Introduction
Oocyte activation, through which the egg can be transformed into an embryo, is an essential feature of fertilization. The earliest signalling event in the activation of an egg by a spermatozoon is a large transient increase in intracellular free calcium ion concentration (Runft et al., Reference Runft, Jaffe and Mehlmann2002).
In amphibians, this Ca2+ signal is sufficient to trigger the events associated with egg activation, including a depolarization of the egg membrane and the exocytosis of cortical granules (Bement & Capco, Reference Bement and Capcom1989; Oterino et al., Reference Oterino, Sanchez Toranzo, Zelarayán, Valz-Gianinet and Bühler2001; Runft et al., Reference Runft, Carroll, Gillett, Giusti, O’Neill and Foltz2004).
In Rhinella arenarum, Ca2+ transient release from intracellular stores is mainly modulated through both agonist-gated channels, the 1-4-5 inositol triphosphate receptor (IP3R) and the ryanodine receptor (RyR) (Ajmat et al., Reference Ajmat, Bonilla, Zelarayán and Bühler2011).
Several observations suggest that phosphoinositide metabolism is a vital intracellular signalling system involved in many cellular functions including fertilization and embryogenesis. PLCs are key enzymes within this system, hydrolyzing the membrane phospholipid substrate, phosphatidylinositol 4,5-bisphosphate (PIP2) (Halet et al., Reference Halet, Marangos, Fitzharris and Carroll2003; Lee et al., Reference Lee, Ono, Miyamoto, Hadama and Arita2006; Whitaker, Reference Whitaker2006) to trigger cytoplasmic Ca2+ oscillations via the inositol 1,4,5-trisphosphate (IP3) intracellular Ca2+ signalling pathway (Berridge, Reference Berridge1993).
The generation of DAG and IP3 may involve the activation of phosphatidylinositol-specific phospholipase C (PI-PLC), and/or the activation of phosphatidylcholine-specific phospholipase C (PC-PLC). The evidence derived from a variety of cell types strongly supports the proposition that U73122 is a relatively specific inhibitor of PI-PLC dependent events. U-73122 has been used to prove PI-PLC involvement in a variety of cellular processes, to date the most noteworthy example of these being the generation of Ca2+ oscillations and transients (Galan et al., Reference Galan, Tramkina, Noel, Sprague and Ward1991; Thompson et al., Reference Thompson, Mostafapour, Denlinger, Bleasdale and Fisher1991; Yamada et al., Reference Yamada, Yamada and Richelson1992).
PC-PLC (66 kDa) hydrolyzes PC to generate phosp-hocholine and 1,2-diacylglycerol (DAG). Tricyclode-can-9-yl-xanthogenate (D609) is known to inhibit phosphatidylcholine (PC)-specific phospholipase C (PC-PLC). Suppressing PC-PLC by D609 stopped proliferation and induced differentiation in various cell systems (Wang et al., Reference Wang, Du and Wang2004, Reference Wang, Sun, Huo, Zhang, Zhao, Zhang and Miao2008). Human epithelial ovarian cancer cells showed activation of PC-PLC while inhibition by D609 reduced the phosphocholine metabolite (Iorio et al., Reference Iorio, Ricci, Bagnoli, Pisanu, Castellano, Di Vito, Venturini, Glunde, Bhujwalla, Mezzanzanica, Canevari and Podo2010). D609, shown to be a competitive inhibitor of bacterial phosphatidylcholine (PC)-specific phospholipase C (PC-PLC), did not inhibit bacterial phosphatidylinositol (PI)-PLC, bovine pancreatic PLA2 or phospholipase D from cabbage (Amtmann, Reference Amtmann1996)
Lithium and neomycin were employed to inhibit phosphoinositide metabolism and generation of second messengers. Lithium decreases the level of cytosolic inositol (Berridge, Reference Berridge1989) and inhibits the enzyme myo-inositol-1-phosphatase, which hydrolyzes inositol phosphates (Gee et al., Reference Gee, Ragan, Watling, Aspley, Jackson, Reid, Gani and Shute1988; Berridge & Irvine, Reference Berridge and Irvine1989). Neomycin, an aminoglycoside antibiotic, binds with high affinity to phosphatidylinositol 4,5-biphosphate (Schacht, Reference Schacht1978), preventing its hydrolysis (Lloyd et al, Reference Lloyd, Davies, Crossley, Whitaker, Houslay, Hall, Marshall and Wakelam1989; Biden et al., Reference Biden, Prugue and Davison1992).
Among its various modulatory actions, calcium can serve as an activator of an 85 kDa cytosolic phospholipase A2 (cPLA2) (Clark et al., Reference Clark, Schievella, Nalefski and Lin1995). Once activated, cPLA2 releases arachidonic acid, a poly-cis unsaturated fatty acid (20:4), from phospholipids of the endoplasmic reticulum as well as from the nuclear envelope. Previous studies have suggested that arachidonic acid is able to modulate the activity of several ion channels and furthermore, that arachidonic acid and/or its metabolites might be involved in the regulation of intracellular calcium homeostasis (Maruyama, Reference Maruyama1993; Rowles & Gallacher, Reference Rowles and Gallacher1996).
Studies in Rhinella arenarum oocyte suggest that PLA2 plays a fundamental role in activation signalling and that this effect is mediated by an increase in PLA2 activity (Ajmat et al., Reference Ajmat, Bonilla, Hermosilla, Zelarayán and Bühler2013). It was found that the addition of melittin, a potent PLA2 activator, and arachidonic acid, the main PLA2 reaction metabolite, was able to induce activation events such as cortical granule exocytosis and subsequent fertilization envelope formation probably through calcium-triggered sequential reactions. Moreover, quinacrine treatment before melittin stimulation was proved to be inhibitory. These findings suggest that the phospholipase A2 pathway is involved in the calcium release mechanism that leads to Rhinella arenarum oocyte activation (Ajmat et al., Reference Ajmat, Bonilla, Hermosilla, Zelarayán and Bühler2013). However, activation of PLA2 at fertilization in Xenopus is a matter of discussion. There were no detectable lipid changes reflecting significant activation of PLA2 during fertilization in Xenopus laevis oocytes (Petcoff et al., Reference Petcoff, Holland and Stith2008).
In the case of fertilization or cloning protocols, different procedures are used to activate oocytes and enable the onset of oocyte development. The most common current activation protocols combine A23187 with protein synthesis or protein kinase inhibitors such as 6-dimethyl aminopurine (6-DMAP) or puromycin (Cibelli et al., Reference Cibelli, Corsi, Diana, Vitiello and Thiel2001; Nakagawa et al., Reference Nakagawa, Yamano, Moride, Yamashita, Yoshizawa and Aono2001; Lu et al., Reference Lu, Chen, Gao, Ma, Li, Hu and Li2006; Iba et al., Reference Iba, Yano, Umeno, Hinokio, Kuwahara, Irahara, Yamano and Yasui2012). However, in many cases flaws are found in the processes after fertilization and the percentages of normal development are low.
Dehydroleucodine (DhL), a sesquiterpenic lactone of the guaianolide group, is the active principle of a wild plant, Artemisa douglasiana Besser. Treatments of mature oocytes of R. arenarum with DhL were able to induce oocyte activation in a dose-dependent manner, the process being independent of extracellular Ca2+ but dependent on intracellular free Ca2+ (Medina et al., Reference Medina, Bühler and Sánchez-Toranzo2014). This DhL-induced activation is genuine, as oocytes are capable of inducing pronuclei formation. Experiments with antagonists of RyRs (ruthenium red) and IP3Rs (heparin) revealed that both Ca2+-mobilizing systems were activated by lactones and that the blockage of either of the systems alone was not sufficient to prevent total activation induced with DhL (Medina et al., Reference Medina, Bühler and Sánchez-Toranzo2014).
In bovines, the use of dehydroleucodine combined with pretreatment with ionomycin was assayed for activation of oocytes in cloning with results similar to those obtained with 6-DMAP (Vichera et al., Reference Vichera, Alfonso, Duque, Silvestre, Pereyra-Bonnet, Fernández-Martín and Salamone2010). They also demonstrated that oocyte activation with ionomycin + DhL was more efficient than with ionomycin alone.
The aim of this work was to analyze the involvement of the phosphoinositide metabolism, especially the participation of PLA2 and PLC, in DhL-induced activation of in vitro matured Rhinella arenarum oocytes.
Materials and methods
Animals
Adult specimens of Rhinella arenarum were collected in the northwestern area of Argentina and kept at 15°C until use, which generally took place 7 days after collection. Experimental manipulation and oocyte culture were conducted at room temperature (22–25°C) using amphibian Ringer solution (AR): 6.6 g NaCl/l, 0.15 g KCl/l, 0.15 g CaCl2/l, containing penicillin G-sodium (30 mg/l), streptomycin sulphate (50 mg/l) and 0.005 M Tris–HCl buffer (pH 7.4).
Hormones and reagents
Progesterone (Sigma) was dissolved in ethanol and added directly to the culture medium to give a final concentration of 2.5 μM. Neomycin was dissolved in ddH2O in order to obtain a stock solution of 400 mM. A stock solution of LiCl (1M) was prepared in ddH2O. U73122 (Sigma) was dissolved in DMSO in order to obtain a stock solution of 1 mM. Aristolochic acid (ATA) (Sigma) was dissolved in DMSO. Quinacrine (Sigma) was dissolved in DMSO (stock solution 4 mM). Dehydroleucodine (DhL) at 93% purity and 11,13-dihydro-dehydroleucodine (2H-DhL) at 95% purity were obtained according to Giordano et al. (Reference Giordano, Guerreiro, Pestchanker, Guzmán, Pastor and Guardia1990, Reference Giordano, Pestchanker, Guerreiro, Saad, Enriz, Rodriguez, Jauregui, Guzman, Maria and Wendel1992). DhL was dissolved in DMSO and different doses were added to the culture medium.
Gamete collection
Fully grown ovarian oocytes (1.7–1.8 mm in diameter) were obtained from adult female specimens of Rhinella arenarum. Oocytes were denuded by manually pulling off the follicle epithelium and theca layer using fine forceps under a stereoscopic microscope. Follicle cells were removed by shaking in AR for 5 min with gentle shaking (100 oscillations/min). Oocytes with only the vitelline envelope were considered as denuded oocytes.
In vitro oocyte maturation
Hormonal maturation was induced by treatment of denuded oocytes with progesterone. Oocyte maturation was assessed 18 h after hormone addition. Meiosis reinitiating was scored by the presence of a transient white spot at the animal pole. In our working conditions, oocytes reached metaphase II at 18 h after progesterone treatment.
Morphological criteria of oocyte activation
All activation experiments were conducted with oocytes matured in vitro (oocytes MIV). The mature oocytes were kept in AR at room temperature to be used in activation assays. The activation experiments were carried out between 1–3 h after the apparition of the white spot.
After 20 min of incubation, a group of oocytes were fixed in Ancel–Vintemberger solution for the morphological and cytological analyses (Bühler et al., Reference Bühler, Petrino and Legname1987). The activation criteria observable under a stereoscopic microscope were: elevation of the vitelline envelope, disappearance of the white polar spot and flattening of the animal pole.
The activation criterium in cytological preparations observed under the optical microscope was exocytosis of cortical granules.
For the observation of cortical granules, the oocytes were destained by immersion of the preparations in H2O2 100 vol. for 24 h. Then, the slices were stained with Alcian Blue-Pas pH 2.5. With this method, the cortical granules exhibited an intense blue staining due to their glycoprotein content (Oterino et al., Reference Oterino, Sanchez Toranzo, Zelarayán, Valz-Gianinet and Bühler2001).
Statistical analysis
Results are expressed as means ± standard error of the mean (SEM). Comparisons among different treatments were carried out using Student's t-test at a 5% significance level.
Results
Participation of PLA2 in DhL-induced oocyte activation
In order to determine the participation of PLA2 in oocyte activation induced by DhL we evaluated the effect of two phospholipase A2 inhibitors, quinacrine and ATA.
Denuded fully grown ovarian oocytes matured in vitro with progesterone (2.5 μM) were exposed to different doses of quinacrine (5–15 μM) or ATA (50–150 μM) for 30 min before addition of DhL. As control we used oocytes treated only with DhL (36 μM). Signs of activation were monitored after 90 min of incubation considered from the moment when the inducer was added.
Results show that the inhibition of PLA2 with quinacrine was able to inhibit DhL-induced activation in a dose-dependent manner (Fig. 1 A).
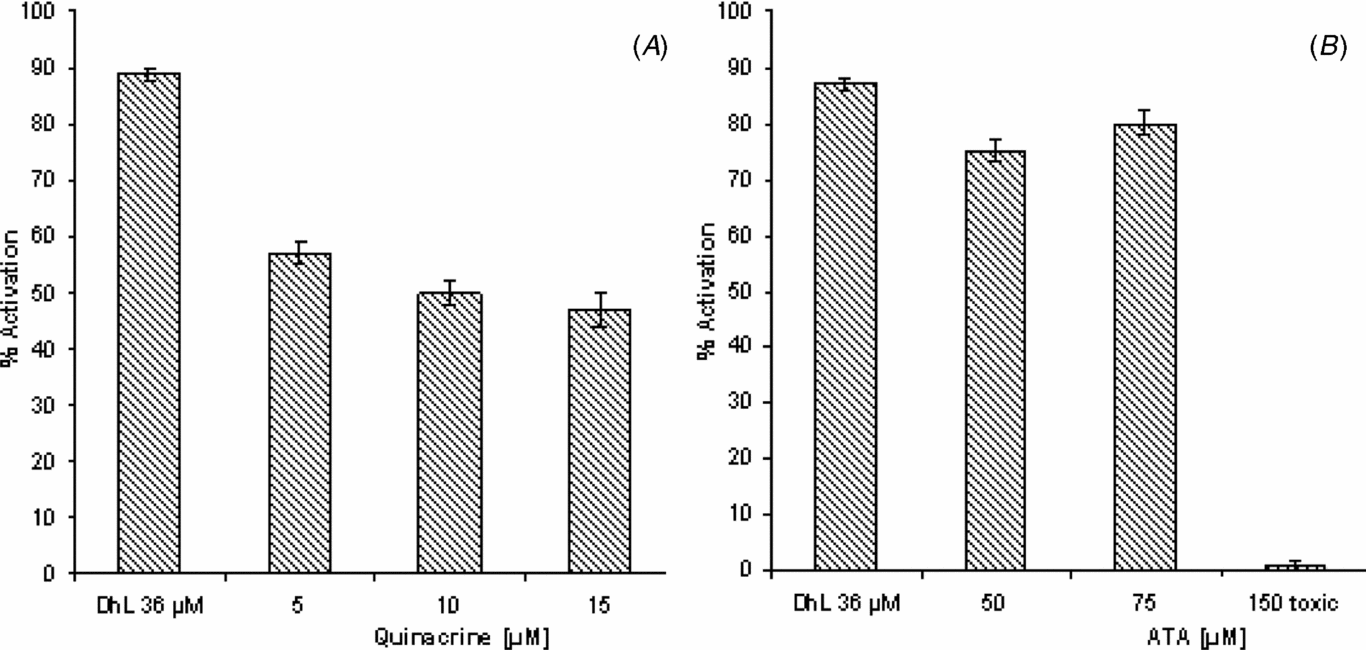
Figure 1 Effect of PLA2 inhibition on DhL-induced oocyte activation. Groups of 20 denuded fully grown ovarian oocytes matured in vitro with progesterone (2.5 μM) were pre-treated with (A) quinacrine (5–15 μM) or (B) ATA (50–150 μM) before addition of DhL (36 μM). Activation was scored after 90 min of culture. Values are the mean ± standard error of the mean (SEM) (n = 3). Each experiment was performed on a different animal.
Maximum inhibition, of about 50%, was obtained with 10 μM of quinacrine, higher concentrations failing to increase the inhibition percentage obtained.
As shown in Fig. 1 B, treatment with ATA at concentrations of 50–75 μM seems to be less effective than treatment with quinacrine in the inhibition of DhL-induced activation. The 150 μM dose had a toxic effect.
Participation of PLC in DhL-induced oocyte activation
In order to analyze the participation of membrane phospholipid hydrolysis during DhL-induced activation, we studied the effect of neomycin, an antibiotic that binds to phosphatidylinositol phosphate (PIP) and phosphatidylinositol 4,5-bisphosphate (PIP2), thus preventing their hydrolysis.
Denuded ovarian oocytes matured in vitro with progesterone (2.5 μM) were pre-incubated in AR with neomycin (5–15 μM) before the addition of DhL (36 μM). Oocyte activation was scored after 90 min of culture. As control we used oocytes which were treated only with DhL (36 μM).
The results presented in Fig. 2 show that treatment with this antibiotic inhibited DhL-induced activation in a dose-dependent manner. With the dose of 5–10μM, a significant decrease of about 70% was observed in DhL-induced activation compared with the control.
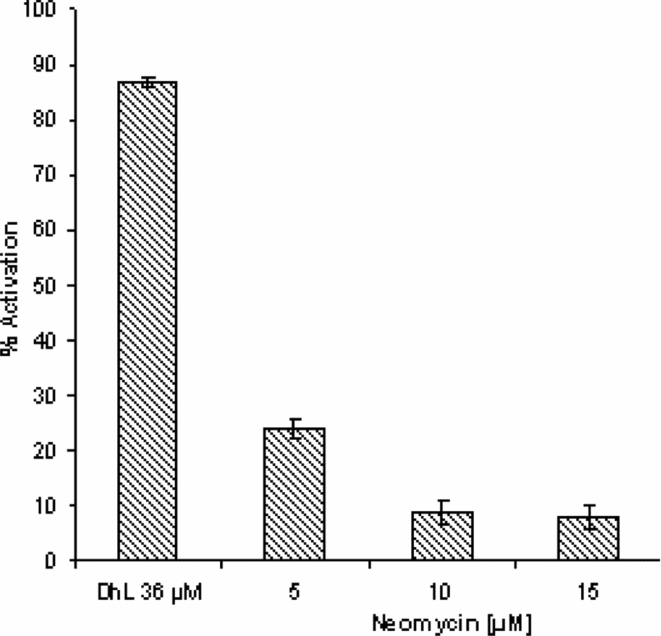
Figure 2 Effect of neomycin on DhL-induced activation. Groups of 20 denuded fully grown ovarian oocytes matured in vitro with progesterone (2.5 μM) were pre-incubated with different doses of neomycin (5–15 μM) and activation was induced by DhL (36 μM). As control we used oocytes treated only with DhL (36 μM). Activation parameters were evaluated after 90 min of culture. Values are the mean ± standard error of the mean (SEM) (n = 4). Each experiment was performed on a different animal.
In order to assay the participation of PI-PLC and PC-PLC we used U73122, a competitive inhibitor of PI-PLC dependent events, and D609, which is known as an inhibitor of PC-PLC.
Matured oocytes were pre-treated for 30 min with U73122 (5–10 μM) or D609 (2.5–10 μM) before addition of DhL (36 μM). Signs of activation were monitored after 90 min of incubation considered from the moment when the inducer was added. The results in Fig. 3 A show that U73122 reduced the activation response in a concentration-dependent manner, 80% inhibition being obtained with a 5 μM dose. The inhibition of activation obtained with D609 was much lower, reaching about 20% at the highest dose used (Fig. 3 B).

Figure 3 Effect of U73122 and D609 on DhL-induced activation. Groups of 20 denuded fully grown ovarian oocytes matured in vitro with progesterone (2.5 μM) were pre-incubated with different doses of (A) U73122 (2.5–10 μM) or (B) D609 (2.5–10 μM) and activation was induced with DhL (36 μM). Activation parameters were evaluated after 90 min of culture. Values are the mean ± standard error of the mean (SEM) (n = 4). Each experiment was performed on a different animal.
In order to confirm whether DhL-induced egg activation is mediated specifically by phospholipid hydrolysis in Rhinella arenarum oocytes we studied the effect of LiCl, which inhibits the turnover of membrane lipids. Denuded fully grown ovarian oocytes matured in vitro with progesterone 2.5μM were pre-incubated with different doses of LiCl (10–30 μM) before treatment with DhL 36 μM. As control, oocytes were treated only with DhL (36 μM). Oocytes activation was scored after 90 min of culture. The results presented in Fig. 4 show that treatment with LiCl significantly inhibited the activation induced by DhL. At the doses tested the inhibition percentage was 60% above control values.

Figure 4 Effect of LiCl on DhL-induced activation. Groups of 20 denuded fully grown ovarian oocytes matured in vitro with progesterone (2.5 μM) were pre-incubated with different doses of LiCl (10–30 μM) and activation was induced by DhL (36 μM). Activation parameters were evaluated after 90 min of culture. Values are the mean ± standard error of the mean (SEM) (n = 4). Each experiment was performed on a different animal.
Discussion
In this paper we analyzed the importance of the lipid metabolism in the DhL-induced activation of in vitro matured oocytes of Rhinella arenarum by testing the effect of inhibitors of PLA2 and PLC.
The results obtained with PLA2 inhibitors show a greater inhibitory effect of quinacrine (about 50%) compared with ATA (15%). This difference could be explained by the fact that quinacrine is not a specific inhibitor for PLA2 (Flower & Blackwell, Reference Flower and Blackwell1976) while ATA is specific for this enzyme.
In Rhinella arenarum, Ajmat et al. (Reference Ajmat, Bonilla, Hermosilla, Zelarayán and Bühler2013) showed that the addition of melittin, a potent PLA2 activator, and arachidonic acid, the main PLA2 reaction metabolite, was able to induce activation events in a bell-shaped manner, indicating the participation of PLA2 in the signalling events of oocyte activation. However, our results suggest that in DhL-induced activation this pathway was not activated.
When the route of phosphoinositides was analyzed, the results showed a significant participation in the effect of the lactone. In this sense, treatment with neomycin, an antibiotic that binds to PIP and PIP2, thus preventing their PLC hydrolysis, caused 70% inhibition in DhL-induced activation.
With these data it is possible to assume that PLC is involved in the signalling pathways used by DhL. The experiment of inhibition of PC-PLC with D609 and IP-PLC with U73122 indicated that IP-PLC has a significant participation in the effect of DhL-induced activation. However, in the present study, we did not observe PC-PLC activation in response to oocyte stimulation by DhL.
DhL could activate PLC with the consequent production of IP3, which would induce Ca2+ release from the endoplasmic reticulum. These possibilities agree with the observation that DhL requires no extracellular Ca2+ to exert its effect; however, intracellular Ca2+ release through IP3Rs and RyRs is critical (Medina et al., Reference Medina, Bühler and Sánchez-Toranzo2014).
The participation of phosphoinositides hydrolysis in the effect of DhL is confirmed by the LiCl assays, which show about 60% inhibition.
Taken together, these results suggest that DhL induces activation of in vitro matured oocytes of Rhinella arenarum by activation of IP-PLC, which in turn induces IP3 formation, causing Ca2+ release from the endoplasmic reticulum. The increase in cytosolic Ca2+ would be responsible for the activation events.
Acknowledgements
This work was supported by a grant from the Science Council of the National University of Tucumán (CIUNT) and the National Agency for Promotion of Science and Technology (FONCYT).






