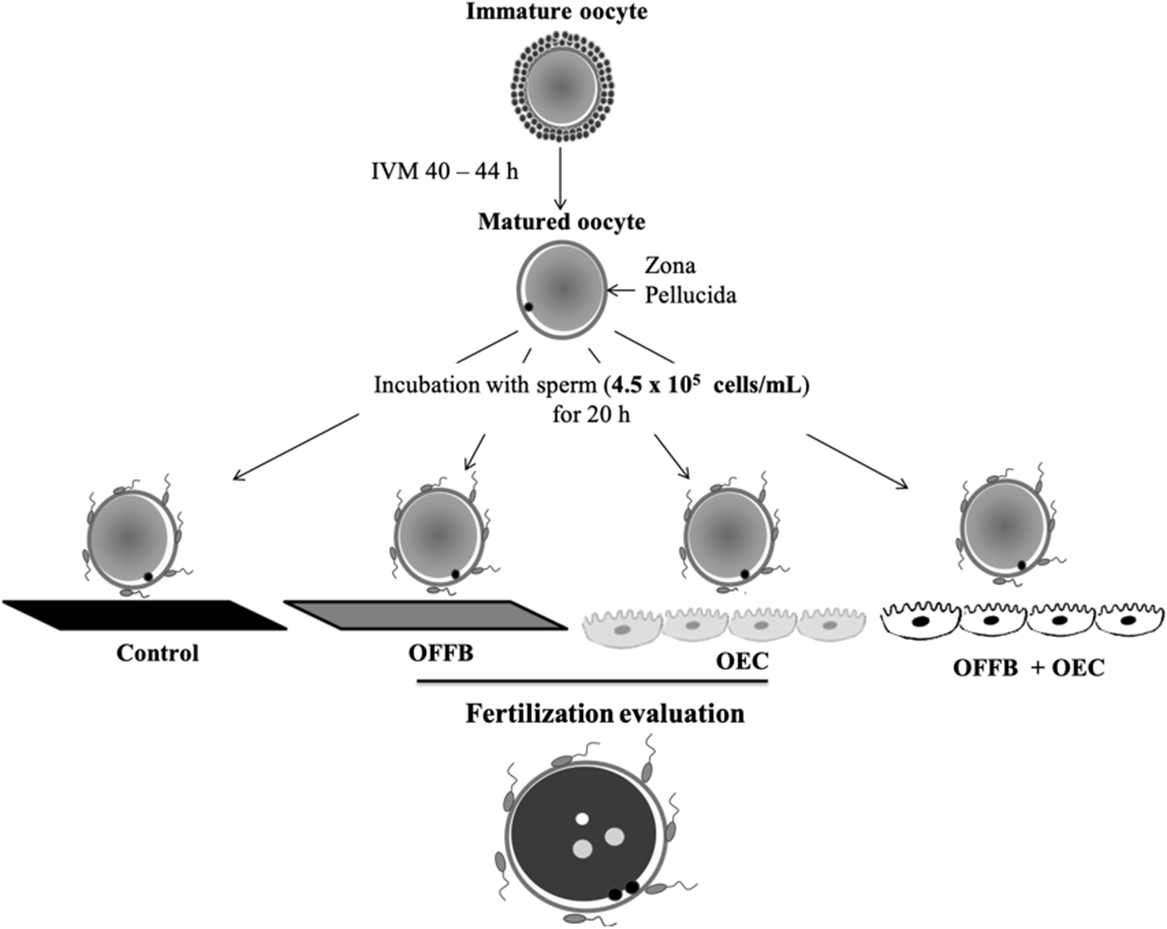
Figure 2. Experimental design of Experiment 2. Effect of association of oviductal fluid flush (OFF) and oviductal epithelial cells (OEC) in the incidence of polyspermy in porcine oocytes.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM) Data for all rates were modelled according to the binomial model of parameters. The rate of oocyte penetration, number of sperm cells per penetrated oocyte, male pronucleus formation, monospermy rate and total numbers of spermatozoa bound to the ZP were tested for normality using the Kolmogorov–Smirnov test. Parameters were compared using one-way analysis of variance (ANOVA) followed by Tukey’s test. Differences were considered significant at P-values < 0.05. Parameters for embryonic development, cleavage and blastocyst rate were compared by chi-squared test.
Results
Experiment 1. Effect of OFFD and OEC on IVF and IVD results
Increase in sperm concentration (0.5; 1.5 and 4.5 × 105 cells/ml) was accompanied by increase (P > 0.05) in the penetration rate (%) in the control group (51 ± 5; 71 ± 9 and 90 ± 4) and 10% OFF during IVF (31 ± 4; 57 ± 6 and 79 ± 10). However, the OEC group was not significantly affected by sperm concentration (72 ± 12; 86 ± 10 and 91 ± 4) (Figure 3A). At the sperm concentration of 0.5 × 105 cells/ml, the highest penetration rate was observed in the OEC group (71% ± 12), compared with control group (51% ± 5) and OFF (31% ± 4), while at the concentrations of 1.5 × 105 and 4.5 × 105 the three groups did not differ. For the monospermy rate (Figure 3B), increasing the sperm concentration led to a significant decrease in the percentage of monospermic zygotes for the OEC group (55 ± 8; 42 ± 3 and 29 ± 5, respectively). However, no significant effect of sperm concentration on monospermy was observed in the control group (48 ± 13; 37 ± 12 and 28 ± 8) and OFF for the IVF group (93 ± 8; 92 ± 6 and 79 ± 5). This resulted in an increased (P < 0.05) efficiency of the IVF system in terms of production of normally fertilized zygotes from total oocytes in OFF (63 ± 5%) compared with the control (25 ± 6%) and the OEC (27 ± 5%) groups, when using a sperm concentration of 4.5 × 105 cells/ml (Figure 3C). In the OFF group, the number of penetrated spermatozoa per oocyte (Figure 3D) and the number of spermatozoa bound to the ZP (Figure 3E) were lower (P < 0.05) compared with the control and OEC groups. However, the number of spermatozoa bound to the ZP increased (P < 0.05) with higher sperm concentration in all groups, and this was more pronounced in the control and OEC groups.

Figure 3. (A–E) Effect of addition of oviductal fluid flush (OFFD) and oviductal epithelial cells (OEC) during in IVF on fertilization results of porcine oocytes. Each bar represents the mean ± standard error of the mean (SEM). For each group approximately 100 oocytes were analyzed. Different letters indicate significant differences (P > 0.05) between (a,b,c) or within (A,B) sperm concentration treatments. (i) Control – IVF in TBM; (ii) OFF – IVF in TBM supplemented with 10% OFF; and (iii) OEC – IVF in TBM medium in the presence of OEC.
The development rates and number of cells in blastocysts are shown in Table 1. The cleavage rate was not significantly affected by sperm concentration, OEC or exposure to OFF. However, IVF in the presence of OEC (0.5 × 105 cells/ml) increased the rate of blastocyst production compared with the control and OFF groups, which did not differ statistically. With the increase in sperm concentration (1.5 × 105 and 4.5 × 105 cells/ml), this difference did not persist between the OEC and OFF groups. The blastocyst formation rate was lower (P < 0.05) in the control group when compared with the OFF groups for sperm concentrations of 1.5 × 105 and 4.5 × 105 cells/ml. For sperm concentration, a negative effect on blastocyst production was observed, with the increase in sperm concentration in the OEC group, whereas in the OFF group a positive effect was observed. The total cell number per blastocyst was similar in all the groups.
Table 1. Effect of oviductal fluid flush during (10% OF) or oviduct epithelial cells (OEC) on the development rate of porcine oocytes fertilized with three different sperm concentrations

n: number of oocytes.
a,b,A,BUppercase letters indicate the comparison between IVF treatments and lowercase letters indicate the comparison between different sperm concentrations. Within a column, values with different superscripts differ significantly (P < 0.05) by chi-squared test.
† Control – IVF in Tris-buffered medium (TBM).
‡ OFFD – IVF in TBM medium supplemented with 10% OFF.
# OEC – IVF in TBM medium in the presence of oviduct epithelial cells on the seventh day of culture.
In Experiment 2: Effect of the combination of OFFB, OEC and heparin on IVF results
The combination of OFF with OEC (OFF 30’ + OEC) during IVF in the medium supplemented with heparin reduced the penetration rate compared with the control group and OEC alone. However, the penetration observed for this group was higher compared with the group exposed to OFF 30’ only (Figure 4A). The monospermy rate in OFF 30’ + OEC was similar to that of the OFF 30’ group, and both were higher compared with the control and OEC groups (Figure 4B). Consequently, the major efficiency (P < 0.05) of production of normally fertilized zygotes was observed in the OFF 30’ + OEC group (Figure 4C). In OFF 30’ and OFF 30’ + OEC groups, the number of pronuclei per oocyte (Figure 4D) and the number of spermatozoa bound to the ZP (Figure 4E) decreased compared with the control and OEC groups.
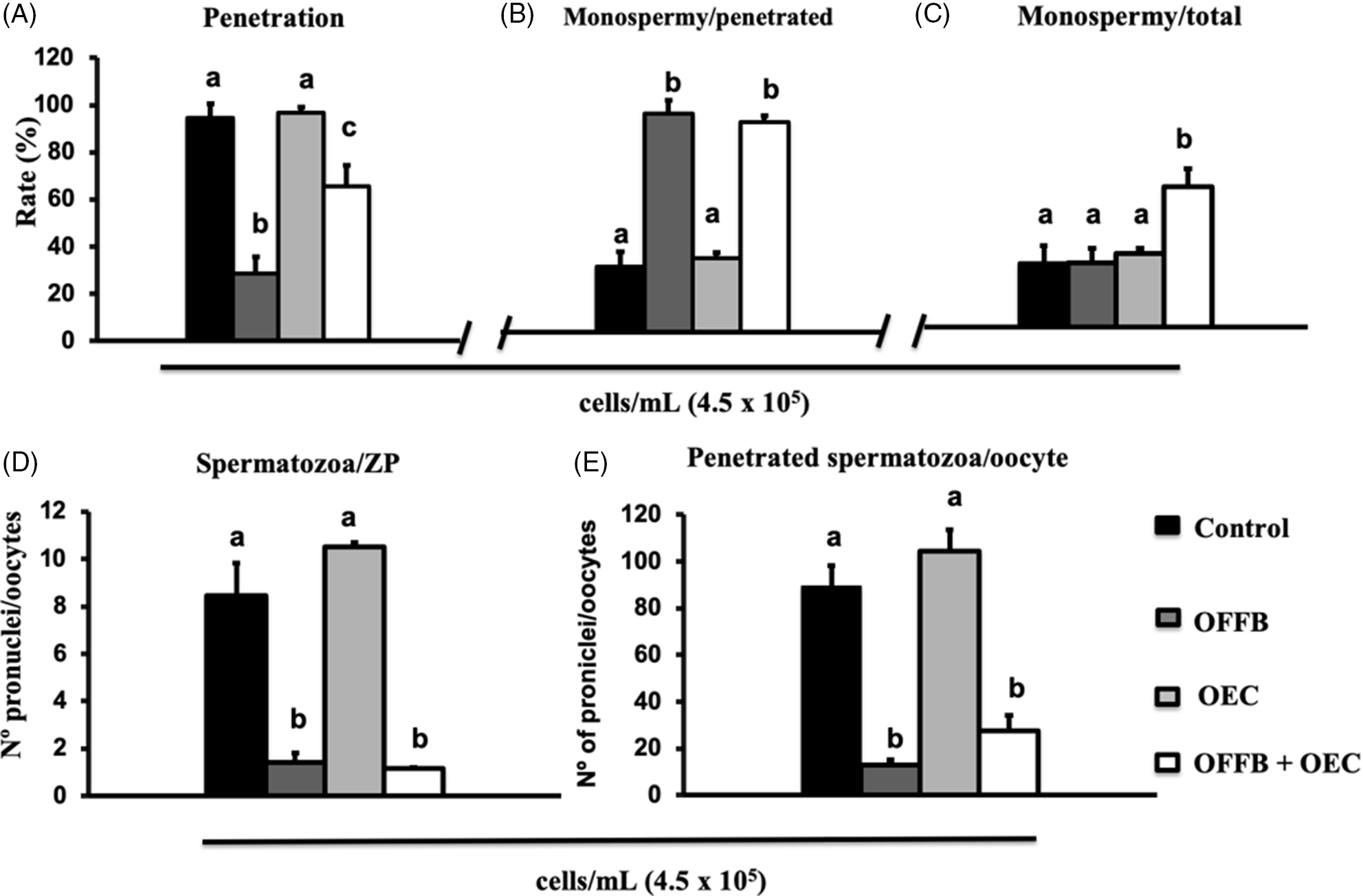
Figure 4. (A–E) Effect of the presence of oviduct fluid flush (OFF) and OEC in association with heparin during porcine IVF on fertilization results. Each bar represents mean ± standard error of the mean (SEM). For each group approximately 100 oocytes were analyzed. Different letters indicate significant differences (P > 0.05). (i) Control – IVF in medium alone; (ii) OEC – IVF in the presence of OEC; (iii) OFFB – oocyte incubation in 10% OFF for 30 min, followed by three washes in TBM medium, and then transferred to fertilization wells; and (iv) OFFB + OEC – oocyte incubation in the OF by 30 min, followed by IVF in TBM medium in the presence of OEC.
Discussion
The results of this study showed the beneficial effects of OFF added before or during IVF on monospermic fertilization, and the ability of OEC to increase the penetration rate; the combination of both improved the efficiency of production of monospermic zygotes. Only OFF from the late follicular phase (pre-ovulatory) was used in this study. However, Dubuc and Sirard (Reference Dubuc and Sirard1995) reported that oviductal cells treated with estradiol were more efficient compared with non-treated or progesterone-treated cells to promote monospermy in pig IVF. In the same way, several studies have shown that gene expression in the oviduct varies both according to the stage of the estrous cycle of the female, and to the presence of gametes or embryos in the oviduct (Cordova et al., Reference Cordova, Perreau, Uzbekova, Ponsart, Locatelli and Mermillod2014; Cerny et al., Reference Cerny, Garrett, Walton, Anderson and Bridges2015; Maillo et al., Reference Maillo, Gaora, Forde, Besenfelder, Havlicek and Burns2015). Recent findings have pointed to OVGP1 protein, whose expression is oestrogen dependent, as one of the main proteins responsible for monospermic fertilization. Its role in fertilization seems to be related to ZP hardening and the control of the number of spermatozoa bound to the ZP (Coy et al., Reference Coy, Cánovas, Mondéjar, Saavedra, Romar, Grullón, Matás and Avilés2008). This information is consistent with the expression and secretion of OVGP1 in the oviduct that occurs mainly during late follicular development (Coy and Avilés, Reference Coy and Avilés2010). This may partly explain the difference observed in the polyspermy rate between OFF and OEC, as OFF was obtained from oviducts in late follicular development, while OEC were cultured for 7 days in the absence of oestrogen, which has been shown to modify the gene expression profile of these cells with, in particular, the loss of OVGP1 expression (Schmaltz-Panneau et al., Reference Schmaltz-Panneau, Locatelli, Uzbekova, Perreau and Mermillod2015).
In addition to the protein content present in the OF, data from the literature have reported the presence of EVs also in the OF of murines (Al-Dossary et al., Reference Al-Dossary, Strehler and Martin-DeLeon2013; Qu et al., Reference Qu, Zhao, Wang, Zhang, Li, Fan and Liu2018), bovines (Alminãna et al. Reference Alminãna, Corbin, Tsikis, Alcântara-Neto, Labas, Reynaud, Galio, Uzbekov, Garanina, Druart and Mermillod2017; Lopera-Vasquez et al. Reference Lopera-Vasquez, Hamdi, Maillo, Gutiérrez-Adán, Bermejo-Alvarez, Ramírez, Yánez-Mó and Rizos2017) and swine (Alcântara-Neto et al., Reference Alcântara-Neto, Fernandez-Rufete, Corbin, Tsikis, Uzbekov, Garanina, Coy, Almiñana and Mermillod2020). These small extracellular compartments carry contents such as proteins, bioactive lipids, various RNAs and DNAs, which are involved in the process of intercellular communication (Zaborowski et al., Reference Zaborowski, Balaj, Breakefield and Lai2015). Using western blotting, Alcântara-Neto et al. (Reference Alcântara-Neto, Fernandez-Rufete, Corbin, Tsikis, Uzbekov, Garanina, Coy, Almiñana and Mermillod2020) identified the presence of proteins (OVGP1 and MYH9) involved in gamete–oviduct interactions in EVs recovered from the oviduct. OVGP1 binds to sperm and oocytes in association with MYH9 through its conserved non-glycosylated N-terminal region (Kadam et al., Reference Kadam, D’Souza, Bandivdekar and Natraj2006), indicating that both proteins participate together in the process of maturation and fertilization of gametes. These data suggested that the EV content reinforced the intercellular communication carried out by the free proteins in the OF, possibly by protecting them against degradation by the proteases present in this fluid.
Previous studies have evaluated the role of the OEC (Romar et al., Reference Romar, Coy, Campos, Gadea, Matás and Ruiz2001), and OF (Coy et al., Reference Coy, Cánovas, Mondéjar, Saavedra, Romar, Grullón, Matás and Avilés2008) in modulating the IVF parameters demonstrated that the effect is an independent species. Similar to data reported in the literature, the results of the present study showed an increased incidence of polyspermy when IVF occurs only in the presence of bovine OEC (Kawakami et al., Reference Kawakami, Kashiwagi, Hori and Tsutsui2001; Gualteri and Talevi, Reference Gualteri and Talevi2003; Romar et al., Reference Romar, Coy, Ruiz, Gadea and Rath2003; Cortés et al., Reference Cortés, Orihuela, Zúñiga, Velásquez and Croxatto2004; Yeste et al., Reference Yeste, Lloyd, Badia, Briza, Bonet and Holt2009). This beneficial effect of OEC on the spermatozoa is associated with sperm capacitation, prolonged sperm survival, enhanced sperm viability and motility, and modified frequency of tail beat. However, some authors have proposed that the effect of the oviductal cell disappears or is masked when high sperm concentrations are used (Romar et al., Reference Romar, Coy, Campos, Gadea, Matás and Ruiz2001; Dubuc and Sirard, Reference Dubuc and Sirard1995). Nevertheless, in the present study, increased sperm concentration, was followed by increase in sperm–zona binding, sperm–oocyte penetration and pronucleus formation in oocytes.
The second experiment demonstrated that the combination of OFF and OEC enhanced the production of monospermic zygotes in the presence of heparin. Heparin has an important role in sperm capacitation, which is required for efficient fertilization in mammals. Heparin, is supposed to bind to the spermatozoa plasma membrane and stimulate: (i) the intracellular increase of calcium, pH, and cAMP, which seem to be necessary to initiate the signalling pathways leading to capacitation (Galantino-Homer et al., Reference Galantino-Homer, Visconti and Kopf1997; Parrish et al., Reference Parrish, Susko-Parrish, Winer and First1988; Visconti et al., Reference Visconti, Galantino-Homer, Moore, Bailey, Ning, Fornes and Kopf1998); and (ii) the removal of seminal plasma proteins adsorbed to the plasma membrane, which are considered to be inhibitors of capacitation (Miller et al., Reference Miller, Winer and Ax1990; Therien et al., Reference Therien, Bleau and Manjunath1995). Conversely, evidence has shown that heparin triggers binding of OVGP1 to the ZP, increasing its resistance to enzymatic digestion by pronase (Coy et al., Reference Coy, Cánovas, Mondéjar, Saavedra, Romar, Grullón, Matás and Avilés2008). For these reasons, heparin was added to the IVF medium in Experiment 2 to test the effect of oviductal environment on fertilization. The treatment of oocytes with OFF before fertilization (OFF 30’) may induce zona hardening which would limit sperm penetration. Then, the presence of OEC during fertilization may enhance sperm capacitation and viability. Altogether, these two effects, that can be dependent on the presence of heparin, would regulate the polyspermy while maintaining a high penetration rate, resulting in increased IVF efficiency (production of monospermic zygotes).
Porcine polyspermic zygotes can develop to the blastocyst stage at the same rate as normally fertilized zygotes (Han et al., Reference Han, Abeydeera, Kim, Moon, Cabot and Day1999a). Depending on the location of the pronuclei, polyploid zygotes may develop into triploid, polyploid, or mixoploid embryos, while only the latter is able to develop to term (Han et al., Reference Han, Wang, Abeydeera, Petersen, Kim and Murphy1999b). This may be confounding while studying the effect of IVF conditions on the rate of embryo production. Nevertheless, in the present study, a reduction in blastocyst production was observed in the control and OEC groups compared with the OFF group when IVF was performed with 1.5 × 105 or 4.5 × 105 cells/ml. Both sperm concentrations resulted in significant increased polyspermy in the control group compared with the OFF 30’ and OFF 30’ + OEC groups, suggesting an adverse effect of polyspermy on embryo development up to the blastocyst stage.
In conclusion, regardless of the spermatic concentration used in this study, exposure of oocytes to diluted OFF during IVF improved the monospermy rate (monospermic/penetrated), while OEC during IVF increased the penetration rate at low sperm concentration, but did not improve the monospermy rate at any concentration. Exposure of oocytes to OFF before IVF increased the monospermy rate but reduced the penetration rate, resulting in monospermic zygote production similar to that of control. This negative effect of OFF on penetration can be alleviated by the presence of OEC during IVF, resulting in improved normal zygote production. These findings will contribute to the development of new approaches to improve the production of monospermic porcine zygotes by IVF.
Financial support
R.I.T.P. Batista was supported by a scholarship from CNPq (Brasília, Brazil). The authors wished to thank the CAPES-COFECUB bilateral framework for financial support for the collaboration between the State University of Ceará and INRA.
Conflicts of interest
The authors declare no conflicts of interest.
Ethical standards
Not applicable.







