Introduction
Mammalian life originates from fertilization, a precisely coordinated process resulting in the fusion of parental genetic and epigenetic information. The sperm was believed to be only responsible for delivery of paternal DNA (Baccetti and Afzelius, Reference Baccetti and Afzelius1976), however accumulating evidence suggests that the sperm affects embryonic, fetal development or even postnatal life of offspring through epigenetic changes (Le Blevec et al., Reference Le Blevec, Muronova, Ray and Arnoult2020), including DNA methylation (Illum et al., Reference Illum, Bak, Lund and Nielsen2018), histone post-translational modifications (Campos et al., Reference Campos, Stafford and Reinberg2014) and non-coding RNA biogenesis (Klastrup et al., Reference Klastrup, Bak and Nielsen2019). It is critical that these epigenetic marks are vulnerable to environmental influences (Legoff et al., Reference Legoff, D’Cruz, Tevosian, Primig and Smagulova2019). Various factors, such as diet (Watkins et al., Reference Watkins, Dias, Tsuro, Allen, Emes, Moreton, Wilson, Ingram and Sinclair2018), pollution exposure (Zhang et al., Reference Zhang, Yang, Lv, Li and Qiang2019) and temperature variation (Weyrich et al., Reference Weyrich, Lenz, Jeschek, Chung, Rübensam, Göritz, Jewgenow and Fickel2016), can induce epigenetic changes in the sperm that have long-term consequences on embryo and offspring development.
Environmental hypoxia is one of the known factors that negatively affects male reproductive health (Jankovic Velickovic and Stefanovic, Reference Jankovic Velickovic and Stefanovic2014). A comprehensive survey of soldiers from military bases at different altitudes in China revealed that hypoxia exposure at 3600 m above sea level for 3 months resulted in a significant decrease in sperm concentration and an increased in sperm abnormality (Zheng et al., Reference Zheng, Liu, Li and Tian2019). Similar results have been reported in men exposed to higher altitudes (He et al., Reference He, Cui, Wang, Gao, Gao, Yang, Zhang, Cao and Yu2015; Gonzales et al., Reference Gonzales, Lozano-Hernandez, Gasco, Gonzales-Castaneda and Tapia2012). Findings from animal experiments fully showed that hypoxia exposure impairs the hypothalamus–gonad axis and disrupts spermatogenesis (Liao et al., Reference Liao, Cai, Chen, Huang, Liu, Jiang and Gao2010). Simulating 4200 m hypoxia led to decreased sperm concentration and teratozoospermia in mice (Vargas et al., Reference Vargas, Bustos-Obregon and Hartley2011). Exposing rats to 5000 m of altitude caused decreases in testis weight and testosterone concentration (Bai et al., Reference Bai, Yang, Tong and Li2018). Exposing rhesus monkeys to an altitude of 4411 m changed sperm count and sperm motility (Saxena, Reference Saxena1995). Interestingly, emerging evidence has suggested that hypoxia causes transgenerational effects in fish (Wang SY et al., Reference Wang, Lau, Lai, Zhang, Tse, Li, Tong, Chan, Wong, Chiu, Au, Wong, Kong and Wu2016) and rats (Dunaeva et al., Reference Dunaeva, Trofimova, Graf, Maslova, Maklakova, Krushinskaya and Sokolova2008). These data suggested that hypoxia produces a dramatic and long-lasting effect on spermatogenesis.
Within the seminiferous tubules, environmental hypoxia resulted in extreme low oxygen supply to developing germ cells, it has been proposed that hypoxia exposure impaired the development of all germ cells including spermatogonial stem cells (Jankovic Velickovic and Stefanovic, Reference Jankovic Velickovic and Stefanovic2014). Genetic deletion of Epas1 in postnatal testis disrupted spermatid development, while the spermatogonia population appeared to be intact (Gruber et al., Reference Gruber, Mathew, Runge, Garcia and Simon2010). Detail analyses uncovered that hypoxia exposure mainly caused apoptosis to increase significantly in spermatocytes, therefore resulting in decreased sperm production (Liao et al., Reference Liao, Cai, Chen, Huang, Liu, Jiang and Gao2010; Reyes et al., Reference Reyes, Farias, Henriquez-Olavarrieta, Madrid, Parraga, Zepeda and Moreno2012). High-altitude exposure changed mitochondrial DNA copy number in sperm, therefore it was clear that hypoxia also dramatically affected sperm quality (Luo et al., Reference Luo, Liao, Chen, Cui, Liu, Jiang, Gao and Gao2011). Impairment of mitochondrial function is likely to be associated with defects in sperm motility of animals exposed to different altitudes (Saxena, Reference Saxena1995; Verratti et al., Reference Verratti, Di Giulio, D’Angeli, Tafuri, Francavilla and Pelliccione2016). Despite findings that have clarified the effect of hypoxia on spermatogenesis, the fertilization ability and developmental competency of sperm from hypoxia-treated animals remain to be examined.
In this study, we hypothesized that environmental hypoxia exposure affected sperm quality, fertilization capacity and preimplantation embryonic development. Because correct establishment of DNA methylation is crucial for fertilization and early lineage segregation, we examined the expression of several genes regulating DNA methylation and demethylation. Together, the results showed that paternal hypoxia exposure greatly affects sperm quality, fertilization, and key events of early development.
Materials and methods
Experimental design
CD-1 mice were purchased from the Charles River Laboratory Animal Centre (Beijing, China) and housed in a controlled environment with food and water ad libitum. For hypoxic exposure, adult male mice were transported to Haibei Experimental Station, Chinese Academy of Sciences (37°33′N, 101°24′E, 3220 m) for 7 d (15–16 weeks old, from this point forwards referred to as hypo-7d group) or 2 m (9–16 weeks old, from this point forwards referred to as hypo-2m group). For the fertility test, litter sizes were counted after mating with control females as described previously (Yan et al., Reference Yan, Li and Yang2020). One male was mated with three females for 1 day and the vaginal plug was tested the next morning. Here, 10 control, nine hypo-7d and 10 hypo-2m animals were used in the fertility test. Average litter size was recorded to assess fertility.
Hypoxia treatment evaluation
Blood samples (n = 3) were collected for blood routine examination using a Prokan PE-6800 VET Fully Auto Haematology Analyzer (Shenzhen, China), red blood cells (RBC), haematocrit (HCT) and haemoglobin (HGB) were measured. After sacrificing, hearts were removed immediately and flushed with normal saline and then moisture was adsorbed onto filter paper. The weights of the right ventricle (RV) and left ventricle plus ventricular septum (LV + S) were recorded to calculate RV/[LV + S] (Chen et al., Reference Chen, Shen, Zhu, Yang, Ye, Liu, Gu and Chen2019).
Histological analysis
Testis and epididymis samples (n = 3) were fixed in 4% paraformaldehyde as described previously (Yan et al., Reference Yan, Li and Yang2020) and dehydrated in a 30, 50, 70, 95 and 100% ethanol series. After embedding in paraffin, samples were cut into 4-µm sections. Rehydrated sections were stained with haematoxylin and eosin (H&E) and observed using a Nikon Eclipse E200 Microscope (Tokyo, Japan).
Computer-aided sperm analysis (CASA)
Sperm released from the epididymis was incubated in 1 ml human tubal fluid (HTF) medium. A 10-µl sperm suspension was loaded into a counting chamber and analyzed using a WeiLi WLJY9000 CASA system (Beijing, China) for sperm parameters, including concentration, motility, viability, linearity (LIN), wobbliness (WOB), straightness (STR), amplitude of lateral head displacement (ALH) and mean angular displacement (MAD) (Krause, Reference Krause1995; Wang G et al., Reference Reyes, Farias, Henriquez-Olavarrieta, Madrid, Parraga, Zepeda and Moreno2019a). At least 12 fields were selected at random for each sample.
Ionophore A23187-induced acrosome reaction
Acrosome reactions were evaluated as described previously with modification (Aitken et al., Reference Aitken, Buckingham and Fang1993). Briefly, sperm were incubated in HTF medium with or without 10 µM A23187 at 37°C in 5% CO2 in air for 1 h. After rinsing in PBS and resuspension, acrosome status was assessed by staining the sample with acrosome marker peanut agglutinin (diluted 1:500) and mitochondria marker MitoTracker (diluted 1:500) for 1 h. After washing, samples were mounted on slides for immunofluorescence analysis on a Leica DMR fluorescence microscope (Mannheim, Germany). The acrosome reactions were calculated using the proportion of sperm without intact acrosome in live sperm with a normal nucleus and tail. At least 700 sperms from three different animals were counted for each group.
Transmission electron microscopy
The caudal epididymis from adult mice was placed in 500 µl PBS and incubated at 37°C for 15 min. After centrifuging at 1500 g at 37°C for 10 min, supernatant was discarded, and 0.5% glutaraldehyde was added slowly to fix the sperm. Sperm were then placed at 4°C for 10 min, centrifuged at 1500 g for 15 min, and the supernatant discarded. After that, sperm were fixed in 2.5% glutaraldehyde at 4°C overnight and then processed in a Lilai Biomedicine Experiment Centre (Chengdu, China) as described (Gu et al., Reference Gu, Zhao, Wang and Sun2019). Images were captured using a JEOL JM-1400Plus electron microscope (Tokyo, Japan) at ×6000 and ×12,000 magnification.
Sperm–zona pellucida binding assay
The sperm–ZP binding assay was performed as described previously with modification (van Wissen et al., Reference van Wissen, Bomsel-Helmreich and Frydman1995). In brief, females were superovulated by injection with 10 IU pregnant mare serum gonadotropin, followed by 10 IU human chorionic gonadotropin (hCG) 48 h later. Cumulus–oocyte complexes were collected from oviducts in M2 medium at 14 h after hCG treatment and cumulus cells were dispersed in 0.1% hyaluronidase. Cumulus-free oocytes were placed in a drop of HTF medium and inseminated with capacitated sperm (2 × 105 sperm/ml). After incubation at 37°C in 5% CO2 in air for 30 min, oocytes were fixed and the number of sperm bound to ZP was counted after DAPI staining. At least 50 oocytes were analyzed for each group.
In vitro fertilization and embryo culture
The in vitro fertilization (IVF) procedure was performed as described previously (Jia et al., Reference Jia, Fu, Cheng, Yue, Jia, Hou and Zhu2015). Briefly, cumulus-free oocytes were inseminated with capacitated sperm and incubated with HTF medium at 37°C in 5% CO2 in air. After 4 h post-fertilization, oocytes were washed and cultured in KSOM medium at 37°C in 5% CO2 in air. Proportions of zygotes, 2-cell embryos, and blastocysts were assessed at 10, 24 and 96 h post-fertilization, respectively. For pronucleus (PN) analysis, zygotes were recovered individually at 4, 6, 8, 10 and 12 h post-fertilization. The classification of PN1 to PN5 stages was performed according to pronuclear morphology and post-fertilization timing.
Immunofluorescence staining
After fixation in 3.7% paraformaldehyde for 20 min, embryos were washed in PBST, permeabilized in 0.5% Triton X-100 for 10 min, denatured in 4 mol/l HCl for 10 min and neutralized in 100 mmol/l Tris–HCl (pH 8.0) for 20 min. Then samples were blocked in 2% BSA for 1 h before incubation with Abcam anti-5mC antibody (diluted 1:500, Cambridge, UK) at 4°C overnight. After several washes, samples were incubated with Abcam anti-rabbit Alexa Fluor 488-conjugated antibody (diluted 1:500; Supporting information Table S1) for 1.5 h at 37°C. Finally, cells were counterstained with PI and mounted on slides for observation. ImageJ software was used to quantify the fluorescence signals. The average methylation intensity for each blastocyst was calculated using the ratio of antibody signal to DNA signal after subtraction of the cytoplasm background fluorescence.
Real-time quantitative PCR (qRT-PCR)
Total RNA was isolated using TRIzol reagent. After removing genomic DNA, RNA was reverse transcribed into cDNA using the GenStar First-Strand cDNA Synthesis Mix with gDNA Remover (Beijing, China). qRT-PCR was performed using the GenStar SYBR Green Fast Mixture on the Applied Biosystems ViiATM7 qRT-PCR System (CA, USA) under standard conditions. The cycle threshold (Ct) value was the average of three replicates and relative gene expression was analyzed based on the 2−∆∆Ct method. All primers are listed in Table S2.
Statistical analysis
Data were statistically analyzed with one-way analysis of variance and least significant difference test using IBM SPSS software (IL, USA). All quantitative data are presented as mean ± standard error of the mean (SEM) for at least three biological replicates. Three sections were used for each animal and 100 seminiferous tubules were used for diameter measurement. Differences between means were considered significant at a P-value < 0.05.
Results
Effects of short- and long-term hypoxia exposure on male fertility and sperm spermatogenesis
Adult male mice were exposed to a hypoxic environment for 7 d or 2 m to determine whether hypoxia had an adverse effect on male fertility (Fig. 1A). The significant elevation of RV/[LV + S] and increased RBC suggested that animals responded to hypoxia treatment (Fig. S1). Fertility testing showed that average litter sizes of hypo-2m (8.70 ± 0.82) (P < 0.001) males were significantly lower than the control (13.70 ± 0.47) or hypo-7d males (11.78 ± 0.76) (P < 0.05) (Fig. 1B). Histological analysis of seminiferous tubules and cauda epididymidis revealed that spermatogenesis appeared to be normal and all types of spermatogenic cells were present (Fig. S2A, C). However, the seminiferous tubule diameters of animals from the hypo-2m group (158.78 ± 1.50 μm) were reduced compared with that of the control and hypo-7d animals (175.48 ± 1.59 and 171.47 ± 1.37 μm) (P < 0.01) (Fig. S2B). Collectively, these data indicated that environmental high-altitude exposure for 2 months affected fertility, but did not significantly disrupt spermatogenesis.
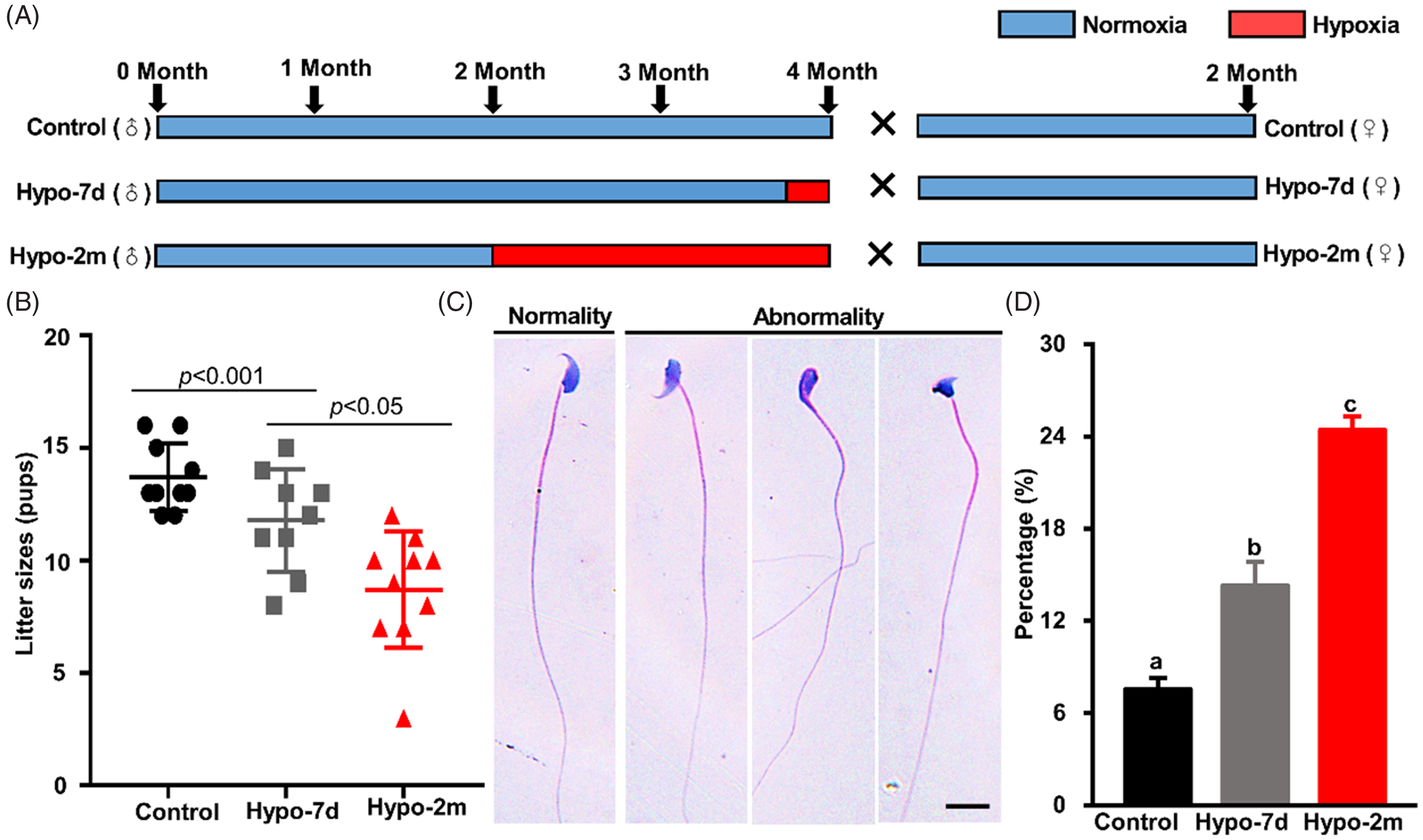
Figure 1. Effects of hypoxia on male fertility and sperm morphology. (A) Flowchart of the hypoxic experiment sets. (B) Litter size per mice from the control, hypo-7d and hypo-2m groups. (C) Representative images of H&E-stained sperm from the control, hypo-7d and hypo-2m animals. Scale bar, 10 μm. (D) Percentages of morphological abnormal sperm from control, hypo-7d and hypo-2m groups. At least 1500 sperms were counted per group. Different letters (a–c) indicate significant difference at P < 0.001.
Hypoxia exposure impairs sperm morphology and motility
To determine the reason of reduced fertility, we measured sperm concentration of animals from three different groups and the results showed that sperm concentration was comparable (Fig. S2D). However, it appeared that hypoxia exposure affected sperm morphology, because only 7.55 ± 0.71% of sperm from control animals showed morphological defects in the head, but this number increased to 14.30 ± 1.56% and 24.43 ± 0.86% in animals treated with short-term or long-term hypoxia (P < 0.001) (Fig. 1C, D). We examined sperm quality in detail using CASA; sperm viability was decreased by more than 50% in animals from the hypo-2m group. Sperm motility was much decreased by hypoxia treatment (47.72 ± 0.65% for control, 35.00 ± 1.86% for hypo-7d and 23.33 ± 1.88% for hypo-2m; P < 0.001). Further analysis indicated that STR and MAD were decreased by hypoxia treatments compared with the control (Fig. 2A).

Figure 2. Effects of hypoxia on sperm motility, structure and gene expression. (A) Sperm kinematic parameters from control, hypo-7d and hypo-2m mice, including motility, viability, LIN, WOB, STR, ALH and MAD. Different letters (a–c) indicate significant difference at P < 0.05. (B) Representative TEM images of epididymal sperm from control, hypo-7d and hypo-2m mice. Scale bars, 200 nm. (C) qRT-PCR analysis of Izumo1, Clgn, Cd36, Tmem95, Adam1a, Adam1b and Spam1 in sperm from control and hypo-2m group. Data were analyzed by mean ± standard error of the mean (SEM) for three mice. *Denotes significance at P < 0.05.
Hypoxia exposure disrupts gene expression and ultrastructure in sperm
Because motility was severely impaired by hypoxia exposure, we conducted transmission electron microscopy (TEM) analysis to examine sperm ultrastructure. The results showed that all animals from the control, hypo-7d and hypo-2m groups had intact double membrane structures, however broken microtubule structure and mitochondrial myelin sheaths were found in sperm from hypo-2m animals (Fig. 2B). Consistent with this observation, we measured the expression levels of several motility-related genes in sperm including Izumo1, Clgn, Cd36, Tmem95, Adam1a, Adam1b and Spam1. Relative expression levels of Izumo1, Clgn, Cd36, Tmem95 and Spam1 were significantly decreased in hypoxia-treated testis (Fig. 2C). These data suggested that hypoxia downregulated the expression of several key regulators of sperm motility to influence sperm function.
Long-term hypoxia treatment impairs acrosome reactions and sperm–zona pellucida binding
Next we asked whether acrosome reactions (AR) of sperm from hypoxia-treated animals were affected by hypoxia treatment. AR occurrence was assessed using sperm incubated in the absence or presence of ionophore A23187, which induces calcium influx in sperm and ARs (Tateno et al., Reference Tateno, Krapf, Hino, Sanchez-Cardenas, Darszon, Yanagimachi and Visconti2013). Similar incidences of spontaneous ARs occurred in control and hypoxia-treated animals. Interestingly, hypoxic exposure for 2 months caused deficient ARs (84.75 ± 2.49% for control, 76.64 ± 4.94% for hypo-7d and 51.05 ± 1.66% for hypo-2m; P < 0.001) (Fig. 3A, B). Furthermore, we examined the binding activity of sperm to ZP and, as expected, compared with the control and hypo-7d sperm (44.72 ± 2.59 and 41.58 ± 4.41), the binding ability of hypo-2m sperm (28.03 ± 1.13) was impaired (P < 0.01) (Fig. 3C, D).

Figure 3. Effects of hypoxia on acrosome reactions and ZP binding ability. (A, B) Representative images (A) and rates (B) of acrosome reactions induced by A23187 from control, hypo-7d and hypo-2m mice. Scale bars, 20 μm. At least 800 sperms were counted per group. (C, D) Representative images (C) and the number (D) of sperm binding to ZP from control, hypo-7d and hypo-2m mice. Scale bars, 50 μm. At least 50 oocytes were counted per group. Bars with different letters (a, b) were significantly different (P < 0.05).
Paternal hypoxia causes impaired fertilization and early embryo development
We then performed IVF experiments to evaluate fertilization and subsequent embryo development of sperm from control and hypoxia-treated animals. Cleavage rates were 91.75 ± 0.84% and 91.43 ± 5.04% for control and hypo-7d animals, indicating that short-term hypoxia treatment did not affect fertilization. However when animals were treated for 2 months, the cleavage rate decreased to 65.08 ± 1.14% (P < 0.001). Blastocyst rates were 76.19 ± 1.45% and 83.49 ± 3.98% for sperm from control and hypo-7d animals, respectively, however this number decreased to 30.79 ± 0.32% for sperm from hypo-2m animals (P < 0.001). These data indicated that paternal hypoxia exposure was detrimental to fertilization and early embryo development (Fig. 5).
Because the fertilization process is sensitive to environmental insults (Colaco and Sakkas, Reference Colaco and Sakkas2018), we first examined the pattern of pronuclear development in zygotes. Compared with the controls, the number of zygotes in PN1–PN2 and PN3 stages at 4 h post-fertilization was increased in hypo-2m animals and this developmental delay was evident at 6 h post-fertilization. The difference was no longer significant at 8 h post-fertilization and thereafter (Fig. 4). This data suggested that the fertilization process of sperm from long-term hypoxia-treated animals was delayed.
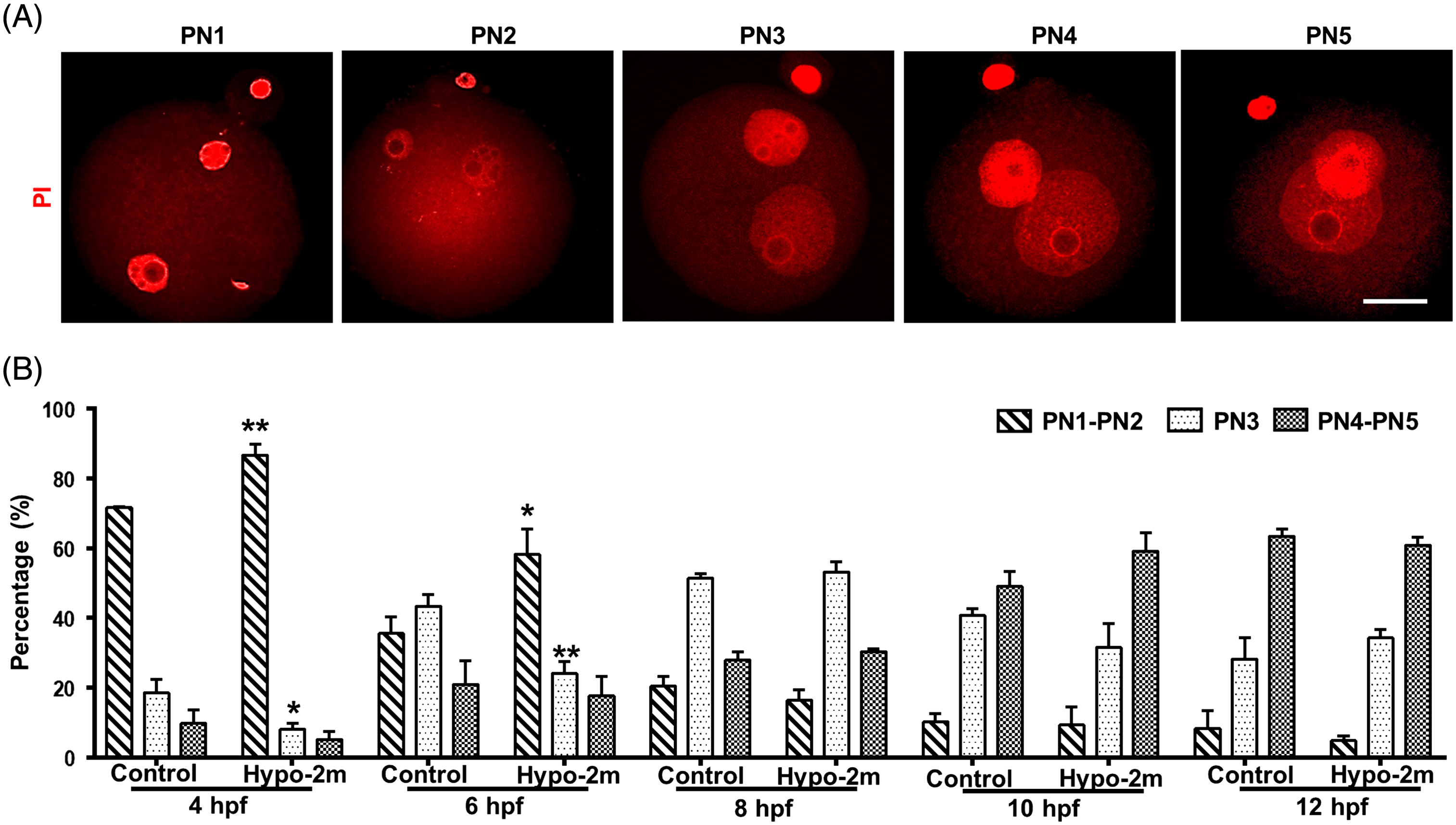
Figure 4. Effects of paternal hypoxia on pattern of pronuclear development in zygotes. (A) Representative images of PN1 to PN5 zygotes stained with PI. Scale bars, 40 μm. (B) Percentages of zygotes at PN1 to PN2, PN3, and PN4 to PN5 stages for every given timepoint from control and hypo-2m groups. At least 50 oocytes were observed for every given timepoint per group. *, **Denotes significant difference at P < 0.05 and P < 0.01, respectively.
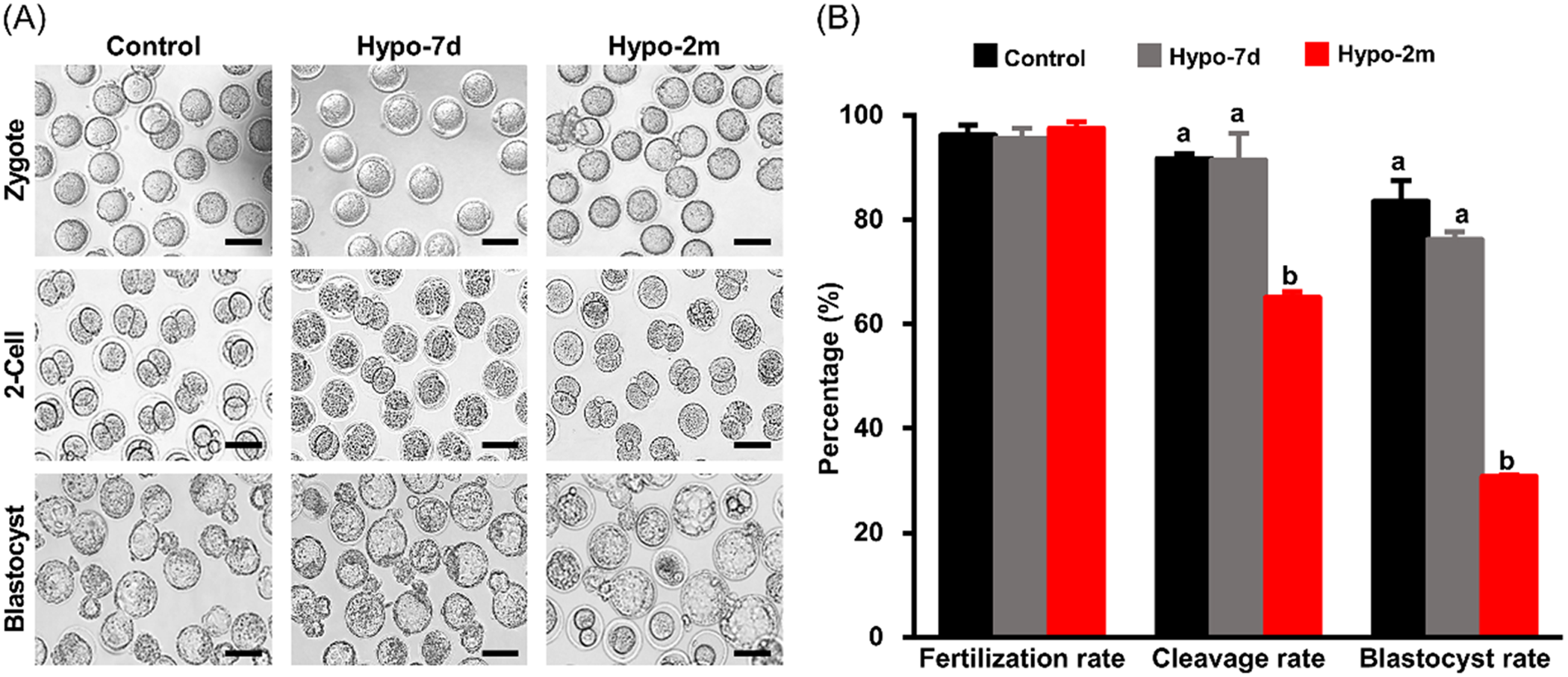
Figure 5. Effects of paternal hypoxia on early embryo development. (A) Representative images of IVF outcome from control, hypo-7d and hypo-2m mouse sperm, n = 3. Scale bars, 100 μm. (B) Fertilization, cleavage and blastocyst rates from control, hypo-7d and hypo-2m groups. At least 300 oocytes were used per group. Bars with different letters (a, b) indicate significant difference (P < 0.001).
Paternal hypoxia exposure affects blastocyst cell number and gene expression
Disrupting the dynamics of DNA methylation during fertilization affects zygotic gene activation and embryo development, although a subgroup of embryos from hypo-2m developed to blastocyst stage, it was unclear whether the quality of these embryos was similar to the ones from the control. Further analysis showed that blastocysts from the hypo-2m group contained fewer cell numbers (53.45 ± 1.72) compared with the control (59.90 ± 2.55) (P < 0.05) (Fig. 6A, B). By comparing the 5mC level in blastocysts, it was clear that the DNA methylation level was reduced by paternal hypoxia exposure (Fig. 6C). This statement was further supported by the fact that expression levels of H19, Igf2, Mest, Peg3 and Dlk1 were significantly decreased in blastocysts produced from hypo-2m sperm (Fig. 6D). Overall, these results illustrated that the quality of blastocysts was negative affected by paternal hypoxia exposure.
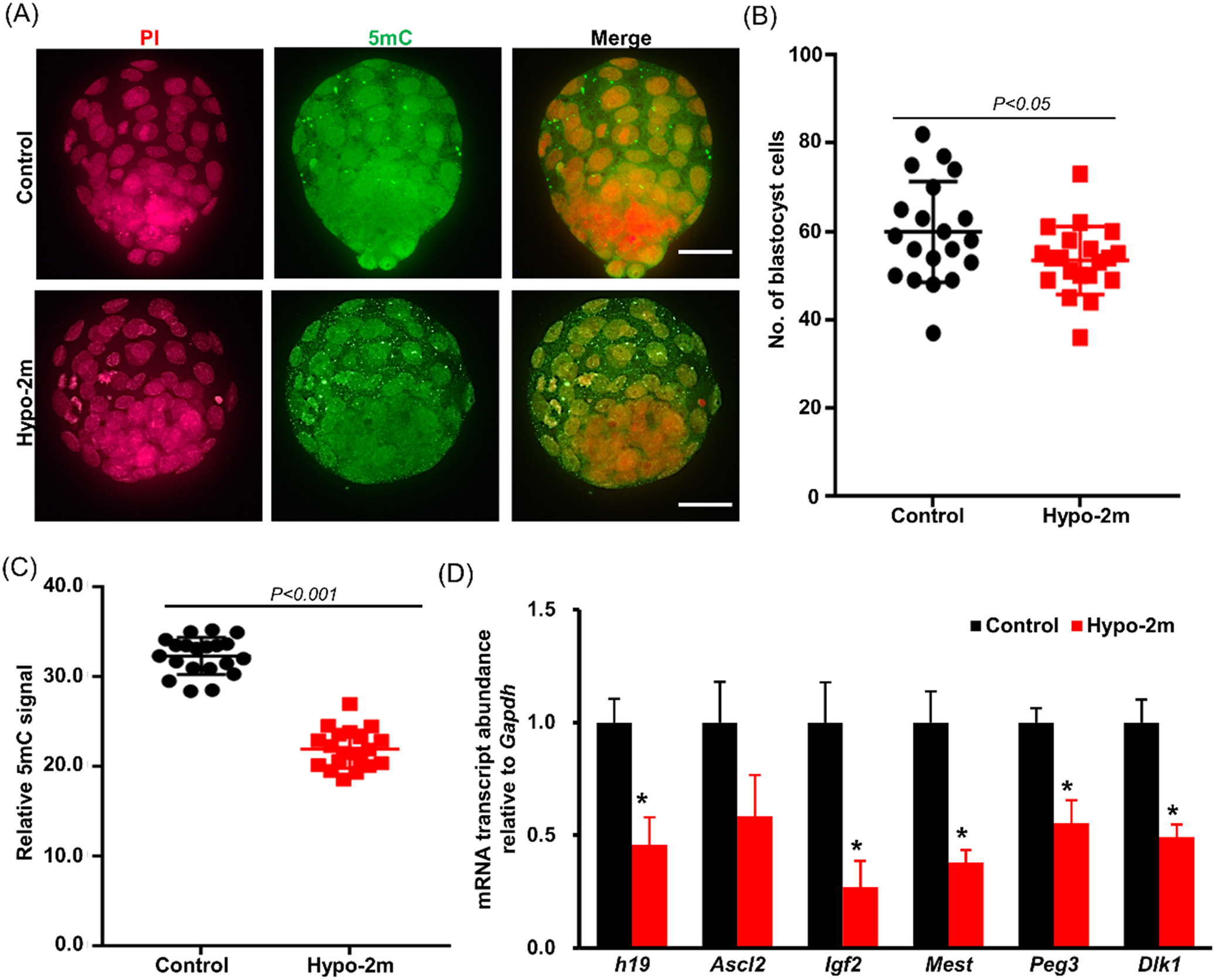
Figure 6. Effects of paternal hypoxia on blastocyst development and gene imprinting. (A) Representative images of blastocysts stained with 5mC or PI from control and hypo-2m groups. Scale bars, 40 μm. (B, C) Statistics of blastocyst cell number (A) and 5mC intensity (C) from control and hypo-2m groups. At least 20 blastocysts were analyzed per group. (D) qRT-PCR analysis of H19, Igf1, Ascl2, Mest, Peg3 and Dlk1 in blastocysts from control and hypo-2m groups. *Denotes significant difference at P < 0.05.
Discussion
In the present study, we showed that the effect of paternal hypoxia exposure was multifaceted and long-lasting. We found that hypoxia exposure did not disrupt spermatogenesis, but instead impaired spermiogenesis and probably sperm maturation. This finding is consistent with previous results (Verratti et al., Reference Verratti, Di Giulio, D’Angeli, Tafuri, Francavilla and Pelliccione2016; Bai et al., Reference Bai, Yang, Tong and Li2018), however these findings provided new evidence that paternal hypoxia exposure had a dramatic effect on fertilization and embryo development.
It has been well documented that hypoxia affects male fertility. Animal studies using environmental hypoxia (He et al., Reference He, Cui, Wang, Gao, Gao, Yang, Zhang, Cao and Yu2015; Verratti et al., Reference Verratti, Di Giulio, D’Angeli, Tafuri, Francavilla and Pelliccione2016) or simulating hypoxic conditions (Liao et al., Reference Liao, Cai, Chen, Huang, Liu, Jiang and Gao2010; Vargas et al., Reference Vargas, Bustos-Obregon and Hartley2011) revealed that the most significant phenotype caused by hypoxia was elevated apoptosis in spermatocytes and defects in sperm motility. One limitation of these studies is that fertility of hypoxia-exposed animals was not thoroughly evaluated. Spermatogenesis was still completed and spermatozoa were generated in this animal. In this study, we conducted fertility tests using control females and provided strong evidence that hypoxia decreased male fertility as a result of sperm function, including impaired motility, abnormal AR and reduced ZP binding. Findings of these analyses uncovered that, although hypoxia appeared to have mild effects on spermatogenesis, it profoundly influenced sperm function.
The sperm delivers DNA and epigenetic information to the oocyte and have crucial roles in regulating embryogenesis. In rats, hypoxia exposure for 5, 15 or 30 days induced DNA damage in sperm possibly by changing expression of 8-oxoguannin glycosylate (OGG1), which serves key functions in the base excision repair pathway (Farias et al., Reference Farias, Zepeda, Castillo, Figueroa, Ademoyero and Pulgar2018). High altitude was reported to impair mitochondrial and nuclear DNA integrity in human sperm (Luo et al., Reference Luo, Liao, Chen, Cui, Liu, Jiang, Gao and Gao2011). It is possible that DNA damage in sperm results in the developmental failure of early embryos. In the present study, we showed that DNA methylation and expression of developmentally important genes in embryos, even at the blastocyst stage, were dysregulated by hypoxia exposure, we proposed that paternal hypoxia exposure induced epigenetic changes in sperm and embryos, and therefore induced subtle but long-lasting consequences in development.
Paternal effects can be delivered to the embryo and influence its developmental process (Chao et al., Reference Chao, Guo, Ou, Luo, Wang, Schatten, Gao and Sun2012; Jia et al., Reference Jia, Fu, Cheng, Yue, Jia, Hou and Zhu2015). Epigenetic marks, especially DNA methylation, have been proven to be important for this process (Lim et al., Reference Lim, Knowles, Solter and Messerschmidt2016). DNA methylation is a reversible modification that is established by DNA methyltransferases (DNMTs) (Lyko, Reference Lyko2018) and removed through DNA replication or DNA demethylation (Guo et al., Reference Guo, Li, Liang, Li, Zhu, Guo, Wu, Wen, Gu, Hu, Walsh, Li, Tang and Xu2014; Wu and Zhang, Reference Wu and Zhang2017). Ten-eleven translocation (TET) methylcytosine dioxygenases actively cause demethylation of 5mC and generate hypomethylation at key regulatory regions of the genome (Iqbal et al., Reference Iqbal, Jin, Pfeifer and Szabo2011). Both maternal and paternal genomes undergo active and passible demethylation in zygotes before reaching the 2-cell stage (Guo et al., Reference Guo, Li, Liang, Li, Zhu, Guo, Wu, Wen, Gu, Hu, Walsh, Li, Tang and Xu2014). In this study, exposing male mice to long-term hypoxia led to decreased global methylation levels in blastocysts. Furthermore, the expression of imprinting genes was aberrant in blastocysts, and may influence placenta formation and fetal development. Findings from these data suggest that paternal hypoxia exposure changed the dynamics of DNA methylation in early embryos.
Long-term hypoxia harms several key developmental events in early embryos, however short-term hypoxia treatment for 7 days was not sufficient to induce such changes. Spermatogenesis is a complex process that in mice requires c. 35 days to complete (de Rooij and Russell, Reference de Rooij and Russell2000). Exposing mice to hypoxia for 2 months covered the whole spermatogenic cycle, and therefore affected every step of development including spermatogonial differentiation, meiosis and spermiogenesis. However, 7 days’ exposure only affected the late stage of spermiogenesis and the maturation process in epididymides. Compared with other types of spermatogenic cell, spermatozoa contain condensed chromatin structures as a result of histone-to-protamine exchange. Therefore, the ability to resist external insults in sperm is stronger than that in spermatogonia and spermatocyte (Fuentes-Mascorro et al., Reference Fuentes-Mascorro, Serrano and Rosado2000; Meyer et al., Reference Meyer, Ketchum and Meyer-Ficca2017; Hao et al., Reference Hao, Ni and Yang2019). We concluded that reduced fertility in animals treated with short-term hypoxia was a result of issues in sperm motility.
We did not observe any dramatic change in histology of seminiferous epithelium; these findings differed from those of a previously published study (Liao et al., Reference Liao, Cai, Chen, Huang, Liu, Jiang and Gao2010). Unlike some studies that simulated an altitude of 5000 m, the present study selected an altitude between 3000 to 4000 m, because it mimicked the geographic distribution of the human population (Cohen and Small, Reference Cohen and Small1998). At this altitude, animals native to the Tibetan plateau have gained adaptive traits to overcome hypoxia and exhibit normal spermatogenesis during their reproductive season (Wang YJ et al., Reference Wang, Jia, Yan, Guo, Tian, Ma, Zhang, Li, Zhang, Du and Yang2019b; Zhao et al., Reference Zhao, Lu, Cheng, Tian, Qiangba, Fu and Ren2019). Non-native animals encounter fertility issue after migration to the high-altitude environment. To gain insight into the molecular mechanisms of hypoxia adaptation, it is essential to dissect the genetic basis of hypoxia resistance using different animal models in the future.
In summary, our data provided evidence that long-term hypoxia caused decreased sperm quality and motility, which finally delayed pronuclear formation and decreased the fertilization ability of the sperm. One novel finding of this study is that paternal hypoxia altered DNA methylation and affected gene expression in blastocysts. Further studies are required to understand the influence of paternal hypoxia on fetal and offspring development.
Conflicts of interest
None.
Financial support
The work was supported by the National Natural Science Foundation of China (81960287) and Natural Science Foundation of Qinghai Province (2020-ZJ-902). QY was supported by CAS ‘100 Talents’ and Qinghai ‘1000 Talents’ programmes. GJ was supported by the Youth Innovation Promotion Association of Chinese Academy of Sciences.
Ethical standards
All animal experiments were approved by the Animal Ethic and Welfare Committee at Northwest Institute of Plateau Biology, Chinese Academy of Sciences.
Supporting information
Supporting information for this paper is available online at the publisher’s website.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0967199421000216








