Introduction
Following the outbreak of bovine spongiform encephalopathy (BSE) in Japan in September 2001, all slaughtered animals have been screened to confirm BSE infection-negative status. Consequently, this hazard analysis and critical control point (HACCP)-based approach for food safety, has lead to the elimination of small-scale abattoirs, and resulted in long distance transportation from large-scale, HACCP criteria-satisfying abattoirs to the laboratories. Both the time-consuming screening assay and the long distance transportation of ovaries to laboratories have made it necessary to optimize transient storage of bovine ovaries, although BSE screening is mandatory only in cattle more than 48 months old (at April 2013). It seemed clear that developmental competence of bovine oocytes retrieved from stored ovaries was lower than that of those retrieved from fresh ovaries (Nakao & Nakatsuji, Reference Nakao and Nakatsuji1992; Matsukawa et al., Reference Matsukawa, Akagi, Adachi, Kubo, Hirako, Watanabe and Takahashi2007). Recommended conditions for storage of bovine ovaries include a decrease in the temperature of the storage solution from 30–37ºC to 15–20°C (Nakao & Nakatsuji, Reference Nakao and Nakatsuji1992; Matsukawa et al., Reference Matsukawa, Akagi, Adachi, Kubo, Hirako, Watanabe and Takahashi2007; Nagao et al., Reference Nagao, Harada, Yamaguchi, Igarashi, Ooshima and Kato2010), and exposure to anti-oxidants (Nagao et al., Reference Nagao, Harada, Yamaguchi, Igarashi, Ooshima and Kato2010), magnesium and raffinose (Iwata et al., Reference Iwata, Hayashi, Sato, Kimura, Kuwayama and Monji2005) in the storage solution.
During the process of fertilization, a bull spermatozoon brings a centrosome into an oocyte and a single sperm aster is formed by polymerization of microtubule components (α- and β-tubulin) from the centrosome (Schatten, Reference Schatten1994). The microtubule network contributes to migration of male and female pronuclei (PN) to the centre of a fertilized oocyte and facilitates the subsequent fusion of the 2PN and mitotic cleavage (Kim et al., Reference Kim, Simerly, Funahashi, Schatten and Day1996; Terada et al., Reference Terada, Nakamura, Simerly, Hewitson, Murakami, Yaegashi, Okamura and Schatten2004). We reported that impaired development of the sperm aster in bovine oocytes was caused by a procedure for intracytoplasmic sperm injection (Hara et al., Reference Hara, Abdalla, Morita, Kuwayama, Hirabayashi and Hochi2011), and that frequent multiple aster formation after in vitro fertilization (IVF) was induced by vitrification of in vitro matured bovine oocytes (Hara et al., Reference Hara, Hwang, Kagawa, Kuwayama, Hirabayashi and Hochi2012), with impaired migration and development of pronuclei prior to the first cleavage. Recently, we have reported that short-term culture of vitrified-warmed bovine oocytes with an inhibitor of Rho-associated coiled-coil kinase (ROCK) suppressed the incidence of multiple aster formation, resulting in an improvement of the blastocyst yield (Hwang et al., Reference Hwang, Hara, Chung, Hirabayashi and Hochi2013).
The purpose of the present study was to investigate: (1) whether multiple aster formation is frequently observed in bovine oocytes retrieved from 1-day stored ovaries after in vitro maturation (IVM) and IVF; (2) whether the incidence of multiple aster formation can be suppressed by short-term ROCK inhibition after IVM; and (3) whether the normalized multiple aster formation rate can contribute to improve the blastocyst yield from stored ovary-derived oocytes.
Materials and Methods
Oocyte preparation
Unless otherwise stated, all chemicals used in this study were purchased from Sigma-Aldrich Chemicals (St. Louis, MO, USA). Abattoir-derived bovine ovaries were transported to the laboratory in saline within 2–6 h at 21–26ºC (defined as ‘fresh ovaries’) or 22–26 h at 12–14ºC (defined as ‘stored ovaries’). The contents of 2–8 mm follicles were aspirated and cumulus–oocyte complexes (COCs) surrounded with at least two layers of compact cumulus cells were collected from the follicular fluid. IVM was conducted in HEPES-buffered TCM-199 (Earle's salt; Gibco BRL, Grand Island, NY, USA) supplemented with 10% (v/v) fetal bovine serum (FBS; SAFC Biosciences, Lenexa, KS, USA), 0.2 mM sodium pyruvate, 0.02 AU/ml follicle stimulating hormone (FSH; Kawasaki-Mitaka Pharmaceutical, Kanagawa, Japan), 1 μg/ml 17β-estradiol and 50 μg/ml gentamycin sulfate for 22 h at 38.5ºC in 5% CO2 in air (8–12 COCs per 100-μl microdrop covered with mineral oil). Cumulus cells were then removed by brief vortex-mixing in HEPES-buffered TCM-199 medium supplemented with 3 mg/ml bovine serum albumin (BSA), 0.2 mM sodium pyruvate, 1000 IU/ml hyaluronidase and 50 μg/ml gentamycin sulfate. Denuded oocytes extruding their first polar body were defined as matured.
Treatment with ROCK inhibitor
HEPES-buffered TCM-199 medium supplemented with 5% (v/v) FBS, 0.2 mM sodium pyruvate, and 50 μg/ml gentamycin sulfate (TCM-199 medium/5% FBS) was used as the base medium for ROCK inhibition of stored ovary-derived oocytes prior to IVF, as reported previously (Hwang et al., Reference Hwang, Hara, Chung, Hirabayashi and Hochi2013). Matured oocytes were incubated in the TCM-199medium /5%FBS containing 10 μM ROCK inhibitor (Y-27632; (R)-(+)-trans-4-(1-aminoethyl)-N-(4-pyridyl)cyclohexanecarboxamide dihydrochloride monohydrate) for 2 h at 38.5ºC in 5% CO2 in air (15–30 oocytes per 100-μl microdrop), while oocytes incubated for the same 2 h in ROCK inhibitor-free medium served as controls.
In vitro fertilization and culture
Spermatozoa were prepared from commercially available frozen semen from a Japanese Black bull. The content of a 0.5-ml straw was layered on the top of a Percoll density gradient consisting of 2 ml of 45% Percoll above 2 ml of 90% Percoll in a 15-ml conical tube, and centrifuged for 20 min at 700 g. The sperm pellet was suspended in modified Brackett and Oliphant (mBO) medium (IVF100; Institute for Functional Peptides, Yamagata, Japan) supplemented with 5 mM theophylline, washed twice (5 min at 300 g each) and then re-suspended in the mBO medium supplemented with 5 mg/ml BSA and 10 μg/ml heparin (IVF medium) to yield a concentration of 1.5 × 107 sperm cells/ml. Next, 20 μl of the sperm suspension was added to 80 μl of IVF medium containing 10–12 matured oocytes (final sperm concentration; 5 × 106 sperm cells/ml) and kept for 6 h at 38.5ºC under 5% CO2 in air. Presumptive zygotes were cultured in modified synthetic oviduct fluid (Holm et al., Reference Holm, Booth, Schmidt, Greve and Callesen1999) supplemented with 30 μl/ml essential amino acids solution (50× Gibco-11130), 10 μl/ml non-essential amino acids solution (100× Gibco-11140) and 5% (v/v) FBS for 8 days at 39.0ºC in 5% CO2, 5% O2 and 90% N2. Cleavage rate was recorded 2 days after IVF, and the number of expanding to hatched blastocysts was recorded at 7 and 8 days after IVF.
Assessment of aster formation
Oocytes released from IVF medium were cultured for an additional 4 h in TCM-199 medium/5%FBS at 38.5ºC in 5% CO2 in air, and subjected to immunostaining as reported previously (Hara et al., Reference Hara, Abdalla, Morita, Kuwayama, Hirabayashi and Hochi2011, Reference Hara, Hwang, Kagawa, Kuwayama, Hirabayashi and Hochi2012; Hwang et al., Reference Hwang, Hara, Chung, Hirabayashi and Hochi2013). Briefly, the oocytes were extracted for 15 min by buffer M (25% glycerol, 50 mM KCl, 0.5 mM MgCl2, 0.1 mM EDTA, 1 mM EGTA and 50 mM imidazole hydrochloride, pH6.8) containing 5% (v/v) methanol and 1% (v/v) Triton X-100 detergent after the zonae pellucidae had been removed with 0.75% protease in M2 medium. The oocytes were then fixed with cold methanol for 10 min and permeabilized overnight in PBS(–) containing 0.1% (v/v) Triton X-100. Sperm aster were labelled with a monoclonal antibody against α-tubulin and the primary antibodies were detected by FITC-conjugated goat anti-mouse IgG. Nuclear DNA was visualized by counterstaining with 2.5 μg/ml DAPI. The preparations were mounted with coverslips in 100 mg/ml 1,4-diazabicyclo[2.2.2]octane dissolved in glycerol:PBS (9:1; v/v) and digital images were taken with a confocal laser scanning microscope (FV1000-D; Olympus, Tokyo, Japan) at a distance of 2 μm and stacked. Normally fertilized oocytes, defined by the presence of 2PN, with detectable microtubule network were selected and classified into either those with single sperm aster (Fig. 1 A) or those with multiple asters (Fig. 1 B) because polyspermically fertilized oocytes exhibit more than two asters.
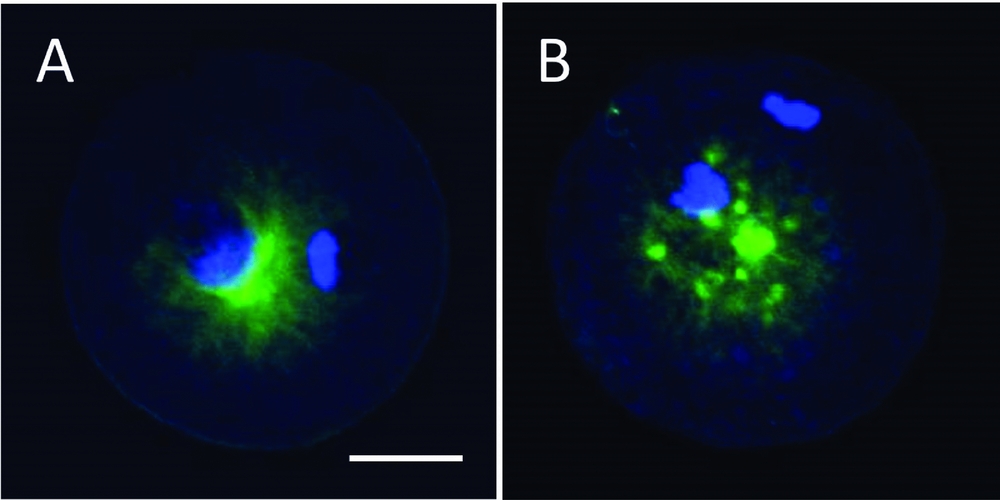
Figure 1 Confocal microscopic observation of bovine IVF oocytes. (A) An oocyte forming a single sperm aster. (B) An oocyte forming multiple asters. Nuclei and microtubules were stained with DAPI (blue) and α-tubulin antibody (green), respectively. The scale bar represents 30 μm.
Statistical analysis
Percentage data in each replicate were arcsine-transformed and subjected to Student's t-test or one-way analysis of variance (ANOVA). When the ANOVA reached significance, differences among means were analyzed by post hoc Tukey's tests. Data for aster formation (single or multiple) were analyzed by chi-squared test. P-values less than 0.05 were considered to be significant.
Results
Under our conventional IVM system for 22 h, the proportion of denuded oocytes extruding the first polar body, defined as matured oocytes, was 83 ± 1% (659/795) and 64 ± 4% (730/1138) in the fresh ovary group and the stored ovary group, respectively (P < 0.05). When these denuded mature oocytes were subjected to IVF, the cleavage rate of the IVF oocytes retrieved from stored ovaries was significantly lower than that of the fresh ovary-derived IVF oocytes (71 versus 85%; Table 1). Significant difference between the stored ovary group and the fresh ovary group was notable in the blastocyst yields at day 7 (33 versus 51%) and day 8 (37 versus 63%).
Table 1 In vitro development of bovine IVF oocytes retrieved from fresh versus 1-day stored ovaries
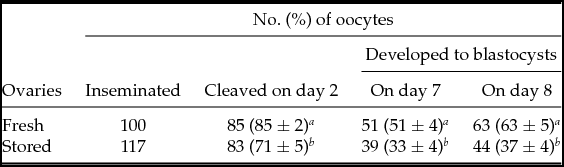
Percentages are expressed as the mean ± standard error of the mean (SEM) of five replicates in each group.
a ,b Significantly different within columns (P < 0.05).
The proportion of IVF oocytes with 2PN in the stored ovary group was significantly lower (P = 0.03) than that in fresh ovary group (Fig. 2 A). In contrast, the incidence of multiple aster formation was more than double in the stored ovary group (31%) compared with the fresh ovary group (15%), while the proportions of aster-forming 2PN zygotes were comparable between the two groups (95 and 99%, respectively). The higher incidence of multiple aster formation in stored ovary-derived IVF oocytes was suppressed significantly by ROCK inhibition over a 2 h incubation after 22 h IVM culture (10 versus 32% in the control group; Fig. 2 B).
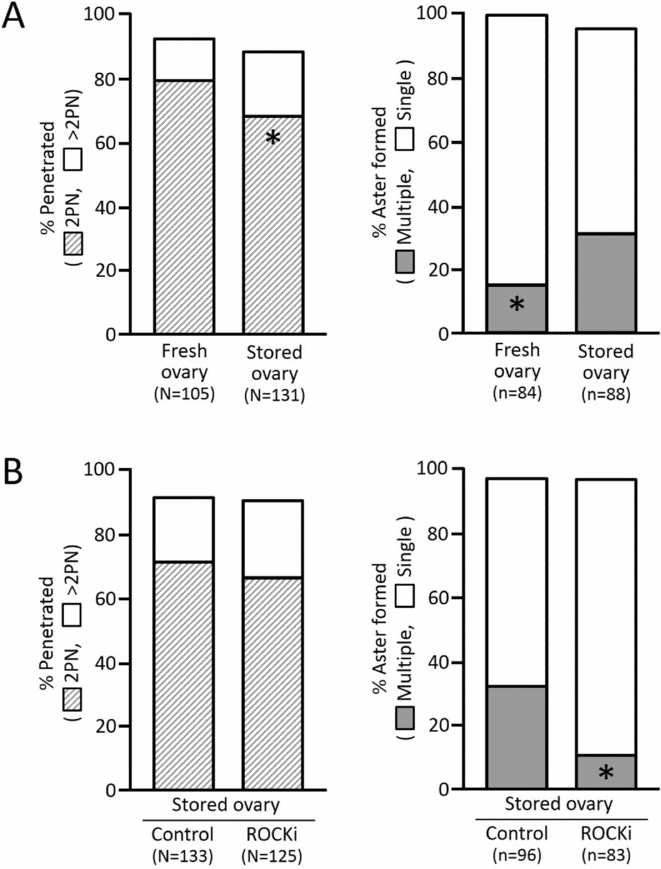
Figure 2 Normal fertilization and aster formation. (A) IVM oocytes retrieved from stored versus fresh ovaries. (B) Stored ovary-derived IVM oocytes with or without ROCK inhibitor (ROCKi) treatment for 2 h. Ten hours after IVF, the oocytes were immunostained against α-tubulin and counterstained with DAPI. N: number of oocytes evaluated. n: number of 2PN oocytes evaluated. Asterisks in the columns denote the significantly lower proportions from the values of counterparts (P < 0.05).
An additional 2 h incubation of post-IVM oocytes had no harmful influence on the cleavage rate and the blastocyst yield (Table 2). The cleavage rate of IVF oocytes in the ROCK inhibitor-treated group (80%) was not different from that in the control group (71%), in addition to yield of the blastocysts on day 7 (both 31%) and day 8 (41 versus 37%). Blastocyst yields from IVF oocytes in the three stored ovary groups were significantly lower than that in the fresh ovary group (61%).
Table 2 Effect of ROCK inhibition during 2 h post-IVM on developmental competence of stored ovary-derived oocytes following IVF
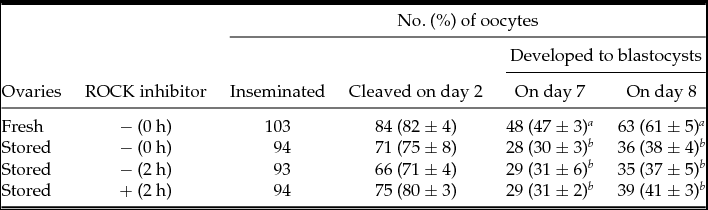
Percentages are expressed as the mean ± standard error of the mean (SEM) of at least four replicates in each group.
a ,b Significantly different within columns (P < 0.05).
Discussion
As reported previously (Nakao & Nakatsuji, Reference Nakao and Nakatsuji1992), developmental competence of stored ovary-derived oocytes was lower than that of fresh ovary-derived oocytes (Table 1). One possible explanation for the decreased developmental competence is a lower rate of normally fertilized oocytes (Fig. 2 A), which may be caused by zona-hardening. Relatively higher yields of bovine blastocysts 7 to 8 days after IVF in the fresh ovary group, compared with general yields of 20–40% reported in conventional bovine IVF (Iwata et al., Reference Iwata, Hayashi, Sato, Kimura, Kuwayama and Monji2005; Rizos et al., Reference Rizos, Ward, Duffy, Boland and Lonergan2002; Sripunya et al., Reference Sripunya, Somfai, Inaba, Nagai, Imai and Parnpai2010), may be due to the selected use of maturation-confirmed oocytes after removal of cumulus cells for subsequent IVF, instead of COCs with an unknown maturation rate.
As shown in Fig. 2 A, stored ovary-derived oocytes showed frequent multiple aster formation after IVF compared with fresh ovary-derived oocytes. This frequent multiple aster formation was suppressed by ROCK inhibition over a 2 h incubation after 22 h of IVM culture (Fig. 2 B). This result was consistent with our previous report (Hwang et al., Reference Hwang, Hara, Chung, Hirabayashi and Hochi2013) in which 2 h treatment of vitrified-warmed bovine matured oocytes with the ROCK inhibitor was found to normalize their aster formation. ROCK has been characterized as a downstream target of the small GTP-binding protein Rho and regulates the number of actin filaments and extent of cellular contractility through actin polymerization and stress fibre organization (Riento & Ridley, Reference Riento and Ridley2003). It has also been reported that ROCK can regulate microtubule acetylation via phosphorylation of the tubulin polymerization promoting protein 1, resulting in microtubule instability (Schofield et al., Reference Schofield, Steel and Bernard2012, Reference Schofield, Garmell, Suryadinata, Sarcevic and Bernard2013). Because the stored ovary-derived oocytes showed abnormal gene expression (Somfai et al., Reference Somfai, Imai, Kaneda, Akagi, Watanabe, Haraguchi, Mizutani, Dang-Nguyen, Inaba, Geshi and Nagai2011), over-expressed ROCK signaling might disturb the microtubule network of the sperm aster in the stored ovary group. Therefore, treatment with the ROCK inhibitor may be useful to suppress the multiple aster formation by stabilizing astral structure.
Hwang et al. (Reference Hwang, Hara, Chung, Hirabayashi and Hochi2013) reported that ROCK inhibition-assisted suppression of multiple aster formation in vitrified-warmed bovine oocytes led to a significant improvement of blastocyst yield from 14 to 21%. However, in the present study, it may be reasonable to conclude that multiple aster formation is not directly involved in the developmental potential of bovine oocytes impaired by ovary storage (Table 2). The difference in multiple aster formation rate between fresh and stored ovary-derived oocytes was small despite the presence of significant difference (Fig. 2 B) when compared with the difference described for vitrified-warmed oocytes with or without ROCK inhibitor treatment (Hwang et al. Reference Hwang, Hara, Chung, Hirabayashi and Hochi2013). Therefore, this result may explain the failure of ROCK inhibition-mediated improvement in blastocyst yield from stored ovary-derived oocytes. In the bovine oocytes that survive vitrification by a short-term recovery culture (Hwang et al., Reference Hwang, Hara, Chung, Hirabayashi and Hochi2013), other cellular functions correlating (or happening simultaneously) with normalization of aster formation through ROCK inhibition might improve their developmental competence. Matsukawa et al. (Reference Matsukawa, Akagi, Adachi, Kubo, Hirako, Watanabe and Takahashi2007) speculated that the oocyte nucleus is sensitive to ovary storage because production of bovine blastocysts by somatic cell nuclear transfer, unlike parthenogenetic activation or intracytoplasmic sperm injection, was not influenced by the use of stored ovaries. Somfai et al. (Reference Somfai, Imai, Kaneda, Akagi, Watanabe, Haraguchi, Mizutani, Dang-Nguyen, Inaba, Geshi and Nagai2011) proposed that the mRNA of the maternal ATP1A1 gene is a possible target for damage induced by storage of bovine ovaries.
Thus, multiple aster formation frequently induced by ovary storage was suppressed by ROCK inhibition of the IVM oocytes. However, the normalized rate of multiple aster formation did not contribute to improve the decreased blastocyst yield from stored ovary-derived IVF oocytes. In conclusion, multiple aster formation was not responsible for the low blastocyst yield from stored ovary-derived bovine oocytes.
Acknowledgements
This study was supported in part by Grant-in-Aids for Scientific Research from JSPS (No. 24580407 to S.H.) and for the JSPS Fellow (No. 25.10439 to H.H.).






