Introduction
Notwithstanding new technologies developed in the last decade to evaluate human embryo viability by assessing objective parameters such as embryo genetic status (Preimplantation Genetic Screening, PGS) or analysis of embryo physiology and function (Proteomics and Metabolomics, OMICS technologies), morphology assessment still remains the primary tool for selecting embryos for transfer. Even though this assessment is recognized as arbitrary and unsatisfactory, it still represents the quickest, cheapest and easiest way to perform embryo selection in routine practice. A considerable amount of literature has been published concerning selection criteria for embryo transfer either at the cleavage stage (day 2 or day 3) (Giorgetti et al., Reference Giorgetti, Vergara, Evangelista, Lo Schiavo, Terzi and Nuti Ronchi1995; Ziebe et al., Reference Ziebe, Petersen, Lindenberg, Andersen, Gabrielsen and Andersen1997; Alikani et al., Reference Alikani, Cohen, Tomkin, Garrisi, Mack and Scott1999; Vilska et al., Reference Vilska, Tiitinen and Hyden-Granskog1999; De Placido et al., Reference De Placido, Wilding, Strina, Alviggi, Alviggi, Mollo, Varicchio, Tolino, Schiattarella and Dale2002; Rienzi et al., Reference Rienzi, Ubaldi, Iacobelli, Romano, Minasi, Ferrero, Sapienza, Baroni and Greco2005) or at the blastocyst stage (day 5) (Gardner & Schoolcraft, Reference Gardner and Schoolcraft1999). Cell morphology, number, size and fragmentation, as well as cleavage rate and blastomeres multinucleation, continue to be the main quality indicators for embryo selection on days 2 or 3, while the expansion grade and morphologies of trophectoderm and inner cell mass (ICM) are indicators for embryo selection on day 5. These parameters have been frequently combined, in some cases incorporating pronuclear scoring of zygotes (Scott & Smith, Reference Scott and Smith1998; Tesarik & Greco, Reference Tesarik and Greco1999; Lan et al., Reference Lan, Huang, Lin, Kung, Hsieh, Huang, Tan and Chang2003), in order to produce a composite scoring system and to improve the ability to predict embryo implantation potential.
Embryo replacement on day 4 has been poorly considered as a possible option in assisted reproductive technology (ART). This situation is probably because the conventional morphological criteria usually scored to select embryos for transfer are lacking for morulas: blastomeres at the morula stage usually number >14 or are in compaction making ti difficult to define their exact number and size. For this reason, when embryo replacement on day 4 is performed, embryo selection is often carried out on the basis of day 3 embryo morphology. Only a few reports performing embryo replacement on day 4 are present in the literature and most are limited to ART cycles in which embryo biopsy was performed on day 3 (Grifo et al., Reference Grifo, Giatras, Tang and Krey1998, Gianaroli et al., Reference Gianaroli, Magli, Munne, Fortini and Ferraretti1999). However, recent papers have demonstrated a correlation between the morphology of compact stage embryos and implantation rates or developmental potential (Ebner et al., Reference Ebner, Moser, Shebl, Sommergruber, Gaiswinkler and Tews2009, Ivec et al., Reference Ivec, Kovacic and Vlaisavljevic2011), and between embryo appearance on day 4 and blastocyst formation/quality and pregnancy (Tao et al., Reference Tao, Tamis, Fink, Williams, Nelson-White and Craig2002; Feil et al., Reference Feil, Henshaw and Lane2008).
In this article, a possible scoring system for morulas is proposed. Two simple parameters (compaction and fragmentation) were observed to test whether morula morphology can be predictive of blastocyst development and blastocyst quality, demonstrating that embryo selection on day 4 can be a feasible alternative to that at cleavage or the blastocyst stage. Secondly, the prediction value of this scoring system was compared with that of the traditional scoring system performed on day 3 and the combination of the two scoring systems (on day 3 and day 4) was then examined.
Materials and methods
Study design
This study is a retrospective analysis of the developmental fate of 519 embryos obtained from 122 patients undergoing infertility treatment from February 2013 to September 2104 at the FertiClinic-Villa Margherita, Italy. In conjunction with the patient in vitro fertilization (IVF) cycle, embryos were scored on day 3 (72 h post injection) and on day 5 (120 h post injection) while embryos that did not reach the early or full blastocyst stage were further cultured for 24 h and re-scored on day 6.
On day 4 (96 h post injection) only the number of visible blastomeres and the presence of compaction were recorded as no day 4 scoring system was in use at that time in this laboratory. However, a picture of each embryo was taken and this action allowed us to perform this analysis. All pictures taken on day 4 were analysed retrospectively using the presented scoring system by the same operator without knowledge of their appearance on day 3 and days 5 or 6.
Once day 4 embryo morphology was assessed, the developmental fate of these embryos was observed in order to see if morula morphology could be predictive of blastocyst development and/or blastocyst quality. Secondly, the prediction value of this scoring system was compared with that of the traditional scoring system performed on day 3. Finally, the prediction power of the combination of the two scoring system on day 3 and day 4 was examined.
Patients and stimulation protocol
The study group included women younger than 38 years old (mean age 36.81 ± 4.54) with basal follicle stimulating hormone (FSH) <10 IU/l (mean value 7.68 ± 3.96), body mass index [BMI = weight (kg)/height (m)2] <27 (mean value 21.74 ± 2.73), menstrual cycle range 24–35 days and normal karyotype of both subjects (Table 1).
Table 1 Patient characteristics

Note: Values are number or mean ± standard deviation (SD).
BMI, body max index; FSH, follicle stimulating hormone.
Patients in which a semen sample was derived from a cryopreserved sample or with disorders such as polycystic ovary syndrome (PCOS), pelvic endometriosis, metabolic and quality autoimmune syndromes, which could affect oocyte competence, were excluded from the data analysis. Ovarian stimulation was conducted in all patients using a gonadotropin-releasing hormone (GnRH) agonist/antagonists and recombinant FSH/hMG. Ovulation was induced with human chorionic gonadotropin at the time when two to three follicles of 18–20 mm diameter were observed by ultrasound examination, and blood 17β-estradiol levels exceeded 1000 pg/ml. Oocyte retrieval was performed 36 h after hCG administration under transvaginal ultrasound-guided follicular puncture.
Laboratory procedures
All media and products were purchased from Sage unless otherwise stated. Embryo culture was performed under oil at 37°C, 5.5% CO2 and 5% O2 in a humified atmosphere. After retrieval, cumulus–corona complexes (COCCs) were incubated in fertilization medium until denudation was performed by brief incubation for 18 s in HEPES-buffered medium containing 20 IU/ml of hyaluronidase followed by mechanical removal of cumulus and corona cells by the use of plastic pipettes (stripper tips, 170 μm and 145 μm; EZ-Tip, RI). MII oocytes were then subjected to microinjection, between 38—39 h post–hCG administration, as described elsewhere (Rienzi et al., Reference Rienzi, Ubaldi, Anniballo, Cerulo and Greco1998). Once ICSI was performed, oocytes and embryos were cultured from day 1 up to day 3 in cleavage medium. On day 3, embryos were placed into blastocyst medium until day 5. All embryos that did not reach the expanding blastocyst stage on day 5 were transferred to fresh drops of blastocyst medium and cultured up to day 6.
Assessment of fertilization and embryo grading
Fertilization was assessed 16–18 h post injection using the scoring system developed by De Placido and colleagues (Reference De Placido, Wilding, Strina, Alviggi, Alviggi, Mollo, Varicchio, Tolino, Schiattarella and Dale2002). Embryo quality (EQ) was evaluated 72 h post injection (day 3) using the scoring system reported elsewhere (Rienzi et al., Reference Rienzi, Ubaldi, Iacobelli, Romano, Minasi, Ferrero, Sapienza, Baroni and Greco2005). Briefly, for each embryo, the number and size of the blastomeres were observed, as well as the percentage of anucleate fragments. Cleaved embryos with 20% anucleated fragments and with equal-sized blastomeres were considered to be type A. When the percentage of anucleate fragments was between 20–50% the embryos were considered type B. Finally, when 50% anucleated fragments were present, embryos were considered type C.
On day 4 (96 h post injection), morulas were re-scored using the following scoring system. We defined morulas as all embryos with ≥14 blastomeres, either non-compacted or compacted. A quality grade was assigned either to compaction or fragmentation and the score was expressed by the following formula M(X, Y) where M indicates morula stage, X the grade of compaction (from 1 to 3) and Y the grade of fragmentation (from 1 to 3) (Fig. 1).
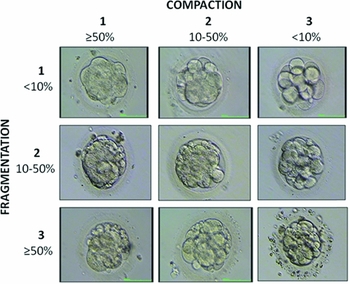
Figure 1 Scoring system developed for morulas on day 4.
A global score from grades A to D was obtained by adding up the single points X and Y: morulas that scored 2 points were considered to be grade A, 3 points grade B, 4–5 grade points C, and 6 points grade D.
Blastocyst development was assessed at 120 h post injection (day 5) using the scoring system of Gardner & Schoolcraft (Reference Gardner and Schoolcraft1999), in which degree of expansion, hatching status, ICM and trophectoderm were scored. A blastocyst was recorded even when a small cavity was visible, a blastocyst that showed a blastocoele <50% of the volume of the embryo was considered an ‘early blastocyst’ (grades I and II according to Gardner & Schoolcraft), a blastocyst showing a blastocoele >50% of the volume of the embryo was a ‘full blastocyst’ (grades III and IV according Gardner & Schoolcraft), and a blastocyst showing a blastocoele >50% of the volume of the embryo together with a grade A trophectoderm and ICM was a ‘top quality blastocyst’ (grades IV and V according Gardner & Schoolcraft). On day 5, embryo transfer was performed and surplus embryos (early and full blastocysts) were cryopreserved in line with Italian legislation. Only embryos that did not reach the early or full blastocyst stage on day 5 were further cultured for 24 h and re-scored on day 6.
Ethics
Board approval was not obtained as the study is a retrospective analysis of previously recorded data.
Statistics
A homogeneity test was used to evaluate differences in odds between ordinate categories, and a score test for trend was used to evaluate trend effects. Logistic regression was applied to evaluate relationships between morulas and embryo categories, and between blastocysts and morulas or embryos categories. An Akaike information criterion (AIC) index, a Bayesian information criterion (BIC) index and a likelihood ratio test were calculated to compare respectively non-nested and nested logistic models. The kappa statistic was used to measure the agreement between embryos and morulas scales. The Landis and Koch interpretation could be used for the kappa statistic: below 0.0 poor, 0.00–0.20 slight, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 substantial, 0.81–1.00 almost perfect. Differences were considered significant at P < 0.05.
Results
A large range of developmental stages was observed on day 4. Out of 519 embryos observed, 75.72% (N = 393) were morulas, 16.38% (N = 88) were embryos with visible distinct blastomeres (<14 blastomeres, but an increased number compared with day 3), while 7.9% o (N = 38) were arrested at the same stage as on day 3 (Fig. S1). On days 5 and 6, the blastocyst formation rate (BFR) per category was evaluated. Embryos with <14 blastomeres on day 4, but exhibiting mitotic activity with respect to day 3, showed a similar BFR (57/88, 64.77%) when compared with morulas (269/393, 68.45%), (P = 0.505). In contrast, embryos arrested on day 4 (i.e. were at the same stage as on day 3) showed a significant reduced BFR (10/38, 26.32%) as compared either with morulas (269/393, 68.45%) or with embryos mitotically active with respect to day 3 (<14 visible distinct blastomeres on day 4) (57/88, 64.77%), (P < 0.01), (Table 2).
Table 2 Developmental stages observed on day 4 and the ability for blastocyst development

Note: Values are numbers with percentages in parentheses.
a versus b: Not statistically significant
a versus c and b versus c : Statistically significant, P-value < 0.01.
All 393 morulas were subsequently evaluated using the scoring system previously described and their developmental fate was examined on day 5 and day 6. Out of 393 morulas, 47.84% (N = 188) were classified as grade A, 26.72% were grade B (N = 105), 20.36% were grade C (N = 80) and 5.09% were grade D (N = 20) (Fig. S2). Grade A morulas had a BFR of 87.2% (164/188), grade B morulas 63.8% (67/105), grade C 41.3% (33/80) and grade D 15.% (3/20). The odds homogeneity test showed a significant correlation between the grade of morula and the blastulation rate. Furthermore, the test for trend pointed out a progressive decrease in the odds and showed that the probability of blastulation was significantly higher for grade A morulas with respect to grades B, C and D morulas (P < 0.001) (Table 3).
Table 3 Different grades of day 4 morulas and their ability to develop in blastocysts

Note: Values are number with percentages in parentheses.
When considering the grading of these embryos on day 3, we did not find the same correlation for morulas between grade and BFR. In fact, the odds homogeneity test did not show any significant differences in term of blastocyst formation for grade A embryos with respect to grade B embryos (P < 0.882), but only for grade A embryos with respect to grades C and D embryos (Table 4).
Table 4 Different grades of day 3 embryos and their ability to develop in blastocysts

Note: Values are number with percentages in parentheses.
NS = Not statistically significant.
To further test the prediction value of this scoring system we compared it to the scoring system used on day 3 and measured the agreement between the two scales using kappa statistics. Figure 1 shows that the 59% grade A embryos were classified as grade A morulas on day 4, the 37% of grade B embryos were grade A morulas on day 4 and the 40% of grade C embryos were classified as grades A or B morulas on day 4. The grading was the same only in 51% of the cases and statistical analysis showed low correlation between the two scores (kappa index = 0.20) (Fig. 2).

Figure 2 Correlation between the embryo scoring performed on day 3 and the morula scoring performed on day 4.
We then compared the relationship between the two logistic regressions, one related to morula grading and blastocyst formation and the other related to embryo grading and blastocyst formation. The analysis showed that the morula scoring system was the best predictive model (AIC index 416.4 vs. 635.3 and BIC index –68.8 vs. –30.0 for morulas and embryos respectively).
We decided to examine if a combination of the two scoring systems on day 3 and day 4 would improve the prediction of blastocyst development with respect to the morula scoring system alone; no supplementary information was obtained from the addition of the two systems (likelihood ratio test comparing the two logistic regression, P = 0.132).
In the second instance, we decided to test if the presented scoring system could be predictive of blastocyst quality. Grade A morulas showed a BFR of 87.2% (164 out of 188): 37.8% of which developed in top quality blastocysts (N = 62), 49.7% in full blastocysts (N = 82) and 12.1% in early blastocysts (N = 20). Grade B morulas showed a BFR of 63.8% (67 out of 105): 22.4% of which developed in top quality blastocysts (N = 15), 49.3% in full blastocysts (N = 33) and 28.4% in early blastocysts (N = 19). Finally, grades C and D morulas showed a cumulative BFR of 36.0% (36 out of 100): 11.1% of which developed in top quality blastocysts (N = 4), 55.6% in full blastocysts (N = 20) and 33.3% in early blastocysts (N = 12). Results showed that the ability to form top quality blastocysts is significantly higher for grade A morulas when compared with grades B, and C and D morulas (P < 0.001) (Table 5).
Table 5 Morphological analysis of blastocysts derived from day 4 morulas

Note: Values are number with percentages in parentheses.
aP-value < 0.001 versus top quality blastocysts derived from grade A morulas.
Discussion
When no genetic screening or metabolic profiling can be performed, evaluation of embryo morphology at different stages remains the only tool by which embryo selection for replacement can be performed. Although data supporting embryo transfer on day 4 have been available in the literature since 1994 (Goto et al., Reference Goto, Kanzaki, Nakayama, Takabatake, Himeno, Mori and Noda1994 Huisman et al., Reference Huisman, Alberda, Leerentveld, Verhoeff and Zeilmaker1994), this practice is still poorly considered in IVF clinical practice. The lack of conventional morphological criteria routinely scored to select embryos for transfer (number, symmetry, fragmentation and multinucleation of blastomeres) makes replacement at the morula stage the less preferred option with respect to that at cleavage or blastocyst stages. In addition, embryo selection at either the cleavage or blastocyst stage also presents some negative aspects. For instance, human transcription from the embryonic genome occurs on about day 3 (Braude et al., Reference Braude, Bolton and Moore1988; Taylor et al., Reference Taylor, Ray, Ao, Winston and Handyside1997), thus embryo selection at the cleavage stage can not be performed if embryonic genome activation (EGA) has occurred, with its high risk of transferring non-viable embryos.
In order to avoid this drawback, many embryologists have switched to a blastocyst culture policy, which allows exclusion from transfer any potentially arrested embryos, i.e. those in which EGA has not occurred. Furthermore, at the blastocyst stage, morphological evaluation can be easily assessed and has been shown to have a better embryo selection and implantation potential (Olivennes et al., Reference Olivennes, Hazout, Lelaidier, Freitas, Franchin, de Ziegler and Frydman1994; Gardner et al., Reference Gardner, Schoolcraft, Wagley, Schlenker, Stevens and Hesla1998a, Reference Gardner, Vella, Lane, Wagley, Schlenker and Schoolcraft1998b; Balaban et al., Reference Balaban, Urman, Alatas, Mercan, Aksoy and Isiklar2001).
Embryo replacement at the blastocyst stage, however, entails higher clinical and laboratory costs (amount of culture media, number and type of incubators with O2 control required, etc.) that are not affordable by all laboratories. Moreover, different studies have shown that prolonged embryo culture is potentially dangerous in terms of correct establishment of epigenetic marks, affecting preimplantation development (Maher et al., Reference Maher, Afnan and Barratt2003; Mann et al., Reference Mann, Lee, Doherty, Verona, Nolen, Schultz and Bartolomei2004; Huntriss & Picton, Reference Huntriss and Picton2008; Market-Velker et al., Reference Market-Velker, Fernandes and Mann2010).
Only a small body of literature has been published concerning selection criteria for morula/compact stage embryos. This paper proposes a scoring system for morulas that differs from those previously published as it assigns two separate scores per morula in relation to grade of compaction and fragmentation respectively, so that each component can be evaluated individually. The global score (from grades A to D) is then obtained by adding up the single points, lowering the probability of a subjective assessment of EQ.
Data showed that this grading system is predictive of blastocyst development as a higher BFR was observed for grade A morulas (87.2%) with respect to grades B, C and D morulas (63.8, 41.3 and 15%, respectively), and the trend test showed that the probability of blastulation was significant higher for grade A morulas compared with the lower grades (P < 0.001). In contrast, when we considered the grading of these embryos on day 3 we did not find a linear correlation between grade and BFR: the odds homogeneity test did not show any significant differences in term of blastocyst formation between grade A and grade B embryos (P = 0.882), but only between grades A and C (P < 0.001), and between grades A and D embryos (P = 0.001).
Our data also revealed that embryo selection at the morula stage is more predictive of blastocyst development with respect to embryo selection on day 3. In fact, when we compared the relationship between the two logistic regressions, one related to morula grading and blastocyst formation and the other related to embryo grading and blastocyst formation, the analysis showed that the morula scoring system had more predictive power (AIC index 416.4 vs. 635.3 and BIC index –68.8 vs. –30.0 for morulas and embryos respectively). Not even the combination of the two scores improved the prediction of blastocyst development: the cumulative prediction power was comparable with that of the morula scoring system alone, suggesting that when embryo replacement is performed on day 4, it is sufficient and strongly preferred that embryos should be selected based on embryo morphology at the morula stage, rather than by evaluating their day 3 appearance.
In this paper, we did not want to state that embryo selection and embryo transfer on day 4 is the best strategy to select embryos for replacement and that it is better than blastocyst transfer, but only that strategy cannot be overlooked.
Embryo selection on day 4 provides advantages similar to those of blastocyst selection as most goals achieved by performing embryo replacement at the blastocyst stage can also be obtained by embryo replacement on day 4, without the need to further extend in vitro embryo culture. For instance, embryo selection at the morula stage can be used to exclude potentially arrested embryos from replacement, performing as well as selection at the blastocyst stage as EGA has already occurred on day 4. As a consequence, the number of embryos to be transferred can also be decreased for morulas, lowering the possibility of multiple gestations such that embryo replacement into the uterus occurs when it physiologically takes place in vivo (Buster et al., Reference Buster, Bustillo, Rodi, Cohen, Hamilton, Simon, Thorneycroft and Marshall1985). Additional positive aspects can be ascribed for the morula stage. For example, assisted hatching is easier and safer because embryos have a large perivitelline space at compaction and there is a low risk of compromising blastomeres. The probability of blastomere escape from the opened ZP is lower compared with that at cleavage stage. Last, but not least, extension of embryo culture is reduced, lowering the possibility of disruption of the epigenetic regulatory processes.
Certainly, morulas can arrest their development as much as embryos at cleavage or blastocyst stage, however the main critical step remains the activation of embryonic genome expression, which affects the further development of embryos from the 4–8-cell stage (Tesarik et al., Reference Tesarik, Kopecny, Plachot and Mandelbaum1986; Braude et al., Reference Braude, Bolton and Moore1988; Tesarik et al., Reference Tesarik, Kopecny, Plachot and Mandelbaum1988; Vanderzwalmen et al., Reference Vanderzwalmen, Bertin-Segal, Geerts, Debauche and Schoysman1991; Janny & Menezo Reference Janny and Menezo1994; Shoukir et al., Reference Shoukir, Chardonnens, Campana and Sakkas1998; Tesarik et al., Reference Tesarik, Greco and Mendoza2004; Tesarik, Reference Tesarik2005). In contrast, morulas seem unlikely to arrest, more often they show delayed compaction that occurs on day 5 instead of day 4 but which does not affect the blastocyst rate (Feil et al., Reference Feil, Henshaw and Lane2008).
Our data show that a delay in morula formation does not affect the blastocyst rate if mitotic activity is detected. In fact, embryos that have not yet reached the morula stage (<14 blastomeres) on day 4, but exhibit mitotic activity with respect to day 3, showed a comparable BFR with respect to those that have reached the morula stage (64.77% vs. 68.45%). In contrast, embryos arrested on day 4 (i.e. that are at the same stage as on day 3) showed a significant reduced blastulation rate (26.32%).
Thus, the overall analysis of the obtained data strongly suggests that embryo selection on day 4 can be taken into account as an option for embryo replacement, representing a better choice with respect to transfers at the cleavage stage and a viable alternative to transfers at the blastocyst stage. However, in order to further validate these results and to examine if day 4 embryo transfer can provide equal or better pregnancy outcome with respect to blastocyst transfer, a prospective randomized study is in progress.
Conflict of interest statement
There are no conflicts of interest in reference to the submitted material.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0967199415000404









