Introduction
The developmental capacity of mammalian cumulus–oocyte complexes (COCs) is defined as their ability for maturation and successful monospermic fertilization. The COC maturation process encompasses several modifications that ultimately lead to the oocyte MII stage. Moreover, the process of oocyte maturation is accompanied by the storage of mRNAs and proteins necessary for further blastocyst formation, embryo growth, and implantation (Jamnongjit & Hammes, Reference Jamnongjit and Hammes2005). It was found that this process, also known as the cytoplasmic maturation of mammalian female gametes, is significantly associated with molecular changes. These changes are part of the main mediators that allow the oocyte to reach full maturity and developmental capacity (Watson, Reference Watson2007). Additionally, reaching the MII stage is required so that proper gamete fusion and nuclear reorganization during zygote formation take place.
During in vivo maturation mammalian COCs undergo growth, and extensive morphological and biochemical alterations (Yokoo & Sato, Reference Yokoo and Sato2004). Morphological modifications include organelle reorganization, extensive mRNA synthesis, protein production and folding, as well as follicle transition from primordial and preantral to antral stage just before ovulation. It has been already shown that proteins involved in transforming growth factor-beta receptor (TGFβR) signaling pathway activation are associated with morphogenesis regulation in mammals. Furthermore, biochemical changes involve nuclear-cytoplasmic shuttling and the formation of gap junctions connections (GJCs) between the oocyte and surrounding cumulus cells (CCs), enabling bidirectional communication between the oocyte and follicle cells, resulting in organization of protein distribution/cytoskeleton in oocytes (Kempisty et al., Reference Kempisty, Ziolkowska, Ciesiolka, Piotrowska, Antosik, Bukowska, Nowicki, Brüssow and Zabel2014). Finally, CCs change their structure from compact to expanded after completed oocyte maturation (Uyar et al., Reference Uyar, Torrealday and Seli2013).
It has been previously shown that the process of oocyte maturation may occur in vitro during COC culture (Casillas et al., Reference Casillas, Teteltitla-Silvestre, Ducolomb, Lemus, Salazar, Casas and Betancourt2014). In vitro oocyte maturation (IVM) differs significantly between each mammalian species. Although it was observed that porcine oocytes could be successfully cultured in vitro, the percentage of gametes that reach the MII stage after 44 h of culture is still very low (Agung et al., Reference Agung, Otoi, Fuchimoto, Senbon, Onishi and Nagai2013). In recent years, several medium supplement modifications have been applied in order to improve the number of pig oocytes that reach the MII stage after IVM. However, the accumulation of mRNA in the cytoplasm is one marker of oocyte maturation ability both in vivo and in vitro. It has been also suggested that mRNA and proteins stored in the cell cytoplasm during maturation are markers of oocyte quality and capability for further embryo growth and development (Revelli et al., Reference Revelli, Delle Piane, Casano, Molinari, Massobrio and Rinaudo2009).
The process of COC maturation both in vivo and in vitro is significantly associated with cell and tissue morphogenesis as well as cellular differentiation. It has been observed that the proper course of mammalian morphogenesis is highly regulated by the expression of target genes involved in cell/tissue growth, and specialization – differentiation and proliferation (Assou et al., Reference Assou, Anahory, Pantesco, Le Carrour, Pellestor, Klein, Reyftmann, Dechaud, De Vos and Hamamah2006). Changes that mammalian COCs undergo in the course of both in vivo and in vitro maturation are reflected by the differential expression of target gene clusters that may be potential oocytes maturational competence markers (Kempisty et al., Reference Kempisty, Piotrowska, Walczak, Śniadek, Dziuban, Bukowska, Antosik, Jackowska, Woźna and Jaśkowski2011).
The satisfactory embryo production is closely related to the IVM success rate. That is why researchers struggle to find the most efficient techniques and gene/protein markers for oocyte selection. A routine tool used to test oocyte quality and maturity is the brilliant cresyl blue (BCB) test, which measures the activity of glucose-6-phosphate (G6PDH) enzyme (Ericsson et al., Reference Ericsson, Boice, Funahashi and Day1993). The enzyme has the ability to convert the BCB stain from blue to colorless. In oocytes that have completed the growth, the activity of the enzyme decreases and the stain cannot be reduced, resulting in blue oocytes (BCB+). So far it has been clearly demonstrated in many species, including bovine (Pujol et al., Reference Pujol, López-Béjar and Paramio2004, Mirshamsi et al., Reference Mirshamsi, Karami-Shabankareh, Ahmadi-Hamedani, Soltani, Hajariana and Abdolmohammadi2013), sheep (Karami-Shabankareh et al., Reference Karami-Shabankareh and Mirshamsi2012), goat (Rodríguez-González et al., Reference Rodríguez-González, López-Béjar, Velilla and Paramio2002), and pigs (Roca et al., Reference Roca, Martinez, Vazquez and Lucas1998), that BCB+ oocytes are more competent in maturation, and have a higher developmental rate, and blastocyst yield in comparison with BCB– oocytes. Apart from the BCB test, for investigation of folliculogenesis, cytoplasmic maturation, and oocyte morphology transmission electron microscopy (TEM) were applied in bovines (Assey et al., Reference Assey, Hyttel, Greve and Purwantara1994), sheep (Cran et al., Reference Cran, Moor and Hay1980), pigs (Cran, Reference Cran1985), equines (Alvarenga, Reference Alvarenga2006), and buffalos (Mondadori et al., Reference Mondadori, Santin, Fidelis, Name, da Silva, Rumpf and Bao2010b). Gene expression profiles were analysed in mouse (Kind et al., Reference Kind, Banwell, Gebhardt, Macpherson, Gauld, Russell and Thompson2013), bovine (Tesfaye et al., Reference Tesfaye, Ghanem, Carter, Fair, Sirard, Hoelker, Schellander and Lonergan2009), humans (Ouandaogo et al., Reference Ouandaogo, Frydman, Hesters, Assou, Haouzi, Dechaud, Frydman and Hamamah2012), and pigs (our unpublished data).
There are only few publications available regarding transcriptomic profile of oocytes before and after in vitro maturation, especially regarding the pig species. Our research is one of the first in this field, providing a better understanding of the selected transcriptomic ontological group role in porcine oocyte in vitro maturation. Worth mentioning is also the fact that selection criteria based on BCB test have never been applied before, in this kind of study. Therefore, the aim of this study was to present the influence of cellular morphogenesis and differentiation pathways on the ability to drive successful nuclear and cytoplasmic maturation in porcine oocytes before and after IVM.
Materials and methods
Experimental design
The experiment was based on a collection of porcine oocytes and two BCB evaluations. The first group (immature) was composed of oocytes graded as BCB negative (BCB–) and not subjected to maturation. The second group (iv-matured) was composed of BCB– oocytes that were matured in vitro, and graded as BCB-positive (BCB+) after IVM.
Animals
In total, 45 pubertal crossbred Landrace gilts, bred on a local, commercial farm, were used in this study. They had a mean age of 155 days (range 140–170 days) and a mean weight of 100 kg (95–120 kg). All animals were housed under identical conditions and fed the same forage (depending on age and reproductive status). The experiments were approved by the Local Ethics Committee.
Collection of porcine ovaries and cumulus-oocyte-complexes (COCs)
Ovaries and reproductive tracts were recovered at slaughter and transported to the laboratory at 38°C in 0.9% NaCl within 40 min. To provide optimal conditions for subsequent oocyte maturation and fertilization in vitro, the ovaries of each animal were placed in 5% fetal bovine serum solution (FBS; Sigma-Aldrich Co., St. Louis, MO, USA) in PBS. Single large follicles (>5 mm) were then opened by puncturing with a 5 ml syringe and a 20-G needle in a sterile Petri dish, and COCs were recovered. The COCs were washed three times in modified PBS supplemented with 36 µg/ml pyruvate, 50 µg/ml gentamycin, and 0.5 mg/ml bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO, USA). COCs were selected under an inverted microscope (Zeiss, Axiovert 35, Lübeck, Germany), counted, and evaluated morphologically using the scale suggested by Jackowska et al. (Reference Jackowska, Kempisty, Antosik, Bukowska, Budna, Lianeri, Rosinska, Woźna, Jagodzinski and Jaśkowski2009). Only grade I COCs with homogeneous ooplasm and uniform, compact CCs were considered for the following steps of the experiment, resulting in 300 grade I oocytes (3 × n = 50 immature group, 3 × n = 50 iv-matured group).
Oocytes selection by BCB staining
The BCB staining test was performed to select oocytes for further experiments (Ericsson et al., Reference Ericsson, Boice, Funahashi and Day1993). Oocytes were washed twice in modified Dulbecco PBS (DPBS) (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 50 IU/ml penicillin, 50 µg/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA), 0.4% BSA [w/v], 0.34 mM pyruvate, and 5.5 mM glucose (DPBSm). Thereafter, they were treated with 13 µM BCB (Sigma-Aldrich, St. Louis, MO, USA) diluted in DPBSm at 38.5°C and 5% CO2 for 90 min. After treatment, the oocytes were transferred to DPBSm and washed twice. During the washing procedure, the oocytes were examined under an inverted microscope and classified as either stained blue (BCB+) or remained colorless (BCB–). Only the granulosa cell-free BCB– oocytes were used for subsequent molecular analysis (immature group) or IVM followed by second BCB test and molecular analysis (iv-matured group).
In vitro maturation of porcine COCs
After the first BCB test, the COCs which remained colorless (BCB–) were subjected to IVM. Immature oocytes have compact cumulus cell layers that required removal for further oocyte evaluation. Thus, COCs were first incubated with bovine testicular hyaluronidase (Sigma-Aldrich, St. Louis, MO, USA) for 2 min at 38°C to separate cumulus and granulosa cells. Cells were then removed by vortexing the BCB– oocytes in 1% sodium citrate buffer followed by mechanical displacement using a small-diameter glass micropipette. The COCs were cultured in Nunclon™Δ 4-well dishes in 500 μl of standard porcine IVM culture medium TCM-199 (tissue culture medium) with Earle's salts and l-glutamine, (Gibco BRL Life Technologies, Grand Island, NY, USA) supplemented with 2.2 mg/ml sodium bicarbonate (Nacalai Tesque, Inc., Kyoto, Japan), 0.1 mg/ml sodium pyruvate (Sigma-Aldrich, St. Louis, MO, USA), 10 mg/ml BSA, (Sigma-Aldrich, St. Louis, MO, USA), 0.1 mg/ml cysteine (Sigma-Aldrich, St. Louis, MO, USA), 10% filtered porcine follicular fluid (v/v), and gonadotropin supplements at final concentrations of 2.5 IU/ml hCG (Ayerst Laboratories, Inc., Philadelphia, PA, USA) and 2.5 IU/ml eCG (Intervet, Whitby, ON, Canada). Wells were covered with a mineral oil overlay and cultured for 44 h at 38°C under 5% CO2. After cultivation, the BCB staining test was performed again, and BCB+ oocytes were used for further experiments.
RNA extraction from porcine oocytes
Oocytes investigated before and after in vitro maturation were pooled into three independent samples for each experimental group. Total RNA was extracted from samples using TRI Reagent® (Sigma, St Louis, MO, USA) and RNeasy MinElute cleanup Kit (Qiagen, Hilden, Germany). The amount of total mRNA was determined from the optical density at 260 nm, and the RNA purity was estimated using the 260/280 nm absorption ratio (higher than 1.8) (NanoDrop spectrophotometer, Thermo Scientific, ALAB, Poland). The RNA integrity and quality were checked on a Bioanalyzer 2100 (Agilent Technologies, Inc., Santa Clara, CA, USA). The resulting RNA integrity numbers (RINs) were between 8.5 and 10 with an average of 9.2 (Agilent Technologies, Inc., Santa Clara, CA, USA). The RNA in each sample was diluted to a concentration of 100 ng/μl with an OD260/OD280 ratio of 1.8/2.0. From each RNA sample, 500 ng of RNA were taken. The remaining amount of isolated RNA was used for the RT-qPCR study.
Microarray expression analysis and statistics
The Affymetrix procedure was previously described by Trejter et al. (Reference Trejter, Hochol, Tyczewska, Ziolkowska, Jopek, Szyszka, Malendowicz and Rucinski2015). Total RNA (100 ng) from each pooled sample was subjected to two round, sense cDNA amplification (Ambion® WT Expression Kit). The obtained cDNA was used for biotin labeling and fragmentation by Affymetrix GeneChip® WT Terminal Labeling and Hybridization (Affymetrix). Biotin-labeled fragments of cDNA (5.5 μg) were hybridized to the Affymetrix® Porcine Gene 1.1 ST Array Strip (48°C/20 h). Then, microarrays were washed and stained according to the technical protocol using the Affymetrix GeneAtlas Fluidics Station. The array strips were scanned employing Imaging Station of the GeneAtlas System. The preliminary analysis of the scanned chips was performed using Affymetrix GeneAtlasTM Operating Software. The quality of gene expression data was confirmed according to the quality control criteria provided by the software. The obtained .CEL files were imported into downstream data analysis software.
All of the presented analyses and graphs were performed using Bioconductor and R programming languages. Each .CEL file was merged with a description file. In order to correct background, normalize, and summarize results, we used the Robust Multiarray Averaging (RMA) algorithm. To determine the statistical significance of the analysed genes, moderated t-statistics from the empirical Bayes method were performed. The obtained P-value was corrected for multiple comparisons using Benjamini and Hochberg's false discovery rate. The selection of significantly altered genes was based on a P-value beneath 0.05 and expression higher than two-fold. These results were presented as a volcano plot. Ten of the genes with the highest fold difference (five from the top and five from the bottom of the expression changes) are shown using a table.
Differentially expressed genes were subjected to the selection of genes involved in morphogenesis and cellular differentiation. The differentially expressed gene list (separated for upregulated and downregulated genes) was uploaded to DAVID software (Database for Annotation, Visualization and Integrated Discovery), in which genes belonging to ‘cell morphogenesis involved in differentiation’ were obtained. Expression data of these genes were subjected to a hierarchical clustering procedure, and their expression values were presented as a heat map.
Interactions between differentially expressed genes/proteins belonging to ‘cell morphogenesis involved in differentiation’ ontology group were investigated by STRING10 software (Search Tool for the Retrieval of Interacting Genes). The list of gene names was used as query for interaction prediction. The search criteria were based on co-occurrences of genes/proteins in scientific texts (text mining), coexpression, and experimentally observed interactions. The results of such analysis generated a gene/protein interaction network in which the intensity of the edges reflected the strength of the interaction score. Besides predicting interactions STRING also allowed performance of functional enrichments of Gene Ontology (GO) terms based on previously uploaded gene sets from the ‘cell morphogenesis involved in differentiation’ GO BP term.
Real-time quantitative polymerase chain reaction (RT-qPCR) analysis
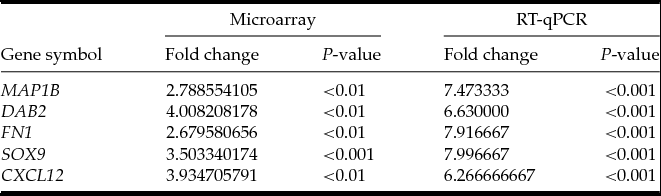
Total RNA was isolated from oocytes before and/or after IVM. The RNA samples were resuspended in 20 µl of RNase-free water and stored in liquid nitrogen. RNA samples were treated with DNase I and reverse-transcribed (RT) into cDNA. RQ-PCR was conducted in a LightCycler real-time PCR detection system (Roche Diagnostics GmbH, Mannheim, Germany) using SYBR® Green I as a detection dye, and target cDNA was quantified using the relative quantification method. The relative abundance of SOX9, MAP1B, DAB2, FN1, and CXCL12 transcripts in each sample was standardized to the internal standards (PBGD, β-actin, 18S rRNA). For amplification, 2 µl of cDNA solution was added to 18 µl of QuantiTect® SYBR® Green PCR (Master Mix Qiagen GmbH, Hilden, Germany) and primers (Table 1). One RNA sample of each preparation was processed without the RT-reaction to provide a negative control for subsequent PCR.
Table 1 Oligonucleotide sequences used for RT-qPCR analysis

Results
Using microarray technology, we analysed oocyte transcriptome changes after in vitro maturation in comparison to the transcriptome profile of freshly isolated oocytes prior to the in vitro procedure. We performed whole gene-expression analysis by Affymetrix® Porcine Gene 1.1 ST Array in which the expression of over 12,000 pig transcripts was examined. The general profile of the entire gene expression was show as a volcano plot (Fig. 1). Each dot presented on the graph corresponds to one transcript. The genes for which the fold change was higher than the cut-off value (fold>|2| and adjusted P < 0.05) were considered differentially expressed. In total, 419 genes fell within the selection criteria. From this group, 379 genes were downregulated and 40 were upregulated in relation to the oocyte transcriptome before the in vivo procedure. Ten of the differentially expressed genes with the highest fold changes (five from the top and five from the bottom of the expression changes) were shown in Table 2. This selected group of genes includes, among others, hydroxy-delta-5-steroid dehydrogenase (HSD3B1) and FBJ murine osteosarcoma viral oncogene homolog (FOS).

Figure 1 Volcano plots of total gene expression profile after in vitro maturation. Y-axis indicates –log10 P-value corrected by false discovery rate (FDR) while log2 fold changes were imposed on the x-axis. Each dot represents a single gene from microarray-normalized dataset. Orange dotted cut-off lines were established using the following parameters fold > | 2 | and adjusted P-value < 0.05. Genes above the cut-off are considered as differentially expressed and marked by turquoise colour. Total numbers of genes in which expression was upregulated or downregulated are written in the corner of the graph.
Table 2 Top 10 genes with the highest and the lowest fold changes after oocyte in vitro maturation. Fold changes and corrected P-values of differentially expressed genes are shown

DAVID (Database for Annotation, Visualization and Integrated Discovery) software was used for extraction of the genes belonging to the Gene Ontology biological process term ‘cell morphogenesis involved in differentiation’. Upregulated and downregulated gene sets were subjected to DAVID searching separately and only gene sets, in which the adjusted P-values were lower than 0.05 were selected. We found that five genes from ‘cell morphogenesis involved in differentiation’ were significantly represented in the upregulated gene set. This set of genes was subjected to a detailed analysis by hierarchical clustering and presented as a heat map (Fig. 2).

Figure 2 Heat map representation of differentially expressed genes belonging to the category ‘cell morphogenesis involved in differentiation’ from GO.BP database. The three columns in each group represent biological replicates. Arbitrary signal intensity acquired from microarray analysis is visually represented (green – higher; red – lower expression). Log2 signal intensity values were resized to Row Z-score scale for any single gene (from −1.5 – lowest expression to +1.5 – highest expression).
Only one interaction was found: between CXCL12 and FN1. Functional enrichment analysis of differentially expressed genes belonging to the ‘cell morphogenesis involved in differentiation’ ontology group revealed five closely related GO term groups (Table 3).
Table 3 Top five GO categories formed by genes differentially expressed belonging to the ‘cell morphogenesis involved in differentiation’ ontology group. GO categories were generated using STRING software. GO ID (pathway ID), GO term description (pathway description), and number of genes belonging to an appropriate category (count in gene set) are shown

RT-qPCR analysis was performed in order to validate microarray results, using the same RNA samples used for PCR and microarray profiling experiments. The result from the RT-qPCR revealed increased expression of SOX9, MAP1B, DAB2, FN1, and CXCL12 in porcine oocytes after IVM as compared with analysis before IVM. The RT-qPCR assay confirmed the fold change and significance of microarray expression profiling (Table 4).
Table 4 Validation of microarray data by RT-qPCR for five genes chosen. Fold changes with their respective P-values for microarray assay and RT-qPCR are presented
Discussion
Proper mammalian female gamete morphogenesis involves a compound process of cell maturation either in vivo or in vitro, as well as cellular growth and differentiation. These processes are regulated on a cellular and molecular level by modifications of organelle morphology and metabolism and also by induction of gene-expression profiles during development (Von Stetina & Orr-Weaver, Reference Von Stetina and Orr-Weaver2011). Additionally, the growth of mammalian oocytes is orchestrated by the storage of a large amount of mRNA and proteins, a main mechanism of proper cytoplasmic maturation. It has been shown in previous studies that both nuclear (achieving of the MII stage) and cytoplasmic maturation of oocytes are crucial steps before successful fertilization, formation of a zygote, and growth and development of early embryos (Jamnongjit & Hammes, Reference Jamnongjit and Hammes2005). The proper maturation ability and fertilizability of oocytes may be ‘fingerprints’ of subsequent embryogenesis (Robertson & Lin, Reference Robertson and Lin2013). Using microarray assays, we analysed expression profiles of morphogenesis-related genes, and we hypothesize that the investigated transcripts may be involved in nuclear and cytoplasmic maturation of oocytes.
SOX9 is one of the main proteins involved in the regulation of mammalian embryogenesis. It is well characterized as a marker of cell growth and differentiation in several species of domestic animals. Additionally, experiments by Kossowska-Tomaszczuk et al. suggested that SOX9 is an important regulator of ovarian granulosa cell differentiation and/or transdifferentiation (Kossowska-Tomaszczuk et al., Reference Kossowska-Tomaszczuk, De Geyter, De Geyter, Martin, Holzgreve, Scherberich and Zhang2009). Therefore, it is recognized as the stemness marker of undifferentiated mammalian cells with stemness plasticity and specificity. There are currently no data that indicate the role of SOX9 gene expression in porcine morphogenesis, however, there are some experiments examining fish, amphibians, and rodents (Dumond et al., Reference Dumond, Al-Asaad, Chesnel, Chardard, Boizet-Bonhoure, Flament and Kuntz2011; Bahamonde et al., Reference Bahamonde, Tetreault, McMaster, Servos, Martyniuk and Munkittrick2014). In most of the cases these experiments included expression of SOX9 in gonads determination and stability (Oreal et al., Reference Oreal, Mazaud, Picard, Magre and Carre-Eusebe2002; Schlessinger et al., Reference Schlessinger, Garcia-Ortiz, Forabosco, Uda, Crisponi and Pelosi2010; Suzuki et al., Reference Suzuki, Kanai-Azuma and Kanai2015). Our results showed significantly increased expression of SOX9 and other investigated genes, i.e. MAP1B, DAB2, FN1, and CXCL12 in porcine oocytes following in vitro maturation (IVM). We proposed that upregulation of SOX9 mRNA in oocytes after IVC is associated with cellular growth during culture and may be accompanied by morphological modifications within the oocyte's cytoplasm during maturation. Hence, the SOX9 protein, a regulator of cellular differentiation plasticity, may also be recognized as the marker of porcine oocyte in vitro maturational competence and/or morphogenesis living ‘in the shadow’ of the proper course of oogenesis and folliculogenesis.
Upregulated expression of SOX9 correlated with expression of other genes, potentially related to process of oocyte's nuclear maturation, cytoplasmic maturation or both. One of these is the chemokine ligand 12 (CXCL12), known as stromal cell-derived factor 1 (SDF1). It belongs to the intercrine family, activating leukocytes by proinflammatory induction pathways. The CXCL12 is identified as an immune system response protein; however, recent findings indicate its role in spermatogenesis during spermatozoa differentiation in both human and animal models (Ara et al., Reference Ara, Nakamura, Egawa, Sugiyama, Abe, Kishimoto, Matsui and Nagasawa2003; Zuccarello et al., Reference Zuccarello, Ferlin, Garolla, Menegazzo, Perilli, Ambrosini and Foresta2011; Yang et al., Reference Yang, Kim, Kaucher, Oatley and Oatley2013; Westernstroer et al., Reference Westernstroer, Terwort, Ehmcke, Wistuba, Schlatt and Neuhaus2014). Although no data discuss the role of CXCL12 during mammalian oocyte maturation, there is information indicating this chemokine as a regulator of follicular growth in humans and gonad development in mice (Ara et al., Reference Ara, Nakamura, Egawa, Sugiyama, Abe, Kishimoto, Matsui and Nagasawa2003; Holt et al., Reference Holt, Jackson, Roman, Aitken, Koopman and McLaughlin2006; Nishigaki et al., Reference Nishigaki, Okada, Okamoto, Sugiyama, Miyazaki, Yasuda and Kanzaki2011, Reference Nishigaki, Okada, Okamoto, Shimoi, Miyashiro, Yasuda and Kanzaki2013). As it is widely accepted that the proper course of folliculogenesis is both synchronized with and accompanied by oogenesis, it is believed these processes are regulated by similar mechanisms and regulatory mediators. Hence, we suggest that the mechanisms involved in regulation of follicle growth may be responsible for reorganization of the oocyte's nuclear or cytoplasmic morphology during in vitro maturation.
Another one, with supposedly similar function during IVM to previously described is DAB2. So far, DAB2 and DAB2 interacting protein (DAB2IP) acting as one of mediators of the Ras induction pathway with GTPase activity, were identified as a tumor suppressors. Wang et al. described the function of DAB2 and DAB2IP as stimulators of GTPase activity in the Ras pathway, both in vivo and in vitro (Wang et al., Reference Wang, Tseng, Pong, Chen, McConnell, Navone and Hsieh2002). These results indicated that interaction between DAB2 and DAB2IP is necessary for inhibition of prostate cancer growth. Recently, Peters investigated the role of zona pellucida laser microdissection during an in vitro fertilization (IVF) procedure (Laser-IVF), examining the DNA methylation profile between IVF and Laser-IVF produced mouse zygotes (Peters et al., Reference Peters, Lepikhov, Rodenacker, Marschall, Boersma, Hutzler, Scherb, Walter and de Angelis2009). The experiment included two groups: zona-intact and Laser-microdissected oocytes. They used semiquantitative RT-PCR to detect a developmental marker expression profile, finding DAB2, OCT4, and DNMT3B in mouse blastocysts derived from IVF- and Laser-IVF oocytes. They did not find differences in the methylation profile or developmental marker expression in embryos collected from both groups of oocytes. Hence, they concluded the IVF procedure did not affect embryo periimplantation growth. Our results suggested that DAB2 is involved not only in embryo development but may also be recognized as the main regulator of porcine oocyte nuclear and cytoplasmic maturation during in vitro culture. Recently, Douville & Sirard (Reference Douville and Sirard2014). used microarray analysis to identify the expression profile of transcripts in bovine granulosa cells isolated from antral follicles of 6 to 9 mm in diameter. They observed a significant increase in DAB2 mRNA expression, an apoptotic marker in granulosa cells collected from atretic vs. plateau follicles. These results indicate that DAB2 may be a developmental, as well as an apoptotic, marker during mammalian embryo periimplantation growth and folliculogenesis.
Consistently, there was correlation between previously described genes and MAP1B protein, also known as microtubule-associated protein 1B, which is highly expressed in brain, spinal cord, and to a lower level in muscle. The human MAP1B amino acid sequence displays 91% similarity in rat and mouse protein structure (Lien et al., Reference Lien, Feener, Fischbach and Kunkel1994). In the study by Allen et al., the role of MAP1B and light chain isoform, MAP1B-LC, was presented (Allen et al., Reference Allen, Ding, Wang, Pramanik, Chou, Yau and Yang2005). They observed that MAP1B-LC, together with gigaxonin, binds to ubiquitin-activating enzyme E1 and influences protein degradation, neural cell metabolism, and survival. The authors demonstrated that the gigaxonin–MAP1B-LC complex significantly mediated the induction and development of human neurodegenerative diseases. There exist no data indicating the role of MAP1B protein during mammalian oocyte maturation, both in vivo and in vitro, or morphogenesis. However, our results identified MAP1B mRNA expression in porcine oocytes before and after IVM. The role of microtubule formation during nuclear and cytoplasmic maturation in mammalian oocytes, particularly in mouse, bovine, and pigs, is well recognized (Chen et al., Reference Chen, Ge, Wang, Sun, Ouyang, Sun and Sun2014; Zhang et al., Reference Zhang, Duan, Cao, Liu, Cui, Kim, Rui and Sun2014; Jeon et al., Reference Jeon, Yoon, Cai, Hwang, Kim, Zheng, Jeung, Lee and Hyun2015; Mahdipour et al., Reference Mahdipour, Leitoguinho, Zacarias Silva, van Tol, Stout, Rodrigues and Roelen2015; Solc et al., Reference Solc, Kitajima, Yoshida, Brzakova, Kaido, Baran, Mayer, Samalova, Motlik and Ellenberg2015). However, most results are related to the oocyte's cytoskeleton, centrosome, or microtubule modifications during cell cycle progression and chromosome segregation during meiotic oocyte maturation. Therefore, our experiments first identified the possible function of MAP1B as a regulator of morphogenesis and mediator of proper porcine oocyte maturation. Our results indicated that upregulation of MAP1B mRNA expression in oocytes after IVM, as compared with immature gametes, may be associated with chromosomal morphology and modifications during genome rearrangement. It is suggested that the nuclear maturation process, which is accompanied by microtubule reorganization, may be identified by the expression of microtubule-related markers such as MPA1B.
Thus, there is a clear trend of expression increase between genes potentially involved in nuclear and cytoplasmic maturation of the oocyte. This result confirms inseparable nature of both processes in successful oocyte's maturation, including IVM. Furthermore, we found positive relation between all four analysed genes and FN1 mRNA. Muro et al. described the role of fibronectin 1 (FN1), also known as the large-external-transformation sensitive protein (LETS), as the glycoprotein present on cell surfaces, basement membranes, and extracellular fluids of connective tissues (Muro et al., Reference Muro, Chauhan, Gajovic, Iaconcig, Porro, Stanta and Baralle2003). Fibronectin 1 may interact with other structural proteins such as collagen, fibrin, and integrins. Additionally, FN1 is a mediator of cell adhesion and migration. Similar to our research, Sreenivas et al. utilized in silico analysis of protein interaction to investigate oocyte maturation ability, fertilizability, and embryo growth in sheep (Sreenivas et al., Reference Sreenivas, Kaladhar, Samy and Kumar2012). The gametes were cultured on TCM199 medium supplemented with epidermal growth factor (EGF), FBS, or wheat peptones. They found that the oocyte maturation rate was higher in the FBS-treated versus BSA-treated group. Furthermore, after using an in silico peptide assay, they observed that interactions between proteins such as epidermal growth factor receptor (EGFR), cholecystokinin (CCK), serum albumin (Alb), estrogen receptor 1 (ESR), transforming growth factor alpha (TGFA), signal transducer (STAT), and FN1 significantly influenced cell growth and development during in vitro culture. This result is in agreement with our experiments and indicates the important role of FN1 in cell growth in vitro, which is also accompanied by oocyte in vitro maturation competence. A recent study by Goossens et al. (Reference Goossens, Van Soom, Van Zeveren, Favoreel and Peelman2009) analysed three different slicing variants of FN1 in bovine preimplantation embryos. Although the role of FN1 during fertilization, gastrulation, and/or implantation is well defined in several species of mammals, the biological function of splicing variant or focusing of FN1 receptors is now of great interest. The RT-qPCR analysis showed increased expression of FN1 slicing variants in both bovine early embryos and CCs. The authors concluded that FN1 might be involved in blastocyst compaction during embryo growth. Based on these findings, we postulate that FN1 may be recognized as a cell rearrangement structure protein involved in both oocyte maturation and embryogenesis. This finding confirms the association between quality and maturational competence of the oocyte with embryogenesis efficiency and embryo production rate.
In this study, we presented gene expression patterns involved in porcine oocyte morphogenesis in relation to maturation status. The upregulated transcripts such as SOX9, MAP1B, DAB2, FN1, and CXCL12 are well known as markers of cell death and survival, follicle growth, or sperm differentiation. Additionally, we showed a new insight into the role of these genes in regulation of porcine oocyte maturational competence after IVC. Using a transcriptomic assay, accompanied by transcript marker examination, we highlighted different reproductive processes that may underlie similar regulation patterns in both females and males.
Conflict of Interest
The authors declare that they have no conflict of interest.
Financial Support
Publication of this article was made possible by grant number 2014/13/D/NZ9/04798 ‘SONATA’ and UMO-2011/03/N/NZ4/00305 from the Polish National Centre of Science.








