Introduction
Mammalian oocyte maturation refers to the developmental period between GVB and the formation of the first polar body (PB) and involves dynamic changes within the cytoskeletal network. Initially, the meiotic spindle forms at a position slightly off-centre, where the germinal vesicle (GV) was located originally. After germinal vesicle breakdown (GVB), microtubules start to polymerize around the condensing chromosomes and, as the chromosomes congress, a bipolar metaphase I spindle is established with chromosomes aligned correctly at the metaphase plate. Meiotic spindle migration occurs after its formation and this process relies on a dynamic microfilament meshwork (Azoury et al., Reference Azoury, Lee, Georget, Rassinier, Leader and Verlhac2008), actin filament nucleator formin-2 (Dumont et al., Reference Dumont, Million, Sunderland, Rassinier, Lim, Leader and Verlhac2007) and RhoGTPase (Na & Zernicka-Goetz, Reference Na and Zernicka-Goetz2006). The peripheral spindle migration is a crucial process that assures conservation of ooplasm through asymmetrical cell division of the PB.
AMP-activated protein kinase (AMPK) is a heterotrimeric protein that contains an α catalytic subunit and β and γ regulatory subunits. It regulates the cellular energy level by sensing the ratio of AMP to ATP and responds to low ATP levels by shutting down the energy-consuming pathways and upregulating ATP production. It is activated via phosphorylation by upstream kinases of threonine 172 on the catalytic α subunit (Hardie, Reference Hardie2003; Carling, Reference Carling2004). Our laboratory has previously demonstrated a role for AMPK in the resumption of meiosis in mouse oocytes by showing that its activation by a variety of means induces GVB and that meiosis can be blocked by its inhibition (Chen et al., Reference Chen, Hudson, Chi, Chang, Moley, Hardie and Downs2006; Downs & Chen, Reference Downs and Chen2006; LaRosa & Downs, Reference LaRosa and Downs2007; Chen & Downs, Reference Chen and Downs2008). Further, AMPK not only has a role in GVB, but participates throughout the entire maturation period, associating with chromosomes and the meiotic spindle and exerting positive and negative effects, respectively, on PB formation and activation (Downs et al., Reference Downs, Ya and Davis2010).
The meiotic spindle association of AMPK is intriguing, because numerous proteins that show a similar localization pattern are known to regulate meiotic spindle assembly and function. AMPK has also been shown to have a role in the maintenance of genome integrity and establishment of cell polarity in Drosophila embryos and other somatic cells (see Discussion). It was, therefore, of interest to test how microtubule and spindle integrity were related to AMPK function in mouse oocytes. Our data indicate that disruption of microtubule integrity blocks hormone-induced maturation in meiotically arrested oocytes while coincidentally blocking AMPK activity. Moreover, active AMPK colocalization with γ-tubulin at microtubule organization centres (MTOC) during metaphase I and metaphase II is dependent on intact microtubules. However, treatment of oocytes with AMPK inhibitor did not prevent spindle formation or migration, despite suppressing the completion of meiotic maturation.
Materials and methods
Oocyte isolation and culture condition
All procedures were approved by the Marquette University Institutional Animal Care and Use Committee. Immature, 19–23-day-old (C57B/ 6J × SJL/J) F1 female mice were used for all experiments. Mice were primed with 5 IU pregnant mare serum gonadotropin (PMSG) 2 days before the experiments. Mice were killed by cervical dislocation and ovaries were removed and placed in a Petri dish containing culture medium. CEO were isolated by puncturing the follicles with sterile needles. DO were obtained by stripping cumulus cell by using a mouth-operated small-bore pipette. Both CEO and DO were washed twice and transferred to plastic culture tubes that contained 1 ml of the appropriate test medium. The culture medium used was Eagle's MEM (Sigma, St Louis, MO, USA) supplemented with penicillin, streptomycin sulphate, 0.23 mM sodium pyruvate and 3 mg/ml BSA (MP Biomedicals, Solon, OH, USA).
Immunofluorescence staining
Oocytes were fixed with microtubule-stabilizing buffer as described previously (Messinger & Albertini, Reference Messinger and Albertini1991) at 4°C overnight. Oocytes were permeabilized for 30 min with 0.1% Triton-100 in blocking solution, which contained 10% donkey serum and 0.5 mg/ml saponin in phosphate-buffered saline (PBS), followed by 1 h in blocking solution minus Triton-100. Oocytes were incubated with primary antibody (1:100) overnight at 4°C, and washed four times at room temperature in blocking buffer. Oocytes were coincubated with FITC-conjugated anti-tubulin antibody (1:100) and Cy3-conjugated secondary antibody (1:100) 1 h at room temperature. After washing, oocytes were placed on slides and mounted with medium that contained 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA, USA).
Confocal microscopy
Oocytes were viewed on a laser scanning confocal microscope (Carl Zeiss Co., Thornwood, NY, USA) with a ×63 objective. During scanning, all settings were kept constant: i.e., laser power, detector gain, amplifier offset, amplifier gain, and pinhole size. Digitally recorded images were exported by LSM Examiner software (Carl Zeiss Co.).
Western blot analysis
Oocytes samples were washed with PBS/polyvinylpyrrolidone (PVP), then twice with a protease inhibitor cocktail (Roche, Indianapolis, IN, USA). Samples were treated with Laemmli's Buffer with 20% beta-mercaptoethanol at 95°C for 5 min. Electrophoresis was carried out using NuPAGE 3–8% Tris–acetate gels (Invitrogen, Carlsbad, CA, USA), and proteins were transferred to nitrocellulose. Membranes were blocked with 5% non-fat milk, followed by incubation with pACC primary antibody (1:250, Cell Signaling) at 4°C overnight. Blots were rinsed with Tris-buffered saline (TBS) (pH 7.4) and TBS–Tween-20 (0.05%), and incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody (1:2000 in 5% non-fat milk, Pierce) at room temperature for 60 min. After washing, protein signals were detected by Super Signal West Pico Chemiluminescent Substrate (Pierce). Blots were stripped with Restore Western Blot Stripping Buffer (Thermo Scientific; Rockford, IL, USA) and reprobed with anti-ACC1 antibody (1:250; Cell Signaling) as a loading control. The pACC/ACC1 ratios were quantified with ImageJ software based on protein band density.
Chemicals
Saponin, dbcAMP, donkey serum, nocodazole, palmitoyl carnitine, and FITC-labelled mouse anti-α-tubulin were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Cy3-conjugated donkey anti-rabbit antibody was supplied by Jackson ImmunoResearch (West Grove, PA, USA). AICAR and compound C were obtained from Toronto Research Chemicals, Inc. (North York, Ontario, Canada). Anti-phospho-AMPK (PT172) antibody was purchased from Cell Signaling Technology (Beverly, MA, USA). Highly purified ovine FSH was from the National Hormone and Peptide Program (NHPP) and Dr A.F. Parlow.
Statistical analysis
All experiments were repeated at least three times and data presented as mean ± standard error of the mean (SEM). Percentages of GVB or nuclear stain underwent arcsin transformation and data were analyzed statistically by analysis of variance (ANOVA) followed by Duncan's multiple range test. A P-value <0.05 was considered significant.
Results
Effect of cytoskeletal disrupting agents on maturation and AMPK activity
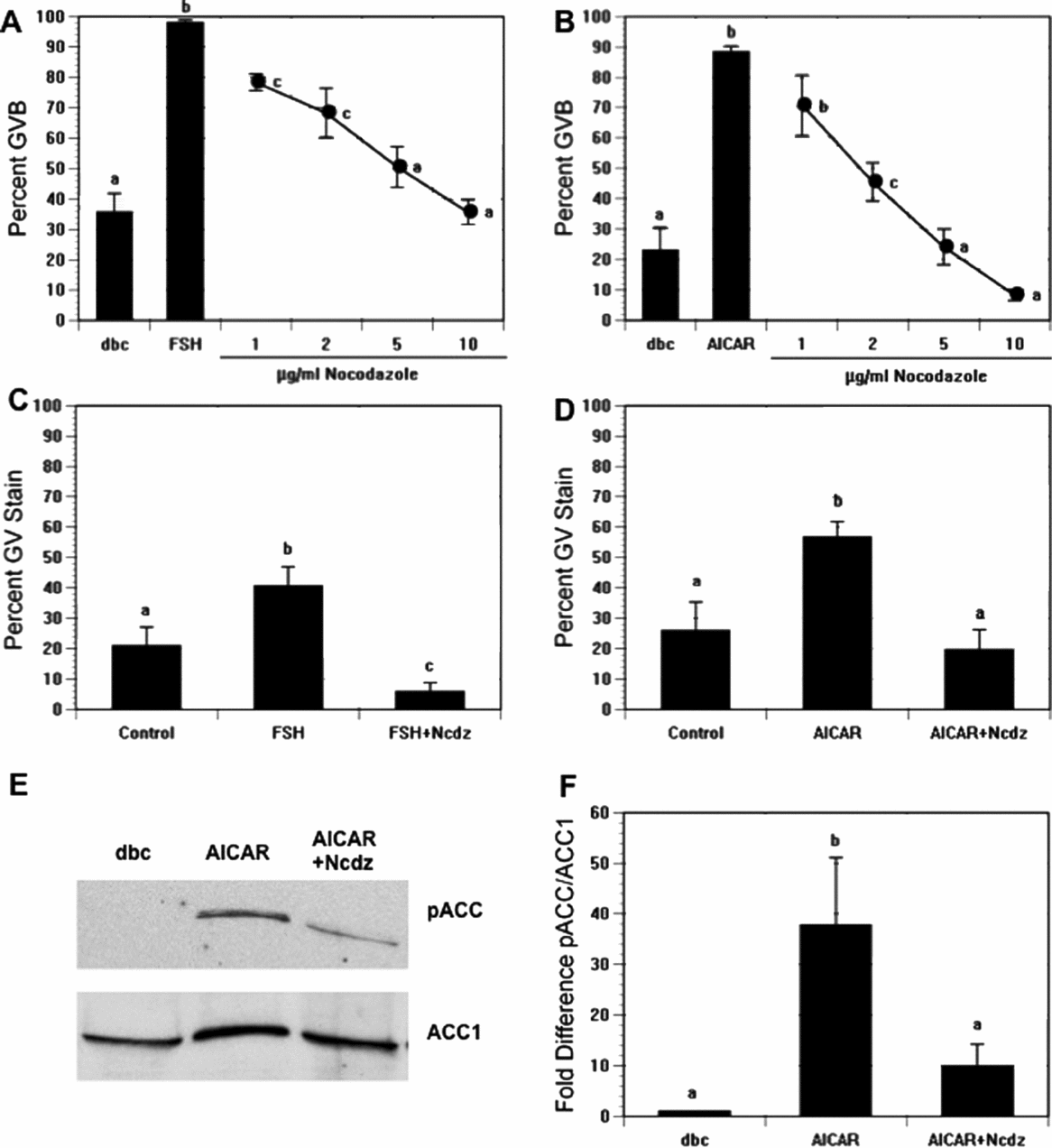
We have shown that AMPK is involved in hormone-induced maturation and that its activation precedes GVB in mouse oocytes (Chen & Downs, Reference Chen and Downs2008). We therefore determined how perturbation of microtubule integrity would affect AMPK activity and meiotic induction. dbcAMP-arrested CEO were treated with 0.1 μg/ml follicle-stimulating hormone (FSH) to induce meiotic resumption, exposed to increasing concentrations of nocodazole, and assessed for GVB after 17–18 h. As shown in Fig. 1A, FSH increased the percentage of GVB by 62%, while supplementation with nocodazole blocked this increase significantly in a dose-dependent manner, by 62% at the highest dose tested. Dimethyl sulphoxide (DMSO) alone, the nocodazole vehicle, had no effect on meiotic resumption (data not presented). To test for a direct effect of nocodazole on the oocyte, dbcAMP-arrested DO were treated with 250 μM AICAR, an AMPK activator, and scored for GVB. AICAR stimulated an increase in GVB from 23 to 89%. This stimulation was abolished by nocodazole, which decreased significantly the percentage of maturation to 8% (Fig. 1B). This inhibition was completely reversible, thereby demonstrating that the drug did not act through toxic means.

Figure 1 Effects of nocodazole treatment on meiotic resumption and AMP-activated protein kinase (AMPK) activation. (A) Cumulus cell-enclosed oocytes (CEO) were cultured in medium that contained 300 μM dbcAMP (dbc) plus follicle-stimulating hormone (FSH) in the presence of increasing concentrations of nocodazole (Ncdz). Germinal vesicle breakdown (GVB) was assessed 17–18 h later. (B) Denuded oocytes (DO) were cultured with 300 μM dbcAMP plus 250 μM 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR) and treated with increasing doses of nocodazole. GVB was assessed after 4 h of culture. (C) CEO were cultured in 300 μM dbcAMP alone (control) or dbcAMP plus FSH with or without 10 μg/ml nocodazole; 4 h later oocytes were fixed and processed for PT172 staining. (D) DO were cultured for 2.5 h in the same medium as CEO but with FSH replaced by 250 μM AICAR; oocytes were then processed for PT172 staining. In both (C) and (D), nocodazole reduced the frequency of the GV stain to control levels. (E) Western blot analysis of pACC of oocytes that were treated as described in (B); acetyl coenzyme A carboxylase 1 (ACC1) was used as loading control. (F) Quantification of phospho-ACC (pACC) and ACC1 from western blot. pACC/ACC1 ratios were normalized to the control dbcAMP-treated group. a,b,cGroups with no common letter are significantly different.
FSH-treated CEO were cultured for 4 h and AICAR-treated DO were cultured for 2.5 h before fixation and immunofluorescence staining for active AMPK, using an antibody against phospho-threonine 172 on the alpha catalytic subunit of AMPK. As shown previously, FSH and AICAR increased phospho-AMPK staining in the germinal vesicle prior to GVB. This staining pattern is characterized by homogeneous staining throughout the GV with absence of label within nucleoli (Chen & Downs, Reference Chen and Downs2008). As nocodazole blocked AICAR- and FSH-induced GVB, it was important to determine if this GV-staining pattern was present after nocodazole treatment of these oocytes. As shown in Fig. 1C, 21% of control CEO arrested in dbcAMP showed nuclear staining, with a 20% increase following FSH treatment; exposure to 10 μg/ml nocodazole eliminated this increase. A similar pattern was evident in DO: GV staining was detected in 26% of control oocytes, with this number increasing to 57% after culture in AICAR; nocodazole again prevented the response (20% with active AMPK accumulation in the GV; Fig. 1D). These data support the idea that activity of AMPK is greatly influenced by microtubule integrity. This idea was confirmed by western blot analysis of nocodazole-treated oocytes that showed suppression of AICAR-induced AMPK activity, using phospho-acetyl coenzyme A (CoA) carboxylase (pACC) as a marker for AMPK activity (Fig. 1E, F).
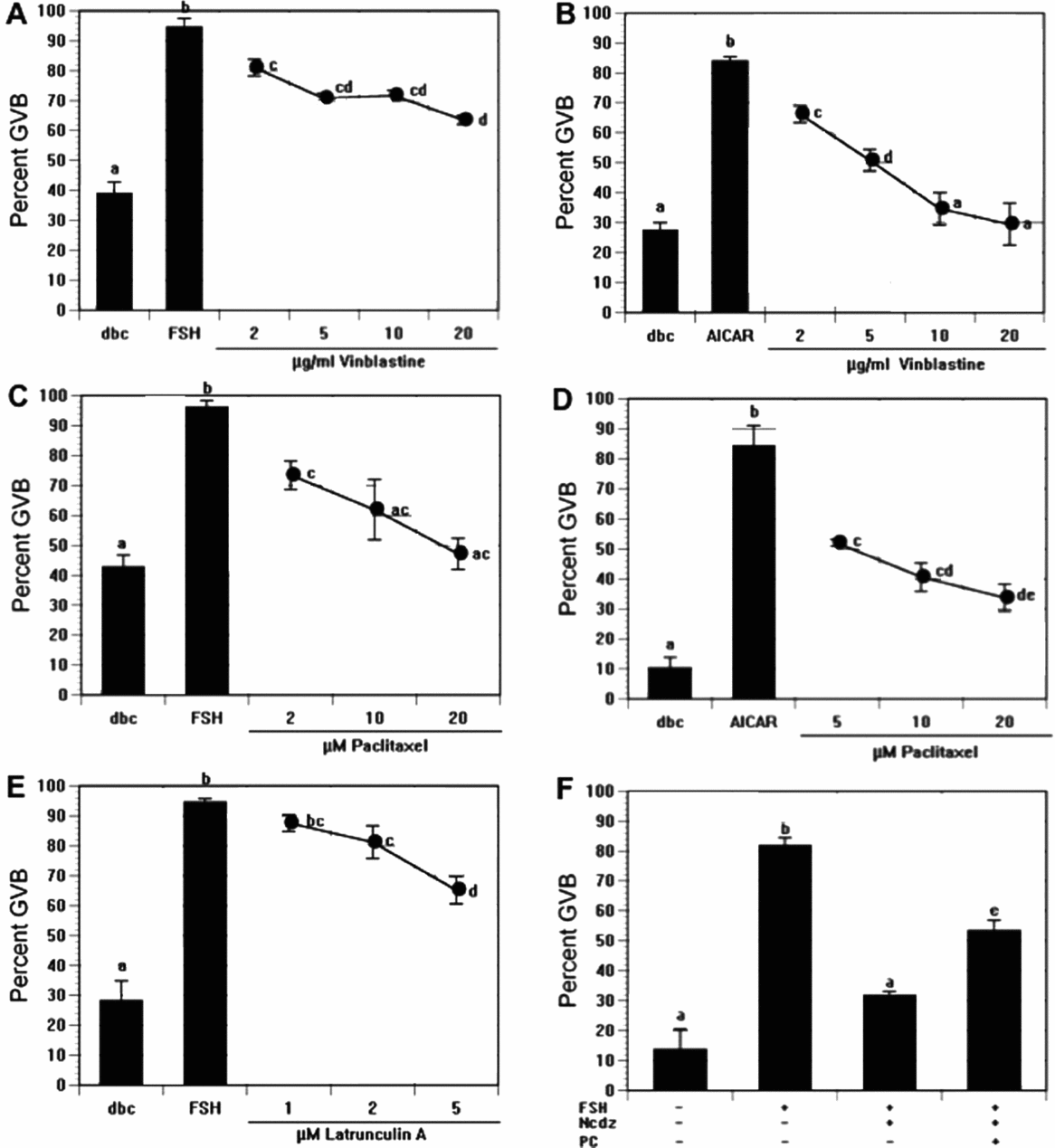
When CEO were treated with another microtubule-destabilizing agent, vinblastine, meiotic induction was again suppressed, with a 31% decrease at the highest concentration tested (Fig. 2A). Inhibition was even more robust in AICAR-treated DO, with 20 μg/ml vinblastine reducing the maturation percentage to control levels (Fig. 2B).

Figure 2 Effects of additional microtubule-targeted agents on follicle-stimulating hormone (FSH)-induced and 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR)-induced maturation in vitro. (A, C) Cumulus cell-enclosed oocytes (CEO) were cultured 17–18 h in medium that contained 300 μM dbcAMP plus follicle-stimulating hormone (FSH) or (B, D) denuded oocytes (DO) were cultured 4 h in 300 μM dbcAMP plus 250 μM AICAR, in the presence of increasing concentrations of vinblastine (A, B) or paclitaxel (C, D). (E) CEO were cultured 17–18 h in 300 μM dbcAMP plus FSH and increasing concentrations of latrunculin A before germinal vesicle breakdown (GVB) assessment. (F) dbcAMP-arrested CEO were induced to undergo maturation with FSH, and this stimulation was suppressed by further treatment with nocodazole (Ncdz). Palmitoyl carnitine (PC) (50 μM) was added to the latter group to trigger GVB via activation of fatty acid oxidation. a,b,c,dGroups with no common letter are significantly different.
The microtubule-stabilizing agent, paclitaxel, was utilized to test how stabilizing microtubules affects meiotic induction. CEO were cultured 17–18 h and treated with FSH plus increasing doses of paclitaxel. Similar to the finding with microtubule-disrupting agents, FSH-induced GVB was blocked in dose-dependent fashion by paclitaxel (Fig. 2C), and the agent was even more potent in AICAR-treated DO (Fig. 2D). No label was observed in the GV when FSH- and paclitaxel-treated oocytes were fixed after 4 h and stained with anti-PT172 antibody; however, punctate staining was present throughout the cytoplasm (data not shown). When paclitaxel was added to AICAR-treated DO, punctate staining of active AMPK was observed near or within the GV, but with no homogeneous staining within the GV. The increased punctate staining of active AMPK after paclitaxel treatment may correspond to excessive stabilization of microtubule polymers.
Cytochalasin D and latrunculin A were added to either FSH-stimulated CEO cultures or AICAR-stimulated DO cultures to determine whether inhibition of actin filament polymerization plays a role in meiotic induction. There was no effect of cytochalasin D on GVB. Latrunculin A showed a modest reduction (29%) of GVB in FSH-treated CEO (Fig. 5E), but it was toxic to AICAR-treated DO.
An experiment was carried out to determine if the nocodazole-sensitive step could be bypassed by stimulating a downstream meiosis-inducing pathway. To this end, we used the fatty acid derivative, palmitoyl carnitine, a compound shown previously to drive meiotic resumption through an increase in fatty acid oxidation (Downs et al., Reference Downs, Mosey and Klinger2009). FSH treatment increased the frequency of GVB by 68% in dbcAMP-arrested CEO, and exposure to nocodazole suppressed this action by 50%. In the presence of nocodazole, palmitoyl carnitine restored the induction by 22% (Fig. 2F). These results demonstrate that the palmitoyl carnitine-stimulated pathway is still active in the presence of nocodazole, thereby indicating nocodazole treatment does not produce a generalized toxicity.
Association of active AMPK with microtubules and effect of microtubule perturbants on localization
As studies in other laboratories have demonstrated that the meiotic spindle-associated localization of numerous proteins is dependent on MT integrity, the next series of experiments were conducted to confirm this relationship for AMPK. Oocytes undergoing in vivo maturation were fixed 8 h after human chorionic gonadotropin (hCG) injection to obtain metaphase I-stage oocytes and stained with antibodies to active AMPK and γ-tubulin. As expected, AMPK colocalized with γ-tubulin at the spindle poles during MI (Fig. 3A).

Figure 3 Immunofluorescence staining of active AMP-activated protein kinase (AMPK) and tubulin. (A) Oocytes were collected 8 h after administration of hCG to pregnant mare serum gonadotropin (PMSG)-primed mice to obtain oocytes at MI. Active AMPK (green) is colocalized at the spindle poles with γ-tubulin (red). Chromatin, blue. Inset: Metaphase I spindle from triple-stained oocyte showing α-tubulin, green; active AMPK, red; chromatin, blue. (B) Effect of microtubule-targeted agents on active AMPK localization. Cumulus cell-enclosed oocytes (CEO) were cultured 17 h in the presence of 300 μM dbcAMP plus follicle-stimulating hormone (FSH); then either the microtubule-destabilizing agent, nocodazole (Ncdz, 0.05 μg/ml), or microtubule-stabilizing agent, paclitaxel (Pac, 20 μM), was added and oocytes were fixed 1 h later and processed for immunostaining for active AMPK (PT172, red) and α-tubulin (green). Nocodazole treatment disrupted the normal spindle pole localization of active AMPK. When treated with paclitaxel, microtubules were excessively polymerized, and this was associated with increased AMPK staining. After CEO were cultured 17 h in the presence of 300 μM dbcAMP plus FSH, oocytes were exposed to nocodazole (5 μg/ml) for 10 min. The nocodazole was then washed out and oocytes were allowed to recover for 1 h before fixation and immunostaining. Upon microtubule repolymerization, active AMPK reestablished its normal spindle pole localization. Active AMPK, red; α-tubulin, green; chromatin, blue. Scale bar, 50 μm.
To test if active AMPK localization is dependent on spindle microtubule integrity, microtubule-targeted agents were tested on CEO during in vitro culture. Isolated CEO were arrested in 300 μM dibutyryl cAMP (dbcAMP) and then treated with 0.1 μg/ml FSH to induce maturation. CEO were cultured for 17 h before addition of the microtubule-depolymerization agent, nocodazole (0.05 μg/ml), or the microtubule-stabilizing agent, paclitaxel (20 μM), and oocytes were fixed 1 h later and stained for active AMPK and α-tubulin. Nocodazole depolymerized the spindle and, as a result, chromosomes became scattered and, not surprisingly, normal spindle pole localization of active AMPK was disrupted (Fig. 3B). When oocytes were treated with paclitaxel, microtubules were polymerized excessively and spindles were larger than normal; formation of a double meiotic spindle was occasionally observed, with two points of active AMPK localization at each pole. Many small asters also appeared in the cytoplasm with colocalized AMPK (Fig. 3C). In vitro matured MII-stage CEO were subjected to nocodazole (5 μg/ml) for 10 min to test whether re-establishment of a bipolar spindle could return active AMPK to its normal localization. The nocodazole was then washed out and oocytes were allowed to recover for 1 h to allow reformation of a bipolar MII spindle before fixation and processing for immunofluorescence staining. The MII spindle completely disappeared after 10 min treatment with nocodazole and active AMPK was dispersed randomly as before (data not shown). Upon spindle repolymerization, active AMPK localization returned to the spindle poles (Fig. 3D), thereby demonstrating reversibility of the nocodazole effect and a microtubule-dependent localization of active AMPK in mouse oocytes.
Effect of AMPK modulators on cytoskeleton dynamics
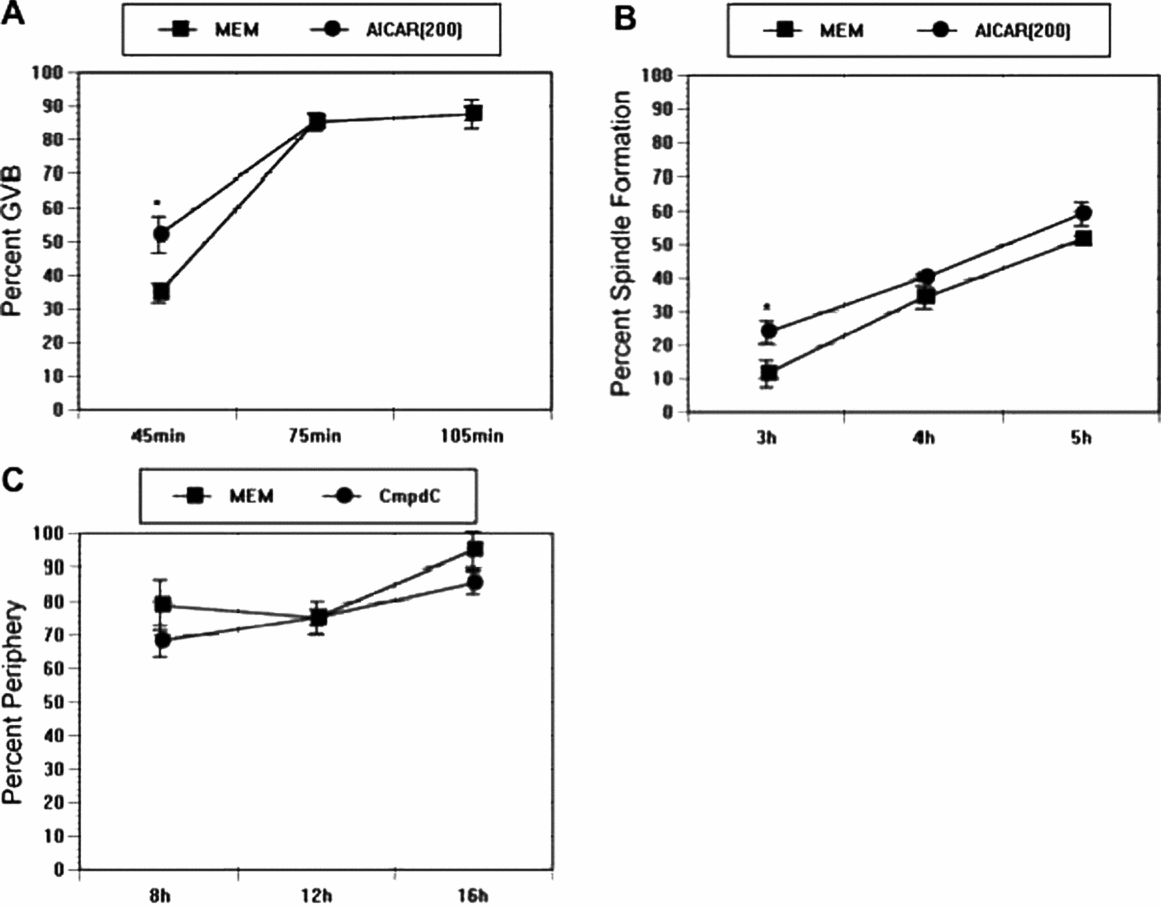
Our data suggest that microtubule integrity regulates localization and activity of AMPK. We next tested if the reverse regulation was true; that is, if AMPK regulates spindle dynamics. AMPK stimulators, AICAR, AMP and 8-Br-adenosine accelerate PB formation of spontaneously maturing CEO (Downs et al., Reference Downs, Ya and Davis2010), and it is possible that this effect is mediated by accelerated spindle formation. First, CEO were matured spontaneously in minimum essential medium (MEM)/bovine serum albumin (BSA) control medium in the presence or absence of 200 μM AICAR and the kinetics of GVB were determined. After 45 min of AICAR treatment, the percentage of GVB was increased from 35 to 52%; thereafter, maturation percentages were comparable up to 105 min of culture (Fig. 4A). CEO that were cultured in the presence or absence of AICAR were fixed at discrete time points and processed for α-tubulin immunofluorescence staining to examine the impact of AICAR on spindle formation. Polymerization of microtubules into a barrel-shaped spindle was the criterion used for assessment. AICAR increased spindle formation significantly at the early time point (an increase from 12 to 24% at 3 h), which is roughly 1 h after GVB (Fig. 4B). A trend towards increased spindle formation in AICAR-treated oocytes continued for the next 2 h, but differences were not significant.

Figure 4 (A) Effect of AMP-activated protein kinase (AMPK) activator, 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR), on spontaneous maturation. Cumulus cell-enclosed oocytes (CEO) were cultured in control MEM or medium supplemented with 250 μM AICAR, and percentage of germinal vesicle breakdown (GVB) was checked at 45, 75 and 105 min after initiation of the culture. (B) Effect of AICAR on early spindle formation. CEO were cultured in minimum essential medium (MEM) or MEM plus 250 μM AICAR for 3, 4, and 5 h before spindle formation was assessed by immunofluorescence staining. (C) Effect of the AMPK inhibitor, compound C, on spindle periphery movement. CEO were cultured in control medium or medium that contained 2.5 μM compound C. Spindle periphery movement was examined at the indicated time points. An asterisk denotes a significant difference from the corresponding control.
Spindle migration is important for asymmetric cell division in oocytes, which helps minimize loss of cytoplasm during PB extrusion. We determined whether blocking AMPK activity could influence the migration of the meiotic spindle. Spontaneously maturing CEO were supplemented with the AMPK inhibitor, compound C, and position of the spindle was checked at 8, 12 and 16 h after initiation of culture. Spindles were considered to have migrated to the periphery if they were located more than one-half the radius of the oocyte from its centre. As shown in Fig. 4C, by 8 h culture 79% of the control oocytes had a spindle located peripherally; this number was 68% in compound C-treated oocytes, which was not different significantly from controls. By 16 h, most CEO in control medium had released the first PB and 95% of the oocyte spindles were localized peripherally. Treatment with compound C significantly blocked PB formation, but did not interfere with the peripheral movement of the spindle.
Finally, we tested whether inhibition of AMPK activity would perturb actin microfilament dynamics, especially the polymerization status of the cortical region. Extensive actin polymerization occurs in the cortical area and facilitates attachment of the outer-most spindle pole to the cortex (Azoury et al., Reference Azoury, Lee, Georget, Rassinier, Leader and Verlhac2008). When CEO were cultured in control MEM or medium that contained 2.5 μM compound C for 9.5 h and 11.5 h and processed for phalloidin staining, normal F actin polymerization was observed in both groups (data not shown). Thus, compound C treatment did not interfere with PB formation by blocking actin microfilament polymerization.
Discussion
In this study, we showed that perturbation of microtubule integrity in mouse oocytes interferes reversibly with meiotic induction of CEO by FSH and DO by AICAR, as well as the localization of active AMPK during maturation, and was accompanied by loss of AMPK activity. Microfilament perturbants had little effect on maturation. While AMPK stimulation accelerated both GVB and spindle formation, blockage of its activity with compound C did not affect actin polymerization or interfere with peripheral spindle migration, although karyokinesis was suppressed.
Disruption of microtubules with nocodazole and vinblastine, as well as their stabilization with paclitaxel, blocked both AICAR-induced maturation in DO and FSH-induced maturation in CEO. This was associated with suppression of AMPK activation, as determined by western blotting, as well as loss of pAMPK staining in the GV prior to meiotic resumption (Chen & Downs, Reference Chen and Downs2008). Compartmentalization of different cellular components is a crucial mechanism for controlling function in discrete microenvironments and influences cell cycle regulation (Pines, Reference Pines1999). For instance, Cdc25B adopts nuclear localization shortly before GVB, where it regulates MPF activity (Oh et al., Reference Oh, Han and Conti2010), and mitogen-activated protein kinase (MAPK) translocates to the GV just before GVB in pig oocytes (Inoue et al., Reference Inoue, Naito, Nakayama and Sato1998). That nocodazole and paclitaxel suppressed GV staining of pAMPK in FSH- and AICAR-treated oocytes, coincident with a reduction in meiotic resumption, suggests a microtubule-dependent step within the oocyte in AMPK regulation of meiosis that involves nuclear localization. Nuclear transport is mediated by nuclear pore complexes that span the nuclear membrane and facilitate the exchange of proteins between the cytoplasm and nucleoplasm (Liu & Liu, Reference Liu and Liu2007; Stewart, Reference Stewart2007). In some instances, successful nuclear import requires the active participation of the cytoskeleton (Campbell & Hope, Reference Campbell and Hope2003; Salman et al., Reference Salman, Abu-Arish, Oliel, Loyter, Klafter, Granek and Elbaum2005; Wagstaff & Jans, Reference Wagstaff and Jans2009). This event has been demonstrated for p53 (Giannakakou et al., Reference Giannakakou, Sackett, Ward, Webster, Blagosklonnyu and Fojo2000; Rathinasamy & Panda, Reference Rathinasamy and Panda2008) and parathyroid hormone-related protein (Lam et al., Reference Lam, Thomas, Loveland, Schilders, Gu, Martin, Gillespie and Jans2002), when microtubule-dependent transport of protein to the nucleus has been implicated. It has been proposed that microtubule-directed relocation of cellular components to a perinuclear location is instrumental in activation of maturation-promoting factor and driving meiotic resumption in oocytes (eg, Van Blerkom, Reference Van Blerkom1991; Albertini, Reference Albertini1992). It is reasonable to propose that a similar mechanism exists for AMPK – i.e. that microtubules aid in the meiotic induction process by facilitating accumulation of AMPK at the nucleus periphery, where it is then imported by conventional means. It is also possible that disruption of microtubule dynamics perturbs the spatial organization of signalling pathway components other than, or in addition to, AMPK that help drive meiotic resumption in response to FSH and AICAR. Consistent with this possibility was the nocodazole-mediated suppression of AMPK activity as assessed by western analysis. It is unlikely that nocodazole acted via non-specific toxicity, because its inhibitory action could be bypassed by treatment with palmitoyl carnitine.
Nocodazole blocks GVB in spontaneously maturing rat oocytes (Albertini, Reference Albertini1987), but we did not observe a similar inhibitory action on spontaneously maturing mouse oocytes (data not shown), results consistent with those of others (Wassarman et al., Reference Wassarman, Josefowicz and Letourneau1976; Van Blerkom & Bell, Reference Van Blerkom and Bell1986). Interestingly, Rime & Ozon (Reference Rime, Jessus and Ozon1988) suppressed GVB in mouse oocytes with estramustine, an anti-mitotic compound that can block microtubule polymerization through its interaction with microtubule-associated proteins. Supplementation with taxol also did not affect the GVB of spontaneously maturing mouse oocytes (data not shown) and this effect has been shown by others for both rat and mouse oocytes; however, like the effects with nocodazole, taxol impairs later meiotic progression, resulting in failure of karyokinesis in mouse oocytes (Albertini, Reference Albertini1987; Combelles & Albertini, Reference Combelles and Albertini2001). These effects of microtubule perturbants imply differences between the two species regarding meiotic regulation (c.f., Downs, Reference Downs2011).
In a previous study (Downs et al., Reference Downs, Ya and Davis2010), we demonstrated that active AMPK associates with chromosomes after GVB, localizes to the spindle pole during metaphase I and II, and moves to the spindle midzone during anaphase. Here we show that active AMPK colocalizes with γ-tubulin, indicating an association with spindle organizing centres during formation of metaphase I spindles. This distribution pattern of pAMPK during oocyte maturation is similar to that occurring during mitosis of somatic cells (Vazquez-Martin et al., Reference Vazquez-Martin, Oliveras-Ferraros and Menendez2009) and is consistent with a reported role for AMPK in cell cycle progression (Bettencourt-Dias et al., Reference Bettencourt-Dias, Glet, Sinka, Mazumdar, Lock, Balloux, Zafiropoulos, Yamaguchi, Winter, Carthew, Cooper, Jones, Frenze and Glover2004; Alessi et al., Reference Alessi, Sakamoto and Bayascas2006; Koh & Chung, Reference Koh and Chung2007; Williams & Brenman, Reference Williams and Brenman2008). Indeed, previous work from this laboratory has demonstrated that AMPK not only induces meiotic resumption in mouse oocytes (Chen et al., Reference Chen, Hudson, Chi, Chang, Moley, Hardie and Downs2006; Chen & Downs, Reference Chen and Downs2008) but also promotes the completion of maturation and prevents premature activation (Downs et al., Reference Downs, Ya and Davis2010). The changes of AMPK localization throughout maturation suggest a dynamic cellular transport process at work. The poleward transport of active AMPK might be dependent on the minus-end directed motor protein, dynein, which has been shown to be critical for spindle pole transport of proteins such as NuMA and Eg5 (Merdes et al., Reference Merdes, Heald, Samejima, Earnshaw and Cleveland2000; Uteng et al., Reference Uteng, Hentrich, Miura, Bieling and Surrey2008).
As AMPK shows a close association with microtubule-containing structures, it is not surprising that perturbation of microtubules dramatically affected the localization of AMPK within the oocyte. Nocodazole destruction of spindle structure led to dispersal of active AMPK in a punctate staining pattern that coincided with staining of microtubule asters, which indicated that the localization pattern of active AMPK is dependent on the integrity of the meiotic spindle and presence of MTOCs. Mouse oocytes have multiple acentriolar MTOCs that can be grouped into two subsets: one subset is associated with the meiotic spindle and the other is cortical (Maro et al., Reference Maro, Howlett and Webb1985; Schatten et al., Reference Schatten, Schatten, Mazia, Balczon and Simerly1986; Messinger & Albertini, Reference Messinger and Albertini1991). Active AMPK was associated with both types of MTOCs. It is possible that active AMPK is more concentrated around MTOCs with nucleating capacity, as stabilization of microtubules with paclitaxel treatment induced further accumulation of AMPK at MTOCs and enlarged spindles.
In many cells, AMPK is regulated by LKB1 through phosphorylation of the threonine 172 activation site on the alpha subunit (Alessi et al., Reference Alessi, Sakamoto and Bayascas2006). LKB1 is a tumour suppressor that has been implicated in establishment of cell polarity through its involvement in AMPK activation (Alessi et al., Reference Alessi, Sakamoto and Bayascas2006; Williams & Brenman, Reference Williams and Brenman2008; Jansen et al., Reference Jansen, Ten Klooster, Offerhaus and Clevers2009). For example, loss of LKB1 led to polarity defects in lung cancer cells (Zhang et al., Reference Zhang, Schafer-Hales, Khuri, Zhou, Vertino and Marcus2008) and epithelial cells (Mirouse et al., Reference Mirouse, Swick, Kazgan, St Johnston and Brenman2007), neuroblasts (Bonaccorsi et al., Reference Bonaccorsi, Mottier, Giansanti, Bolkan, Williams, Goldberg and Gatti2007) and developing embryos (Lee et al., Reference Lee, Koh, Kim, Kim, Lee, Karess, Lee, Shong, Kim, Kim and Chung2007) of Drosophila. Moreover, Szczepańska & Maleszewski (Reference Szczepańska and Maleszewski2005) reported that LKB1 associates with the meiotic spindle in mouse oocytes and proposed that it has a role in oocyte polarity; thus, it was of interest to test whether AMPK is also involved in a similar capacity in mouse oocytes. In oocytes, the spindle forms at the centre of the oocyte after GVB, and then migrates toward the oocyte periphery where the PB is extruded, a process that is crucial to asymmetric cell division and differentiation of cortex into a cortical granule-free domain (Sun & Schatten, Reference Sun and Schatten2006).
Inhibition of AMPK activity in spontaneously maturing CEO with compound C did not perturb the peripheral movement of the spindle. Although some recent studies have also suggested the participation of microtubules (Ai et al., Reference Ai, Li, Schatten and Sun2009), spindle movement in the oocyte is known to be highly dependent on a dense actin microfilament meshwork (Azoury et al., Reference Azoury, Lee, Georget, Rassinier, Leader and Verlhac2008). Our results suggest that interfering with AMPK activity does not affect the integrity of the cortical actin network, because the extensive F actin polymerization in the cortex near the spindle appeared unperturbed by compound C treatment. Myosin regulatory light chain (MLC) is an important component of myosin II that participates in actomyosin-mediated cytokinesis at the cleavage furrow (Dumont et al., Reference Dumont, Million, Sunderland, Rassinier, Lim, Leader and Verlhac2007) and becomes phosphorylated in somatic cells in response to AMPK activation (Lee et al., Reference Lee, Koh, Kim, Kim, Lee, Karess, Lee, Shong, Kim, Kim and Chung2007). However, it is unclear whether MLC is a physiological substrate for AMPK under energy-depleted conditions (Bultot et al., Reference Bultot, Horman, Neumann, Walsh and Hue2009). It will be interesting to determine if AMPK influences the interaction of actin and myosin at the time of cytokinesis.
Acceleration of the kinetics of PB extrusion by AMPK-stimulating agents (Downs et al., Reference Downs, Ya and Davis2010) indicates an active role for AMPK throughout the entire maturation process, and this role might be accomplished by action on earlier meiotic events. Indeed, we show herein that the rate of GVB and spindle formation is increased by AICAR treatment. These data indicate that AMPK manifests an early effect during maturation that shortens the time required to complete maturation. In support of this indication, the increase in PB formation is eliminated when AICAR treatment is delayed, while delaying compound C treatment decreases its inhibitory action (Downs et al., Reference Downs, Ya and Davis2010). The early increase in spindle formation is likely due to the accelerated kinetics of GVB, but it should be pointed out that, in addition to faster maturation kinetics, AMPK activation also leads to increased PB formation.
The close association of AMPK with microtubules suggests a functional role with the meiotic apparatus. Numerous proteins exhibit a similar localization pattern and have important roles in spindle organization and function. For instance, aurora-A kinase induces GVB in Xenopus oocyte and has to be dephosphorylated for the MI to MII transition (Ma et al., Reference Ma, Cummings and Liu2003). In mouse oocytes, aurora-A is critical for spindle assembly, centrosome maturation and chromosome segregation. Furthermore, its role is not limited to oocyte maturation, as it also contributes to early embryo spindle organization (Yao et al., Reference Yao, Zhong, Zhang, Chen, Schatten and Sun2004; Ding et al., Reference Ding, Swain and Smith2011). Another aurora kinase family member, aurora-B, is one of the chromosome passenger proteins and localizes specifically to the metaphase chromosomes, where it mediates chromosome segregation and spindle kinetochore attachment (Ruchaud et al., Reference Ruchaud, Carmena and Earnshaw2007; Uzbekova et al., Reference Uzbekova, Arlot-Bonnemains, Dupont, Dalbies-Tran, Papillier, Pennetier, Thélie, Perreau, Mermillod, Prigent and Uzbekov2008). Aurora-C kinase localization is similar to that of active AMPK; moreover, injection of deficient kinase-induced failure of meiosis I (MI) and disrupted proper localization of kinetochore proteins, BubR1 and Bub (Yang et al., Reference Yang, Li, Chang, Tang, Lin, Lee and Tang2010). Astrin is associated with meiotic spindle microtubules with particular concentration at the spindle poles, and perturbation of its function greatly compromises normal spindle integrity and chromosome segregation (Yuan et al., Reference Yuan, Li, Wei, Yin, Xiong, Li, Lin, Schatten and Sun2009). The apoptosis inhibitor protein, survivin, mimics AMPK localization in mouse oocytes, and its depletion adversely affects chromosome alignment and alters normal PB extrusion (Sun et al., Reference Sun, Wei, Li, Lin, Xu, Liang, Kim, Schatten, Lu and Sun2009). Another similarly localized kinase, Polo-like kinase-1 (Plk1), was implicated in spindle assembly in oocytes and later mitosis in the embryo after fertilization (Wianny et al., Reference Wianny, Tayares, Evans, Glover and Zernicka-Goetz1998; Tong et al., Reference Tong, Fan, Lian, Li, Chen, Schatten and Sun2002). In addition to regulation of spindle organization, Plk1 also promotes M phase entry by participating in the MPF amplification loop (Karaiskou et al., Reference Karaiskou, Leprêtre, Pahlavan, Pasquier, Du, Ozon and Jessus2004), and blocking its activity with antibody reduces the GVB rate (Tong et al., Reference Tong, Fan, Lian, Li, Chen, Schatten and Sun2002). Of particular relevance to the present study is the recent finding that pharmacological inhibition of Plk1 blocks AMPK activation and cytokinesis in HeLa cells (Vazquez-Martin et al., Reference Vazquez-Martin, Oliveras-Ferraros, Cufi and Menendez2011). Another kinase, protein kinase C delta (PKCδ), associates with γ-tubulin and pericentrin, and disruption of its function leads to spindle disorganization and misalignment of chromosomes (Ma et al., Reference Ma, Koch and Vivieros2008). PKC has also been shown to be involved in the MI to MII transition (Viveiros et al., Reference Viveiros, Hirao and Eppig2001). Finally, phospho-MEK1/2, the upstream kinase for MAPK activation, displays a prominent presence at the spindle poles in mouse oocytes, and blocking MAPK leads to meiotic spindle abnormalities and poor chromatin condensation (Lu et al., Reference Lu, Dunn, Angeles and Smith2002; Yu et al., 2007; Sun et al., Reference Sun, Xiong, Lu and Sun2008). It may be that AMPK interacts with one or more of these proteins to influence meiotic progression. An alternate scenario is that the kinase docks at MTOCs without contributing to spindle assembly or function.
In conclusion, both AMPK localization and activity in the mouse oocyte are dependent on intact microtubules. Further, while perturbation of microtubule integrity blocks meiotic induction at the level of the oocyte, it has no effect on spontaneous maturation. This situation demonstrates that additional microtubule-sensitive modalities are required to overcome induction of maturation in meiotically arrested oocytes and lends further support to the idea that these are distinct processes.
Declaration of interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Acknowledgements
This work was funded by a grant from the NIH (R15D061858) to SMD.