Introduction
Octamer-binding transcription factor 4 (Oct4) is a transcription factor of the POU family that can be characterized as a critical molecule for modulating the self renewal and pluripotency of embryonic stem cells (Okamoto et al., Reference Okamoto, Okazawa, Okuda, Sakai, Muramatsu and Hamada1990; Mountford et al., Reference Mountford, Nichols, Zevnik, O’Brien and Smith1998; Nichols et al., Reference Nichols, Zevnik, Anastassiadis, Niwa, Klewe-Nebenius, Chambers, Scholer and Smith1998). The precise regulation of Oct4 is very important for maintaining and differentiating embryonic stem cells; thus, both the overexpression (Hochedlinger et al., Reference Hochedlinger, Yamada, Beard and Jaenisch2005; Fan et al., Reference Fan, Gu, Zhang, Zhong, Wang, Zhou, Wang, Jia and Wang2013) and downregulation (Pesce et al., Reference Pesce, Gross and Scholer1998; Adjaye et al., Reference Adjaye, Bolton and Monk1999; Goto et al., Reference Goto, Adjaye, Rodeck and Monk1999; Niwa et al., Reference Niwa, Miyazaki and Smith2000; Fan et al., Reference Fan, Gu, Zhang, Zhong, Wang, Zhou, Wang, Jia and Wang2013) of Oct4 induce a loss of pluripotency and induce differentiation. Apart from embryonic stem cells, the expression of Oct4 is also found in other cell types including mesenchymal stem cells (Fan et al., Reference Fan, Gu, Zhang, Zhong, Wang, Zhou, Wang, Jia and Wang2013), early embryonic tissues (DeVeale et al., Reference DeVeale, Brokhman, Mohseni, Babak, Yoon, Lin, Onishi, Tomilin, Pevny, Zandstra, Nagy and van der Kooy2013) and cancer cells (Li et al., Reference Li, Yan, Ji, Bao, Qian, Chen, Wu, Chen, Li and Su2012), although its roles and underlying mechanisms are still unclear. Recently, several reports have shown that the expression of Oct4 controls cell-cycle progression and enhances the proliferation of the cells for both embryonic stem cells (Lee et al., Reference Lee, Go, Kang, Han and Kim2010) and other cell types (Li et al., Reference Li, Yan, Ji, Bao, Qian, Chen, Wu, Chen, Li and Su2012; DeVeale et al., Reference DeVeale, Brokhman, Mohseni, Babak, Yoon, Lin, Onishi, Tomilin, Pevny, Zandstra, Nagy and van der Kooy2013; Fan et al., Reference Fan, Gu, Zhang, Zhong, Wang, Zhou, Wang, Jia and Wang2013).
The high proliferation of nuclear donor cells is important in transgenic animal production using the somatic cell nuclear transfer (SCNT) technique, as the transgenesis and selection procedures of the donor cells require multiple subcultures and expansion steps. Because only primary cultured cells (fibroblasts in most cases) can be used for SCNT, it is critical to maintain the highly proliferative properties of the cells that are intended for use in SCNT after gene modification. From this point of view, we wondered whether the expression of Oct4 in primary cultured porcine fibroblasts enhances proliferation, as has been suggested by previous reports. However, the role of Oct4 expression in porcine fibroblasts is still unclear.
Hence, we hypothesised that the expression of Oct4 in porcine fibroblasts might play a role in cell proliferation. In this study, we cloned porcine endogenous Oct4 cDNA and established and analysed Oct4-overexpressed porcine fibroblasts. In addition, we produced cloned embryos using the Oct4-overexpressed fibroblasts by the SCNT technique and observed the effects of Oct4 expression on the development of porcine embryos.
Materials and methods
Primary culture of porcine fibroblasts
Primary culture was performed using ear skin biopsies from neonatal piglets. The lumps of ear skin were minutely homogenised and then cultured overnight in collagenase IV (Sigma-Aldrich Corp., St. Louis, MO, USA) in a 37°C incubator. The homogenised ear tissues were washed more than three times with phosphate-buffered saline (PBS; Life Technologies, Carlsbad, CA, USA) and collected by centrifugation at 43 g, for 2 min. The collected clusters of cells were cultured in 60-mm culture dishes with Dulbecco's Modified Eagle Medium (DMEM, Life Technologies), supplemented with 10% fetal bovine serum (FBS, Life Technologies) and 1% penicillin/streptomycin (Pen/Strep; Life Technologies).
Porcine Oct4 cloning and the construction of an expression vector
For cloning the coding domain sequences (CDS) of porcine Oct4, total RNA was extracted from pig ovaries using an RNA extraction kit (Qiagen, Hilden, Germany). One microgram of total RNA was used for reverse transcription with Superscript III (Life Technologies). The CDS of the porcine Oct4 was amplified by PCR using specific primer sets (Table 1). The PCR fragments were cloned into the NT-GFP Fusion TOPO-cloning vector (Life Technologies) and sequenced for confirmation. The vectors containing porcine Oct4 CDS were digested using two restriction enzymes, NheI and XhoI (New England Bio Labs, Inc., Beverly, MA, USA), and ligated into the pIRES2 DsRed-Express2 vector (CLONTECH Laboratories Inc., Palo Alto, CA, USA). The map of the expression vector is illustrated in Figure 1A.
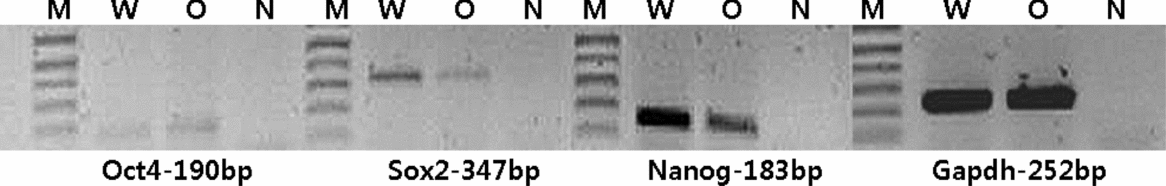
Table 1 Primer sequences used in the study

aControl for RT-PCR; bControl for real-time RT-PCR.

Figure 1 (A) Vector construct used in the study. (B) Morphology and Oct4 expression of wild-type (Wt; Ba, Ba′ and Ba′′) and Oct4-overexpressed (Oct4; Bb, Bb′ and Bb′′) porcine fibroblasts. Cell shape was changed after transfection of Oct4 (Ba versus Bb). Wild-type and Oct4-overexpressed cells were stained with DAPI (Ba′ and Bb′), and Cy3 conjugated secondary antibody was attached to Oct4 primary antibody (Oct4′; Ba′′ and Bb′′). Expression of Oct4 was only detected in transfected cells and localised in the nucleus. (C) The cell growth curves of wild-type and Oct4-overexpressed porcine fibroblasts. A significant difference in growth patterns was observed between two groups.
Transfection and establishment of a stable Oct4-overexpressed cell line
One day before transfection, fibroblasts were plated at a density of 1 × 105 cells/ml in a 35-mm culture dish and cultured overnight to achieve 50–70% confluence. Next, 1 μg of the Oct4 vector (linearised by AflII; New England Bio Labs, Inc.), 3 μl of the transfection reagent (Fugene HD; Roche Inc., Switzerland) and 96 μl of DMEM were incubated for at least 20 min at 25°C in a 1.5-ml microtube and were then overlaid on a prepared porcine fibroblast. After 2 days of culture, 1 mg/ml neomycin (G418, Life Technologies) was added for another 2 weeks of culture to select the stable Oct4-expressing cells.
Immunocytochemistry validation of Oct4 expression
To determine the transfected cells that expressed the Oct4 protein, the cells were immunostained. The cells were fixed with 4% paraformaldehyde for 20 min, permeabilised with 0.1% Triton X-100 (Sigma) for 10 min and blocked with 10% normal goat serum for 1 h. The fixed cells were incubated overnight with primary antibody (Oct3/4, Santa Cruz Biotechnology, Inc., CA, USA) and then with secondary antibody (Cy3-AffiniPure Goat Anti-Mouse IgG, Jackson ImmunoResearch Laboratories, Inc., PA, USA) for 2 h. In addition, DAPI was used as a counter-stain. The stained cells were examined under ultraviolet light using a fluorescence microscope (Nikon, Japan).
Analysis of cell growth and proliferation
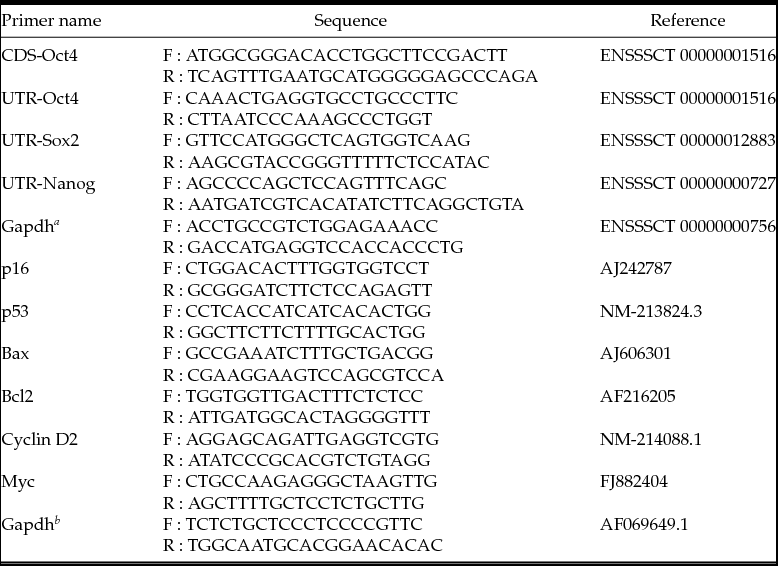
Cell growth was analysed using an automated cell counter (Countess® Cell Counter, Invitrogen). For analysis, 0.5 × 105/ml cells were plated and cultured in 12-well plates (Falcon, NJ, USA), and the cultured cells were collected every 24 h by 0.25% trypsin–EDTA treatment. The collected cells were resuspended in 1 ml of PBS, and 10 μl of the sample were mixed with 10 μl of trypan blue and then pipetted into a Countess® Chamber slide for analysis. Cell number results were analysed with population doubling time (PDT) calculating software (http://www.doublingtime.com/compute.php). To determine the cell proliferation ability, a cell migration assay was performed. Monolayers of the wild-type and Oct4-transfected cells were prepared by seeding 2 × 104 cells onto Oris™ Cell Migration Assay kit 96-well plates (PLATYPUS Technologies, WI, USA). After 24 h, following the removal of the stopper, phase-contrast images were taken for a pre-migration reference (t = 0 h), and then cells were allowed to grow for 36 h to permit cell migration. After 36 h of culture, images of migrated cells were recorded. The images were analysed to establish the migration range from pre-migration (t = 0 h) to post-migration (t = 36 h), using NIH Image J freeware, and the Z factor was evaluated as previously reported (Zhang et al., Reference Zhang, Chung and Oldenburg1999).
Quantitative gene expression analysis
To analyse the expression of the endogenous stemness-related genes, Oct4, Sox2 and Nanog, quantitative RT-PCR was performed as described previously (Kong et al., Reference Kong, Jing, Yan, Li, Gong, Zhu, Li, Zhang, Zheng, Wang, Xie and Zhang2009) with some modification. Briefly, total RNA was extracted from wild-type or Oct4-transfected cells, and cDNA was synthesised and used for PCR amplification as described earlier. To distinguish the endogenous genes from those transfected with Oct4, specific primers including the untranslated region were designed and used (Table 1). For comparing the range of expression levels, Gapdh was used as a housekeeping control. For analysing gene expression related with the cell-cycle progression and apoptosis, real-time RT-PCR was used as described previously (Kinikoglu et al., Reference Kinikoglu, Kong and Liao2014) with some modification. For the analysis, SYBR® Green PCR master mix and 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) were used. Details of specific primers used in the study are shown in Table 1. The expression levels of different genes were normalised to the housekeeping gene Gapdh using the ΔΔCt method, as previously described (Livak and Schmittgen, Reference Livak and Schmittgen2001).
Somatic cell nuclear transfer and embryo culture
To produce Oct4-expressing porcine embryos, SCNT was performed as described in the previous report (Park et al., Reference Park, Cho, Koo, Kim, Kang, Hurh, Kim, Yeom, Moon, Lee, Choi, Hong, Jang, Hwang, Yang, Lee and Ahn2014) with a slight modification. Briefly, in vitro matured and enucleated oocytes were placed in TCM-199, which was supplemented with 10% (v/v) FBS. Either wild-type fibroblasts or the Oct4-transfected fibroblasts were used as donor cells for SCNT. The donor cells were injected into the perivitelline space of the prepared oocytes and then electrically fused using an electro cell fusion generator (LF101; Nepa Gene Co., Japan). After 40 min of fusion, the artificial activation of the fused embryos was performed with a single direct current electric pulse of 1.5 kV/cm for 60 μs using a BTX Electro Cell Manipulator 2001 (BTX Inc., San Diego, USA). The activated SCNT embryos were cultured in Porcine Zygote Medium-5 (PZM-5) (Yoshioka et al., Reference Yoshioka, Suzuki, Tanaka, Anas and Iwamura2002) under mineral oil at 39°C in 5% CO2, 5% O2 and 90% N2. Embryos were evaluated for cleavage on day 2 and for blastocyst formation on days 5 and 6.
Statistical analysis
All experiments were replicated at least three times and statistically analysed using Prism 5 software (GraphPad, La Jolla, CA, USA). Student's t-test was used to analyse any differences between two groups. One-way analysis of variance (ANOVA) was used to analyse any differences between three or more groups. A P-value less than 0.05 was considered to be statistically significant.
Results and Discussion
In this study, we cloned the CDS of porcine endogenous Oct4 and used it for overexpression in porcine fibroblasts. Several studies have used human-derived Oct4 in porcine cells to generate induced pluripotent stem cells (Kwon et al., Reference Kwon, Jeon, Oh, Ock, Im, Lee, Im, Lee, Oh, Park and Hwang2013; Park et al., Reference Park, Cha, Ahn and Woo2013; Yuan et al., Reference Yuan, Lee, Park, Spate, Prather, Wells and Roberts2014; Zhang et al., Reference Zhang, Wei, Zhang, Li, Liu, Pu, Li, Cao, Cao, Liu and Zhang2014). However, a few previous reports have tried to use porcine endogenous Oct4 (Liu et al., Reference Liu, Ji, Mao, Liu, Wang, Chen and Liu2012). The sequencing results of our cloned porcine endogenous Oct4 showed that the homologies of the amino acid sequence of porcine Oct4 and human Oct4 were 93% identical (data not shown).
After transfection and selection, the expression of Oct4 was validated with immunochemistry (Fig. 1B). Because Oct4 is a transcription factor, most of the cells showed Oct4-positive staining in the nucleus (Fig. 1B) as expected. Interestingly, the morphology of the fibroblasts was remarkably changed in Oct4-overexpressed cells. (Fig. 1Ba, Bb) The Oct4 cells were smaller in size with sharper and clearer margins compared to the wild-type cells. These changes in morphologies were similar to those seen in less senescent cells presented by previous reports (Bayreuther et al., Reference Bayreuther, Rodemann, Hommel, Dittmann, Albiez and Francz1988; Cho et al., Reference Cho, Ryu, Oh, Park, Lee, Kim, Kim, Jang and Park2004). The overexpression of porcine Oct4 protein also facilitated cellular doubling and proliferation. The PDT was noticeably reduced from 55.77 ± 1.32 h in the wild-type cells to 42.67 ± 0.84 h in the Oct4-overexpressed cells and, as a result, the growth curves were significantly different between the two groups (Fig. 1C). A cell migration assay was also performed to analyse the proliferation capacity of Oct4-overexpressed cells. The migrations of the cells were more active in the Oct4 cells (Fig. 2). Therefore, the migration speed of the Oct4 cells (2.10 × 106 ± 3.67 × 104 μm2/h) was significantly faster than that of the wild-type cells (1.66 × 106 ± 2.21 × 104 μm2/h). These data exhibited high reliability, evidenced not only by a CV of 2.3 and 3.0 for wild-type and Oct4-overexpressed cells, respectively, but also a Δ migration of 0.5< Z<1.0.

Figure 2 (A) Cell migrations of wild-type (Wt) and Oct4-overexpressed (Oct4) porcine fibroblasts. White circles are open areas on culture plate at 0 h (Aa and Ab) and 38 h (Aa′ and Ab′). (B) Oct4-overexpressed cells showed significantly smaller open areas after cell migration, which reveals higher proliferation capacity compared with wild-type cells. The symbol (*) indicates a significant difference (P < 0.05).
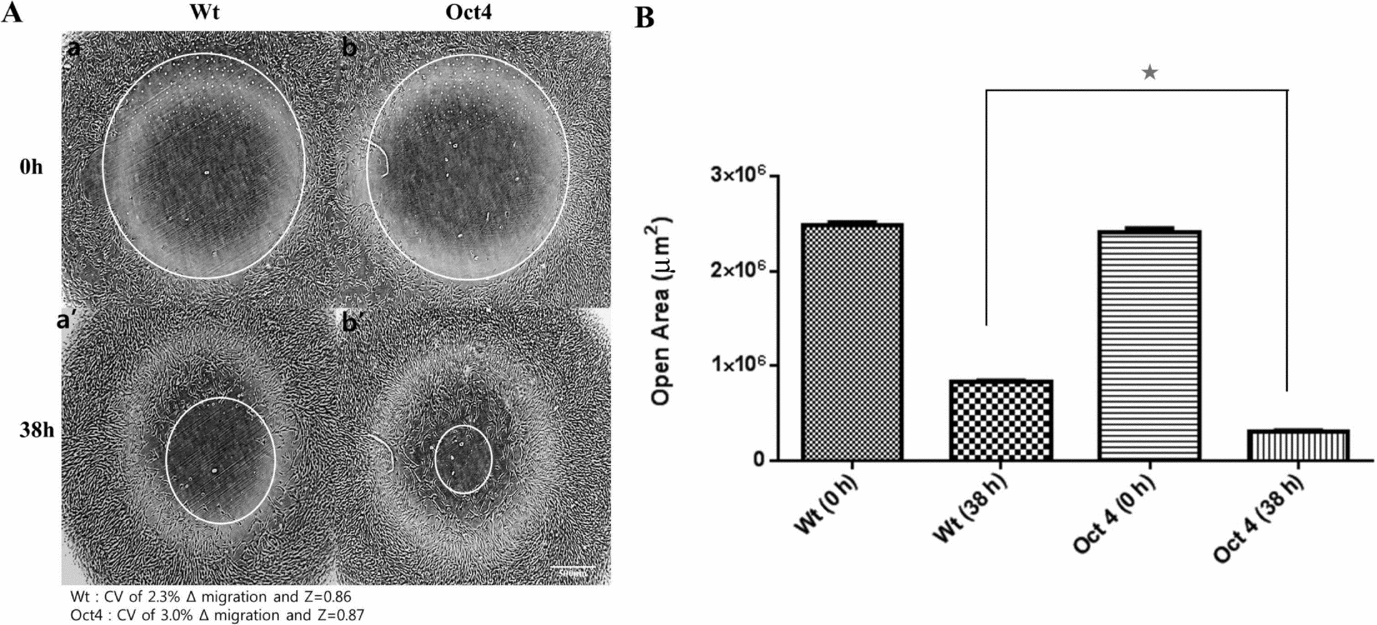
To investigate the underlying mechanism of enhanced proliferation by Oct4 overexpression, we examined the transcription level of the cell-cycle-related genes, p16, Cyclin D2, p53 and Myc, and the apoptosis-related genes, Bax and Bcl2 (Fig. 3). Results showed that p53, Cyclin D2 and Bax expression was not significantly different between the two groups. However, p16 transcription was increased more than four-fold and Myc was increased about three-fold in the Oct4-overexpressed cells. Usually, in highly proliferative cells, p16 and p53 expression is reduced. If p16 and p53 expression is increased, cells are likely undergoing cell senescence and cell-cycle arrest (Weinberg, Reference Weinberg1989; Ruley, Reference Ruley1990; Hinds et al., Reference Hinds, Dowdy, Eaton, Arnold and Weinberg1994; Downward, Reference Downward1997; Serrano et al., Reference Serrano, Lin, McCurrach, Beach and Lowe1997). Thus, the upregulation of p16 in Oct4-overexpressed cells was somewhat unexpected. However, a previous report showed that the expression of Myc bypassed the p16 pathway and promoted cell proliferation even in the presence of p16 (Alevizopoulos et al., Reference Alevizopoulos, Vlach, Hennecke and Amati1997). The present study also showed that the expression of Myc is upregulated by the expression of Oct4. Thus, we suggest that Myc is one of the key molecules of proliferation in Oct4-overexpressed cells. On the other hand, the expression of the anti-apoptosis gene Bcl2 was also increased about two-fold in the Oct4-overexpressed cell line. This means that the reduced apoptosis by Bcl2 expression also contributed to faster proliferation in the Oct4-overexpressed cells.

Figure 3 Transcriptional levels of cell cycle genes (p16, p53, Cyclin D2 and Myc) and apoptosis-related genes (Bax, Bcl2) were analysed. In Oct4-overexpressed cells (Oct4), transcription levels of p16, Myc and Bcl2 were significantly higher compared with that in wild-type cells (Wt). The symbol (*) indicates a significant difference between groups (P < 0.05).
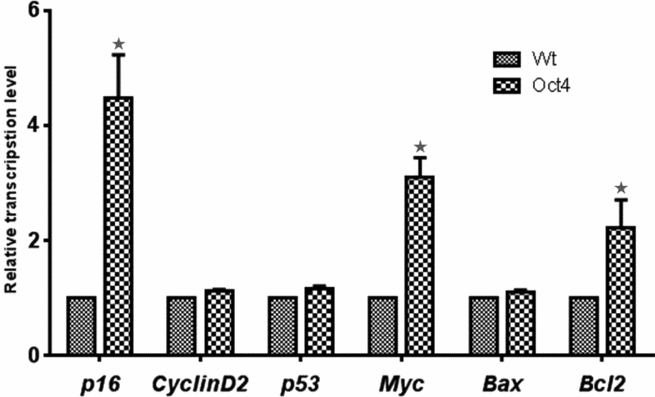
We also analysed the expression of the endogenous stemness-related genes, Oct4, Sox2 and Nanog, in Oct4-overexpressed cells. As shown in Fig. 4, the expression of endogenous Oct4 in porcine fibroblasts was very weak and did not change after transfection of the Oct4 vector. This means that the cellular and molecular changes found in the present study were affected by transfected Oct4 and not by the expression of endogenous Oct4. Sox2 and Nanog are also known as molecules that modulate cell-cycle progression and promote proliferation (Zhang et al., Reference Zhang, Neganova, Przyborski, Yang, Cooke, Atkinson, Anyfantis, Fenyk, Keith, Hoare, Hughes, Strachan, Stojkovic, Hinds, Armstrong and Lako2009; Fan et al., Reference Fan, Gu, Zhang, Zhong, Wang, Zhou, Wang, Jia and Wang2013). However, the present study showed that the expression of endogenous Sox2 and Nanog was downregulated (about 20 and 30%, respectively). Therefore, we conclude that enhanced proliferation in the Oct4-overexpressed cells is not related with the effect of Sox2 and the Nanog pathway. Current results were similar to previous reports indicating that Oct4 overexpression induced negative feedback to Sox2 and Nanog expression (Lee et al., Reference Lee, Kim, Rho, Han and Kim2006b; Boer et al., Reference Boer, Kopp, Mallanna, Desler, Chakravarthy, Wilder, Bernadt and Rizzino2007).

Figure 4 Transcription level changes of endogenous Oct4, Sox2 and Nanog in wild-type and Oct4-overexpressed porcine fibroblasts. Endogenous Oct4 expression was very weak and did not change after Oct4 vector transfection. Expression of Sox2 and Nanog was reduced in Oct4-overexpressed cells compared with wild-type cells. M: 1 kb plus marker; W: wild-type cells; O: Oct4-overexpressed cells; N: blank template.
The effects of Oct4 overexpression in porcine embryos were also investigated in the study. For this purpose, we produced cloned embryos reconstructed with wild-type or Oct4-overexpressed cells. Oct4 overexpression improved both the cleavage and blastocyst formation rate of cloned embryos (Table 2). As we reported earlier, the expression of Oct4 in cloned embryos was significantly lower compared with fertilised embryos (Lee et al., Reference Lee, Lee, Kim, Jeong, Kim, Koo, Park, Hashem, Hossein, Son, Lee, Hwang, Kang and Lee2006a). Thus, the overexpression of Oct4 in donor cells might be helpful for the proper development of cloned SCNT embryos. In addition, the higher expression of Bcl2 in Oct4-overexpressing donor cells may affect the development of SCNT embryos. Interestingly, developmental velocity was also increased in the Oct4 group, and the formation of blastocysts could be observed as early as day 5 of culture, while blastocysts were observed from day 6 in the wild-type group. This phenomenon is similar to the result of increased PDT in Oct4-overexpressed fibroblasts in the present study. Therefore, we conclude that the overexpression of Oct4 can facilitate cell-cycle progression in both fibroblasts and cloned embryos.
Table 2 Development of porcine cloned embryos reconstructed with wild-type (Wt) or Oct4-overexpressed (Oct4) cells

a,bValues with different superscripts within the same column are significantly different (P < 0.05). BL: blastocyst.
In conclusion, we cloned the porcine endogenous Oct4 gene and established a stable Oct4-overexpressed fibroblasts cell line. We found that the overexpression of Oct4 promotes the proliferation of fibroblasts. This phenomenon seems to be related to the upregulation of the Myc and Bcl2 genes. The development of cloned embryos reconstructed with Oct4-overexpressed cells was improved, and blastocysts were observed as early as day 5 in culture.
Acknowledgements
This study was supported by grants from IPET (#111078–03–1-CG000), NRF (NRF-2011–0014941), Biogreen (PJ0090962012), RDA (#PJ009802), MOTIE and BK 21 plus program for veterinary science.