Introduction
Morphometric analysis is a practical tool to measure male gametes and to identify changes within a determined animal group (Micklem & Sanderson, Reference Micklem and Sanderson2001). This procedure can be used to rapidly determine sperm quality based on gold standard measurements (Valle et al., Reference Valle, Nayudu, Leal and Garcia-Herreros2012) and to compare sperm morphology from different species (Phetudomsinsuk et al., Reference Phetudomsinsuk, Sirinarumitr, Laikul and Pinyopummin2008; Steinberg et al., Reference Steinberg, Nieves, Ascunce, Palermo and Mudry2009).
Several studies have reported species-specific variation in the form and size of sperm (Gage & Freckleton, Reference Gage and Freckleton2003). These findings are supportive for taxonomic and phylogenetic studies, as well as for understanding reproductive evolution (García et al., Reference García, Montiel, Pérez, Pichardo, Reza and García2003). Furthermore, such knowledge will support the development of assisted reproductive techniques to preserve genetic material from endangered species (Andrabi & Maxwell, Reference Andrabi and Maxwell2007). Studies in primates suggest that increase in sperm size is positively correlated with sperm competition, as observed in males from the geni Macaca and Papio, in which evolution selected the sperm with the longest tail facilitating a more rapid swimming to reach the oocyte (Gomendio & Roldan, Reference Gomendio and Roldan1993). This fact was also recently demonstrated by the sperm morphometry comparison of the monogamic (low male-male competition) night monkeys (Aotus lemurinus) with the polygamic squirrel monkey species (Saimiri boliviensis) (high male–male competition). It was clear that the sperm from the night monkeys were much smaller than those from squirrel monkeys (Nakazato et al., Reference Nakazato, Yoshizawa, Isobe, Kusakabe, Kuraishi, Hattori, Matsumoto, Fukui, Kuwahata, Ochi, Kiso and Kai2015). Other studies indicated a relationship between sperm morphometry and male body mass, female reproductive tract dimensions, oestrous length (Gage, Reference Gage1998), and sperm quality (Valle et al., Reference Valle, Nayudu, Leal and Garcia-Herreros2012, Reference Valle, Arakaki, Carvalho, Muniz, Leal and García-Herreros2013a,b; Nakazato et al., Reference Nakazato, Yoshizawa, Isobe, Kusakabe, Kuraishi, Hattori, Matsumoto, Fukui, Kuwahata, Ochi, Kiso and Kai2015). Besides the sexual evolution, sperm morphometry indicates also evidences when searching for common ancestors for primate groups (Gould & Martin, Reference Gould and Martin1978).
In recently published revisions on phylogeny and gender systematics of neotropical primates from genus Saimiri (Lynch Alfaro et al., Reference Lynch Alfaro, Boubli, Paim, Ribas, Silva, Messias, Rohe, Merces, Silva Junior, Silva, Pinho, Koshkarian, Nguyen, Harada, Rabelo, Queiroz, Alfaro and Farias2015; Mercês et al., Reference Mercês, Alfaro, Ferreira, Harada and Júnior2015), some subspecies were recognized as new species, for example the S. collinsi previously considered a subspecies of S. sciureus (Paglia et al., Reference Paglia, da Fonseca, Rylands, Herrmann, Aguiar, Chiarello, Leite, Costa, Siciliano, Kierulff, Mendes, Tavares, Mittermeier and Patton2012; Rylands et al., Reference Rylands, Mittermeier and Silva2012). Consequently, all the reproductive aspects from the new species are now under investigation.
The population of the S. collinsi is usually located at Marajó island and in the south of the Amazonas river (Brazil). In Brazil there is a colony of captive S. collinsi at the National Primate Center (1°22′57′′S 48°22′52′′W; Ananindeua). Some of these animals are maintained in captivity for scientific research purposes. However, there is still a lack of information on the reproductive biology of these species (Oliveira et al., Reference Oliveira, Santos, Leão, Brito, Lima, Sampaio and Domingues2016a,Reference Oliveira, Santos, Leão, Queiroz, Paim, Vianez-Júnior and Dominguesb). Another species, S. vanzolinii presents a much more restrict geographic location, being considered the smallest geographic distribution and one of the smallest population among all the other neotropical primates (Paim et al., Reference Paim, Valsecchi, Harada and Queiroz2013). Almost the entire population of this species is located in the Mamirauá Sustainable Development Reserve, in Brazil (03°08′–02°36′S, 65°45′–67°13′W) (Paim et al., Reference Paim, Valsecchi, Harada and Queiroz2013). A combination of the restricted habitat and small population resulted in the inclusion of the S. vanzolinii in the IUCN list as a threatened species (IUCN, 2016). Both squirrel monkey species, together with the S. ustus, form a monophyletic group within the genus Saimiri (Lynch Alfaro et al., Reference Lynch Alfaro, Boubli, Paim, Ribas, Silva, Messias, Rohe, Merces, Silva Junior, Silva, Pinho, Koshkarian, Nguyen, Harada, Rabelo, Queiroz, Alfaro and Farias2015). Hence, reproductive aspects studied and determined in captive S. collinsi most probably will be valid for extrapolations to S. ustus and S. vanzolinii.
The aims in the present study were: (i) to describe sperm morphometry from the species S. collinsi and S. vanzolinii; (ii) to verify if the morphometric sperm patterns are similar or different between both species; and (iii) to determine if the sperm morphometry is affected by the levels of sperm defects using the S. collinsi as a model. For all these analysis, only morphologically normal sperm were measured.
Materials and methods
Ethical aspects
S. collinsi
All experimental protocols were approved by environmental authorities (Ministry of the Environment – System of Authorization and Information on Biodiversity – SISBIO/ICMBio/MMA no. 31,542-2, for captive animals/no. 29,906-3, for wild animals), by the Ethical Committee in Animal Research (no. 0010/2011/CEPAN/IEC/SVS/MS, for captive animals).
S. vanzolinii
All experimental protocols were approved by the Ethical Committee in Animal Research of the Mamiraua Institute for Sustainable Development (no. 002/2012) and by the System of Authorization and Information in Biodiversity (SISBIO/ICMBIO/MMA no. 299006-1). All procedures were supervised and controlled by a veterinarian.
Study site
We conducted our study at two different locations. The captive males (S. collinsi) were examined and maintained at the National Primate Center, Ananindeua, Brazil (1°22′57′′S, 48°22′52′′W), where the climate is tropical humid, with an average annual temperature of 28°C. The free-living males (S. vanzolinii) were captured at Mamirauá Sustainable Development Reserve. The reserve is a protected area located at the confluence of the Solimões and Japurá rivers (03°02′22′′S to 64°51′41′′W). Monthly average precipitation is 131.1 mm, and the climate is also tropical humid, with an annual average temperature of 27.5°C.
Animals
Sexually mature and healthy S. collinsi males (n = 10; >5-year-old) were collectively housed in cages of 4.74 m × 1.45 m × 2.26 m (length, width, and height, respectively), under natural photoperiod (i.e., 12 h of light and 12 h of dark). The diet consisted of fresh fruit, milk and commercial pellet chow (MEGAZOO® P18, Protein 18%, Fiber Maxi. 6.5%, Betim, MG, Brazil), and cricket larvae (Zophobas morio). Vitamins, minerals, and eggs were supplied once a week, and water was available ad libitum.
Sexually mature and healthy S. vanzolinii males (n = 2; >5-year-old) were captured using a Tomahawk Live Trap (0.7 m × 0.4 m × 0.4 m; length, width, and height, respectively) according to Paim & Rabelo (Reference Paim and Rabelo2015). Traps were set up in the early morning and checked after 4 h and at mid-afternoon. The animals caught were handled by a trained animal caretaker wearing leather gloves. Semen collection was performed at the site of capture and, after anaesthetic recovery, animals were left free at same local. For these animals, semen collection was performed at capture points to avoid the removal of the caught animals from their places of origin.
The age of all animals was estimated on the basis of dentition considering tooth eruption, intraosseous tooth formation, and tooth wear (Smith, Reference Smith1989).
Semen collection
Physical restraint was done with netting and leather glove, by a trained animal caretaker. Semen was collected at the same period of the day, i.e. in the morning before feeding and throughout 5 months for S. collinsi (July to December, 2011) and during two expeditions at Mamirauá Reserve for S. vanzolinii (November 2012 and October 2013). After physical restraint, all studied animals were anesthetized with ketamine hydrochloride (Vetanarcol 15 mg/kg IM; König S.A., Avellaneda, Argentina) and xylazine hydrochloride (1 mg/kg IM; König S.A.) by a veterinarian (Oliveira et al., Reference Oliveira, Leão, Almeida, Santos and Domingues2015).
After total anaesthetic effect, the males were placed in dorsal recumbence, and the genital region was then sanitized with a mild soap and distilled water (1:10) and gauze. The prepuce was retracted with the thumb and index fingers for a more efficient cleansing of the penis with saline solution.
Animals were stimulated by electro-ejaculation (EEJ; Autojac-Neovet, Uberaba, Brazil) with a rectal probe as indicated by Oliveira et al. (Reference Oliveira, Leão, Almeida, Santos and Domingues2015): 0.6 cm diameter and 12.5 cm length with a rounded end, bearing two metal plates (2 cm in length and 0.8 cm wide) on opposite sides. The probe was smeared with a sterile lubricant jelly (KY Jelly, Johnson and Johnson Co., Arlington, TX, USA), introduced in the rectum (~5 cm deep), and electrical stimuli were delivered. The stimulation session consisted of three series (8 min), composed of 35 electrical stimuli (12.5–100 mA) within an interval of 30 s between series (Oliveira et al., Reference Oliveira, Leão, Almeida, Santos and Domingues2015). Ejaculate (liquid and coagulated fractions) was collected into microtubes (1.5 ml). If a male did not ejaculate after the session, no further attempts were made to collect semen in the case of wild animals. In the case of captive animals, another EEJ was attempted after intervals of at least 30 days. A veterinarian monitored the animals during EEJ and after recovering from anaesthesia.
Seminal analysis
Immediately after ejaculation in a graduated microtube, semen was placed in a water bath at 37°C. The seminal liquid fraction was measured with the aid of a pipette. Coagulum volume was calculated as the total volume minus the liquid volume. Only the liquid fraction in natura was used, once coagulated fraction must be liquefied mechanically or enzymatically, which could affect morphometric parameters (Maree et al., Reference Maree, Du Plessis, Menkveld and Van der Horst2010). Therefore, coagulated fraction was discarded in the present study. Vigour was subjectively evaluated on a scale of 0 to 5 as previously described (Oliveira et al., Reference Oliveira, Santos, Leão, Brito, Lima, Sampaio and Domingues2016a). In brief, no sperm motility was considered 0, slight movement with >75% of sperm showing vibration only was represented by 1, moderate forward movement in about >50% of sperm was represented by 2, forward movement in about 70% of sperm was represented by 3, and when ~90% or >95% of sperm presented very active forward movement, scales 4 and 5 were used, respectively. Sperm motility was expressed as the percentage of cells actively moving in a forward direction. Sperm vibrating in place were not considered to be motile (Dong et al., Reference Dong, Rodenburg, Huang and VandeVoort2008). To measure percentage of progressive forward sperm motility, 10 µl of semen was placed in a pre-warmed (37°C) glass slide with cover slip and 200 sperm were counted. Normal sperm morphology and plasma membrane integrity were evaluated by a smear prepared adding 5 µl of eosin nigrosin stain (Vetec, Rio de Janeiro, Brazil) to 5 µl of semen on a pre-warmed (37°C) glass slide.
Morphologic defects detected in sperm were classified according to Bloom (Reference Bloom1973), as major or minor. Major defects are those that affect fertility, and minor defects are of less importance. All evaluations were performed under a light microscope (Nikon, Tokyo, Japan), at a magnification ×100, by the same measurer.
Selection of samples
Collected samples (n = 10 from S. collinsi; n = 2 from S. vanzolinii) from each species were classified for their morphological quality according to Bloom (Reference Bloom1973). During the analysis, it was found that half of the S. collinsi males presented less than 70% of morphologically normal sperm. Therefore, we divided the animals of this group into two subgroups as follow: the group denominated as high quality (n = 5 males) was characterized by S. collinsi males presenting ≥70% normal sperm, with not more than 10% of major defects and not more than 20% of minor defects, while the group denominated as poor quality (n = 5 males) was characterized by S. collinsi males presenting <70% normal sperm. Both S. vanzolinii males presented ≥70% normal sperm, with not more than 10% of major defects and not more than 20% of minor defects. Therefore, no subgroups were used for this species. Both S. vanzolinii studied males presented more than 70% of morphologically normal sperm.
Sperm morphometry
Sperm analysis was performed with the help of a light microscope (Leica DM LS 2, Leica Microsystems, Aargau, Switzerland) with a ×1000 magnification. All the images were recorded with a Canon® Power Shot S50 and processed with the software Canon® Utilities Zoom Browser (EX 690 1996–2004, 5.0, Australia). Only sperm without morphological defects were evaluated, and when it was possible to achieve an optimal resolution for head and tail. Measurements were performed with the software Image J® 1.46r (Wayne Rasband National Institutes of Health, IH, Bethesda, MD, USA) using the tools Threshold, Wand (tracing) tool and Straight (segmented line). Five parameters related to linear dimensions from spermatic head and tail were measured as described in Table 1. Area (µm²), perimeter (µm), length (µm), and width (µm) of sperm head, as well as tail length (µm) and sperm length (µm) were firstly expressed as pixels and transformed to micrometres by ImageJ®. Besides these linear parameters, derived parameters such as head shape (ellipticity end elongation), were measured (Yániz et al., Reference Yániz, Soler and Santolaria2015).
Table 1 Description of the selected morphometric parameters for sperm head and tail

Adapted from Martí et al. (Reference Martí, Aparicio and García-Herreros2011).
Statistical analysis
Due to the number of S. vanzolinii animals used in this study (n = 2), data from these species are included in the results tables as means of visual comparison. All data are expressed as mean ± standard deviation (SD) and analysed by the Minitab software (Minitab Inc., Copyright© 2005, version 14.20). Plasma membrane integrity, normal sperm morphology, motility, vigour and morphometry were compared within the high quality and poor quality S. collinsi groups. Data were evaluated using one-way analysis of variance (ANOVA) followed by Fisher's protected least significant difference (LSD) as a post hoc test, and a P-value < 0.05 was considered as statistically significant.
Results
From the 10 S. collinsi, 32 ejaculates were obtained, but only 10 were suitable for the study, while from the two S. vanzolinii, one ejaculate from each male was used for evaluation (see details in Table 2).
Table 2 Data on semen collection by electro-ejaculation and the distribution (number of animals, number of attempts per animal, ejaculates obtained per animal and samples used in the study

a Only those with semen in natura for analysis, and when it was possible to achieve an optimal resolution for sperm head and tail.
Table 3 shows the percentages of sperm with normal morphology and the percentages of major and minor defects within the S. collinsi subgroups (high and poor quality), as well as for S. vanzolinii. The S. collinsi group with the lowest percentages of morphologically normal sperm also presented more than 10% of major defects and almost 40% of minor defects, being significantly different from the group with high quality sperm. Based on these data, collected sperm from S. vanzolinii could be considered of high quality. When considering the morphologic defects in S. collinsi, the poor quality semen contained a significantly higher percentage of sperm with coiled tail, when compared with high quality semen.
Table 3 Mean (± SD) percentages of normal sperm and sperm morphology (with major and minor pathologic defects) of in natura semen of S. collinsi (high and poor quality group) and S. vanzolinii. Data from distinct males (n = 12)
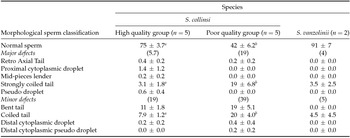
a,b Different letters in a row indicate significant statistical differences among high and poor quality groups in S. collinsi (P < 0.05).
The seminal volume was highly variable and did not differ among species or S. collinsi samples. Although plasma membrane integrity tended to be lower in sperm from poor quality semen, this difference was not significant. Importantly, sperm motility and vigour were significantly lower in poor quality sperm S. collinsi than in those presenting high quality semen. S. vanzolinii presented sperm with motility and vigour similar to those S. collinsi of high quality semen (Table 4).
Table 4 Mean (± SD) of the volume of liquid and coagulated fractions of ejaculate (μl), sperm plasma membrane integrity (%), sperm motility (%) and vigour (grade) of fresh semen of S. collinsi (high and poor quality group) and S. vanzolinii

a,b Different letters in a row indicate significant statistical differences among high and poor quality groups in S. collinsi (P < 0.05).
Table 5 Mean (± SD) of area (μm2), perimeter (μm), length (μm), width (μm), ellipticity, elongation, tail length and total sperm length of in natura semen of S. collinsi (high and poor quality group) and S. vanzolinii. Data from distinct males (n = 12)

a,b Different letters in a row indicate significant statistical differences (P < 0.05).
When morphometry of morphologically normal sperm was evaluated, it was observed that head parameters were significantly larger in S. collinsi from the high quality sperm group when compared with the one with poor quality sperm S. collinsi group. The inverse was observed when comparing sperm head ellipticity. Usually, sperm from S. vanzolinii presented a larger head than those from S. collinsi (independently on the group), but tail lengths were similar (Table 5).
Discussion
Sperm morphometric studies were performed in many primate families (Cercopithecidae; Hylobatidae; Pongidae; Hominidae; Callithricidae and Cebidae) (Gage & Freckleton, Reference Gage and Freckleton2003), including S. sciureus (Bennett, Reference Bennett1967; Martin et al., Reference Martin, Gould and Warner1975; Laverde et al., Reference Laverde, Medina and Casallas2001) and S. boliviensis (Steinberg et al., Reference Steinberg, Nieves, Ascunce, Palermo and Mudry2009; Nakazoto et al., Reference Nakazato, Yoshizawa, Isobe, Kusakabe, Kuraishi, Hattori, Matsumoto, Fukui, Kuwahata, Ochi, Kiso and Kai2015). For the best of our knowledge, up to now no studies reported sperm morphometric characteristics from the neotropical primates S. collinsi and S. vanzolinii, and its relationship with sperm quality, e.g. motility and vigour. In the present study we used the captive S. collinsi as a model to determine the importance of morphometric analysis to access sperm quality. Samples from two S. vanzolinii specimens were collected to compare with the S. collinsi data.
We showed that sperm from all studied S. collinsi presented similar tail length, corroborating with Humphries et al. (Reference Humphries, Evans and Simmons2008) who demonstrated that the swimming speed is not dependent on the size of the tail, but from the head size, which may retard the propulsion given by the sperm tail. We also observed a variable morphometry for sperm head when high and poor quality sperm groups were compared, where sperm of poor quality presented a more elongated head, which was 100% more elongated than S. collinsi high quality sperm and similar to S. vanzolinii sperm and not higher. S. collinsi sperm from the poor quality group presented a smaller head when compared with the high quality group. It has been shown that the most common defect in morphologically normal sperm occurs in the chromatin (Saacke, Reference Saacke2008). Beletti et al. (Reference Beletti, Costa and Guardieiro2002) reported that, in bovine, such chromatin defect is accompanied by a decrease in the sperm head area. Although we did not evaluate sperm chromatin, our data on S. collinsi substantiates these previous findings in bovine. Sailer et al. (Reference Sailer, Lorna and Donald1996) pointed that a variation in sperm morphometry can be considered a potential marker for male sub-(in)fertility and abnormal chromatin structure.
Regarding other neotropical primates, sperm morphometry was described for Callithrix jacchus (Moore et al., Reference Moore, Hartman and Holt1984; Cummins & Woodall, Reference Cummins and Woodall1985; Valle et al., Reference Valle, Nayudu, Leal and Garcia-Herreros2012; Swanson et al., Reference Swanson, Valle, Carvalho, Arakaki, Rodas-Martínez, Muniz and García-Herreros2016), Lagotthrix sp. (Cummins & Woodall, Reference Cummins and Woodall1985), Cebus apella (Gage & Freckleton, Reference Gage and Freckleton2003), Callimico goeldii (Valle et al., Reference Valle, Carvalho, Muniz, Leal and García-Herreros2013b, Swanson et al., Reference Swanson, Valle, Carvalho, Arakaki, Rodas-Martínez, Muniz and García-Herreros2016), Alouatta caraya (Valle et al., Reference Valle, Arakaki, Carvalho, Muniz, Leal and García-Herreros2013a; Swanson et al., Reference Swanson, Valle, Carvalho, Arakaki, Rodas-Martínez, Muniz and García-Herreros2016), Aotus lemurinus (Nakazoto et al., Reference Nakazato, Yoshizawa, Isobe, Kusakabe, Kuraishi, Hattori, Matsumoto, Fukui, Kuwahata, Ochi, Kiso and Kai2015) and Atelles geofroyi (Swanson et al., Reference Swanson, Valle, Carvalho, Arakaki, Rodas-Martínez, Muniz and García-Herreros2016). Comparing these reported data with our study, we can show that linear parameters from sperm head and tail from S. collinsi and S. vanzolinii are larger than in other species cited above, except for Cebus apella that presents larger heads (Gage & Freckleton, Reference Gage and Freckleton2003) and S. boliviensis (Nakazato et al., Reference Nakazato, Yoshizawa, Isobe, Kusakabe, Kuraishi, Hattori, Matsumoto, Fukui, Kuwahata, Ochi, Kiso and Kai2015) that presents longer sperm. This brings in evidence that sperm morphometry can vary within neotropical primates. However, comparisons among different studies are not completely accurate once fixation and staining methods may affect sperm morphometry if reagents are not isosmotic (Maree et al., Reference Maree, Du Plessis, Menkveld and Van der Horst2010). Therefore, it is important to check if different studies adopted the same or similar protocols. The observed sperm morphometric differences between S. collinsi and S. vanzolinii can be an indicative that this parameter may be used to characterize species (Martin et al., Reference Martin, Gould and Warner1975), being a tool to support taxonomic and phylogenetic studies (Steinberg et al., Reference Steinberg, Nieves, Ascunce, Palermo and Mudry2009; Sousa et al., Reference Sousa, Santos, Bezerra, Lima, Castelo, Fontenele-Neto and Silva2013).
It was stated that there is a relationship between sperm size and male–male competition. Gomendio & Roldan (Reference Gomendio and Roldan1991) described that within primates and rodents, sperm length is larger in accordance to the degree of male-male competition in the species under analysis. Comparative studies indicate that, in mammals, larger sperm are faster swimmers (Gomendio & Roldan, Reference Gomendio and Roldan1991, Reference Gomendio and Roldan2008; Tourmente et al., Reference Tourmente, Gomendio and Roldan2011), as an evolutionary adaptation for post copulatory male-male competition (Simmons & Fitzpatrick, Reference Simmons and Fitzpatrick2012).
Although no complete information is available on the mating system from S. collinsi and S. vanzolinii, it is known that the Saimiri genus presents a multimale-multifemale system, probably with high sperm competition (Izar et al., Reference Izar, Stone, Carnegie, Nakai, Garber, Estrada, Bicca-Marques, Heymann and Strier2009). This may explain the larger sperm from these species when compared with monogamic neotropical species.
Sperm morphometry can be used as a complementary tool to predict sperm motility and vigour for the S. collinsi species. This can be used as an extra parameter when selecting males for reproductive programs and biotechniques. Furthermore, S. collinsi appear as a good model for S. vanzolinii.
Competing interests
The authors declare that there is no conflict of interest that can be perceived as prejudicing the impartiality of the research reported.
Acknowledgements
The authors thank National Primate Center (CENP), Brazil and Mamiraua Institute for Sustainable Development (IDSM), Brazil for the logistical support. W.V. Sampaio was supported by CNPq, Brazil.







