Introduction
Cholesterol has significant effects on the organization of membranes, including the order of the acyl chains of phospholipids, stabilization of membranes and reduction of their permeability, thus influencing phase transitions and providing a suitable microenvironment for membrane proteins (Crockett, Reference Crockett1998). Moreover, cholesterol decreases membrane fluidity at temperatures above the phase transition but increases it at temperatures below the phase transition, and this effect is more pronounced in membranes rich in saturated phospholipids compared with those containing unsaturated phospholipids (Crockett, Reference Crockett1998; Mocé et al., Reference Mocé, Blanch, Tomás and Graham2010a).
During cryopreservation, partly irreversible damage of sperm membranes is induced, which decreases the motility and fertilizing ability of spermatozoa after freezing–thawing (Curry, Reference Curry2000; Holt, Reference Holt2000). These injuries are related to changes in temperature, ice formation, modifications in sperm membranes, oxidative damage, harmful effects of cryoprotectants and osmotic stress (Watson, Reference Watson1995, Reference Watson2000; Holt, Reference Holt2000). The damage occurs when cell membranes undergo the transition from liquid-crystalline to a gel phase (Drobnis et al., Reference Drobnis, Crowe, Berger, Anchordoguy, Overstreet and Crowe1993).
Cyclodextrins, cyclic oligosaccharides of glucose containing a hydrophobic core and an external hydrophilic face, can be used to investigate the role of cholesterol. When cholesterol is loaded into cyclodextrins (CLCs), they can insert it into cell membranes (Purdy & Graham, Reference Purdy and Graham2004a). CLCs have been used prior to sperm cryopreservation in many mammalian species in order to increase the cholesterol/phospholipid ratios of sperm plasma membranes, and this treatment increases the percentage of motile and membrane-intact spermatozoa in comparison with untreated spermatozoa (buck: Farshad et al., Reference Farshad, Amidi, Koohi Khor and Rashidi2011; ram: Mocé et al., Reference Mocé, Purdy and Graham2010b; Motamedi-Mojdehi et al., Reference Motamedi-Mojdehi, Roostaei-Ali Mehr and Rajabi-Toustani2014; goat: Konyali et al., Reference Konyali, Tomás, Blanch, Gómez, Graham and Mocé2013; bull: Award & Graham, Reference Award and Graham2002; Purdy & Graham Reference Purdy and Graham2004a; Moraes et al., Reference Moraes, Graham, Torres, Meyers and Spizziri2010; stallion: Moore et al., Reference Moore, Squires and Graham2005; Oliveira et al., Reference Oliveira, Vasconcelos, Souza, Martins-Filho, Silva, Varago and Lagares2010; Spizziri et al., Reference Spizziri, Fox, Bruemmer, Squires and Graham2010; Murphy et al., Reference Murphy, English, Holden and Fair2014; boar: Galantino-Homer et al., Reference Galantino-Homer, Zeng, Megee, Dallmeyer, Voelkl and Dobrinski2006; Tomás et al., Reference Tomás, Blanch, Hernandez, Gil, Roca, Vazquez, Martinez and Mocé2011, Reference Tomás, Blanch, Fazeli and Mocé2013; Blanch et al., Reference Blanch, Tomás, Graham and Mocé2012). Alternatively, cyclodextrins alone have a high affinity for sterols in vitro and stimulate the removal of sperm plasma membrane cholesterol and promote mammalian sperm capacitation (Christian et al., Reference Christian, Haynes, Phillips and Rothblat1997; Visconti et al., Reference Visconti, Galantino-Homer, Ning, Moore, Valenzuela, Jorgez, Alvarez and Kopf1999; Oliveira et al., Reference Oliveira, Vasconcelos, Souza, Martins-Filho, Silva, Varago and Lagares2010). Although delivering CLCs to spermatozoa improves their cryosurvival rates (bull: Award & Graham, Reference Award and Graham2002; stallion: Pamornsakda et al., Reference Pamornsakda, Pojprasath, Suwimonteerabutr and Tharasanit2011), this addition may inhibit sperm capacitation after thawing, affecting the fertilizing potential of spermatozoa, since cholesterol efflux from the sperm plasma membrane is essential for fertilization (Travis & Kopf, Reference Travis and Kopf2002; Sheriff & Ali, Reference Sheriff and Ali2010).
In avian spermatozoa, it is believed that a period of capacitation within the female reproductive tract is not required in order to fertilize ova, as it is in mammals (Howarth, Reference Howarth1970). The hen oocyte is not surrounded by cumulus cells that would require a different mode of sperm motility. It may therefore be suggested that there is no need for motility hyperactivation to prepare for the acrosome reaction in chicken spermatozoa and that this special motility pattern seen in mammals has not been developed in birds. Moreover, Lemoine et al. (Reference Lemoine, Grasseau, Brillard and Blesbois2008) showed that classical inducers of mammalian capacitation and acrosome reactions have no stimulating effect in chicken spermatozoa.
Therefore, we were interested in the changes in chicken sperm plasma membranes fluidity and polarity as lipid packing arrangement induced by CLC and how that changes would improve sperm cryopreservation outcomes.
Materials and methods
Reagents
The fluorescent dyes 1,6-diphenyl-1,3,5-hexatriene (DPH) and Laurdan probe (6-dodecanoyl-2-dimethylaminonaphtalene) were purchased from Life Technologies Ltd, Grand Island, NY, USA. All media components were purchased from Sigma–Aldrich, St. Louis, MO, USA.
Cyclodextrin and cyclodextrin–cholesterol complex (CLC) preparation
2-Hydroxypropyl-β-cyclodextrin (HBCD) was pre-loaded with cholesterol as described by Mocé et al. (Reference Mocé, Purdy and Graham2010b). Briefly, a 0.45 ml aliquot of cholesterol dissolved in chloroform (200 mg cholesterol per 1 ml chloroform) was added to 2 ml of methanol containing 1 g of HBCD and the mixture was stirred until the resulting solution was clear. The solvents were removed using nitrogen gas, and the remaining solution was allowed to dry in a desiccator, forming crystals that were stored at 22°C until use. A working solution of CLC was prepared by adding 50 mg of CLC to 1 ml of EK diluent at 37°C and mixing the solution using a vortex mixer for 30 s. In addition, HBCD was prepared similarly but no cholesterol solution was added.
Animals
The experiment was carried out using 10 mature (2-year-old) roosters of the Greenleg Partridge breed kept individually in cages (70 × 95 × 85 cm) at 18–20°C, under a 14 L:10 D photoperiod. Birds were fed with commercial feed for breeding flocks. Water was provided ad libitum.
All procedures were performed with the agreement of the 2nd Local Ethical Committee in Wroclaw.
Semen collection and processing
Semen was collected twice a week by the dorso-abdominal massage method (Burrows & Quinn, Reference Burrows and Quinn1937) and then pooled from 10 individuals to obtain a sufficient sample for analysis. Only clean ejaculates were used for experiments.
The pooled semen was diluted at a ratio of 1:2 with EK diluents (1.4 g sodium glutamate; 0.14 g potassium citrate × H2O; 0.7 g glucose; 0.2 g d-fructose; 0.7 g inositol; 0.1 g polyvinylpyrrolidone; 0.02 g protamine sulfate; 0.98 g anhydrous sodium hydrogen phosphate; 0.21 g anhydrous sodium dihydrogen phosphate were diluted to 100 ml with distilled water; pH 7.3; osmotic pressure 390 mOsmol/kg; Partyka et al., Reference Partyka, Niżański and Łukaszewicz2010) and then split into four samples. After evaluation of the sperm concentration in each sample, two of them were mixed with 1 or 2 mg of CLC per 120 × 106 cells, one sample was treated with 2 mg of HBCD (negative control) per 120 × 106 cells and one sample was used as the control (untreated). The three treated samples were incubated for 15 min at 21°C and then subjected to cryopreservation. To the control sample, an equivalent volume of EK diluent was added and sample was incubated before cryopreservation too.
Semen cryopreservation
The semen samples were frozen in accordance with a procedure adapted from Tselutin et al. (Reference Tselutin, Narubina, Mavrodina and Tur1995). Diluted semen samples were stored for 15 min at −8°C, then dimethylacetamide (DMA) at a final concentration of 6% was added. After 3 min of equilibration, the solution was pipetted and plunged drop-by-drop directly into the liquid nitrogen. After 1 month of storage in liquid nitrogen, frozen samples (control, and treated with 2 mg HBCD, 1 mg CLC or 2 mg CLC) were thawed in a water bath at 60°C and each was divided into three aliquots for Experiments 1, 2 and 3.
Ten replicate semen collections and freezing procedures were performed.
Experimental design
Experiment 1: Effect of CLC on sperm cryosurvival and motility
One aliquot of each thawed sample (control, treated with 2 mg HBCD, 1 mg CLC or 2 mg CLC) was subjected to viability and motility evaluations.
Examination of sperm viability was performed on histological smears stained with eosin–nigrosin (4% eosin solution and 8% nigrosin solution in sodium citrate) (Łukaszewicz et al., Reference Łukaszewicz, Jerysz, Partyka and Siudzińska2008). Spermatozoa were considered to be viable when they were not stained (white), and eosin-stained sperm were considered to be dead (pink). For each slide, 300 spermatozoa were evaluated. Viability of spermatozoa was examined under an oil immersion objective (×1000 magnification) by light microscopy (Nikon Eclipse E200; Nikon, Tokyo, Japan).
Sperm motion characteristics were evaluated in sperm diluted in Dulbecco's modified medium with low glucose (DMEM) warmed to 40°C, at a ratio of 1:50 (Blesbois et al., Reference Blesbois, Grasseau, Seigneurin, Mignon-Grasteau, Saint Jalme and Mialon-Richard2008) using a computer-assisted semen analyser (CASA) Hamilton-Thorne Sperm Analyser IVOS version 12.2l (Hamilton-Thorne Biosciences, MA, USA) under 1.89 × 10 magnification as described before (Partyka et al., Reference Partyka, Niżański, Bajzert, Łukaszewicz and Ochota2013). The parameters measured were: the percentage of motile sperm (MOT), the percentage of progressive spermatozoa (PROG), path velocity (VAP, average velocity/smoothed average position of the spermatozoa), progressive velocity (VSL, straight-line distance between the beginning and the end of the track), curvilinear line velocity (VCL, average velocity measured over the actual point-to-point track followed by the cell), straightness (STR, a measure of VCL side-to-side movement determined by the ratio VSL/VAP × 100), linearity (LIN, a measure of the departure of the cell track from a straight line; the ratio VSL/VCL × 100), and percentage of rapid spermatozoa (RAPID, percentage of sperm moving with VAP > 50 µm/s).
Experiment 2: Effect of CLC on sperm plasma membrane fluidity and polarity
Fresh diluted semen after 15 min of incubation with HBCD, CLCs or control group and also a second aliquot of each thawed sample (control, treated with 2 mg HBCD, 1 mg CLC or 2 mg CLC) was subjected to sperm plasma membrane fluidity and polarity evaluations.
Membrane fluidity was assessed by measuring the fluorescence anisotropy (ANISO) with the fluorescent dye 1,6-diphenyl-1,3,5-hexatriene (DPH) inserted into the lipid fraction of the plasma membranes. The samples were suspended in 2 ml of EK diluent at a sperm concentration of 2 × 106/mL with DPH working solution (2.5 μM prepared from a DPH stock solution of 2 mM in tetrahydrofuran (THF)) in quartz cuvettes. The uptake of DPH did not increase the percentage of non-viable cells and did not affect sperm motility. The measurements were conducted with a fluorimeter (CARRY Eclipse, VARIAN, California, USA) at 5, 21, 25 and 40°C. The excitation and emission wavelengths were λex = 360 nm and λem = 425 nm, respectively. Fluorescence anisotropy (ANISO) for the DPH probe was calculated using the formula:
where III and I⊥ = fluorescence intensities observed in directions parallel and perpendicular, respectively, to the polarization direction of the exciting wave. G is an apparatus constant dependent on the emission wavelength.
Anisotropy is directly proportional to membrane rigidity and consequently inversely proportional to fluidity.
Characterization of membrane polarity was evaluated on the basis of changes in the packing order of the hydrophilic part of the membrane, investigated using the Laurdan probe (6-dodecanoyl-2-dimethylaminonaphtalene). Generalized polarization (GP) was calculated using the formula (Parasassi et al., Reference Parasassi, Di Stefano, Loiero, Ravagnan and Gratton1994):
where Ib = fluorescence intensity at λ= 440 nm, Ir = fluorescence intensity at λ= 490 nm.
The samples were suspended in 2 ml of EK diluent at a sperm concentration of 2 × 106/ml with Laurdan working solution (2.5 μM prepared from a Laurdan stock solution of 2 mM in dimethyl sulphoxide (DMSO)) in quartz cuvettes. The measurements were conducted with the fluorimeter (CARRY Eclipse, VARIAN) at 5, 21, 25 and 40°C. The excitation wavelength was 360 nm, and the emitted fluorescence was recorded at two wavelengths, 440 and 490 nm.
Experiment 3: Effect of CLC on thawed sperm viability and motility after 2 h storage at 5°C
A third aliquot of each thawed sample (control, treated with 2 mg HBCD, 1 mg CLC or 2 mg CLC) was stored for 2 h at 5°C. After this time, sperm viability and motility were assessed, as described in Experiment 1.
Statistical analysis
Statistical analyses were performed using STATISTICA (StatSoft, Inc. (2001), version 6, Tulsa, OK, USA). The results obtained are presented as means ± SD of measurements on samples from 10 replicate determinations and were analysed by analysis of variance (ANOVA) and Duncan's multiple range test. All percentage data were transformed to arc sin prior to analyses.
Results
Experiment 1: Effect of CLC on sperm viability and motility after freezing–thawing
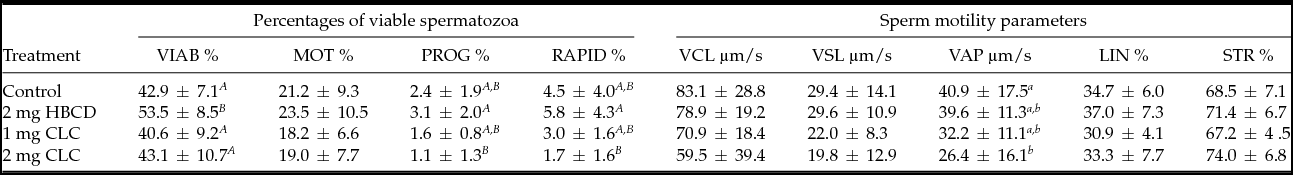
Following cryopreservation, spermatozoa treated with 2 mg of HBCD exhibited the highest (P < 0.01) percentage of viable spermatozoa compared with the control and to spermatozoa treated with the two concentrations of CLC. There were no significant differences in sperm viability between the control and spermatozoa incubated with CLC (Table 1).
Table 1 Influence of pre-freezing incubation of chicken spermatozoa with 2-hydroxypropyl-β-cyclodextrin (HBCD; 2 mg/120 × 106 sperm) or cholesterol-loaded cyclodextrins (CLC; 1 mg or 2 mg/120 × 106 sperm) on post-thawing percentages of viable spermatozoa and sperm motility parameters
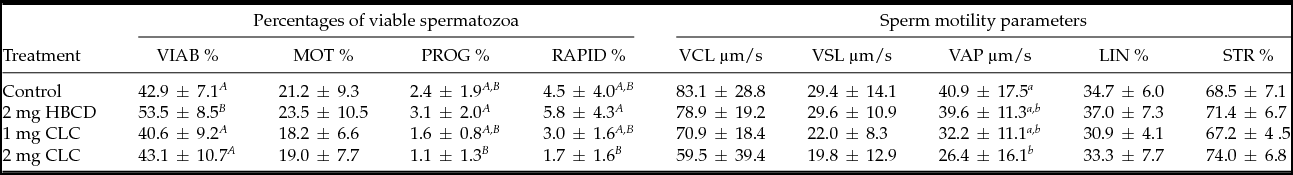
Different superscripts within the same column represent significant differences: A,BP < 0.01; a,bP < 0.05.
VIAB: viable sperm, MOT: motile sperm, PROG: progressively motile sperm, RAPID: rapid sperm, VCL: curvilinear velocity, VSL: straight-line velocity, VAP: average path velocity, LIN: linearity, ratio VSL/VCL, STR: straightness, ratio VSL/VAP.
The sperm sample incubated with 2 mg HBCD exhibited the highest percentage of progressive and rapid cells compared with the other samples, although this difference was significant only with relation to the spermatozoa treated with 2 mg CLC (Table 1). Moreover, the spermatozoa incubated with 2 mg CLC exhibited the lowest VAP parameter compared with the control sample (P < 0.05). None of the other parameters of motility (MOT, VCL, VSL, LIN, STR) was significantly different among the groups.
Experiment 2: Effect of CLC on sperm plasma membrane fluidity and polarity
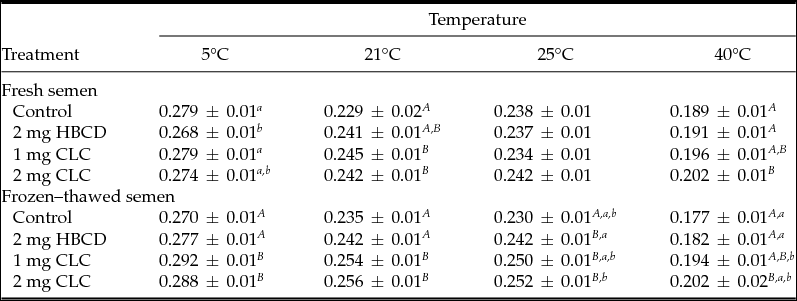
The anisotropy value measured at 40°C in fresh semen was significantly higher (lowest fluidity) (P < 0.01) in the group treated with 2 mg CLC. However, the lowest anisotropy (highest fluidity) in fresh semen measured at 5°C was noted in the HBCD group, although there were no differences with the control group and 1 mg CLC (Table 2).
Table 2 Influence of pre-freezing incubation of chicken spermatozoa with 2-hydroxypropyl-β-cyclodextrin (HBCD; 2 mg/120 × 106 sperm) or cholesterol-loaded cyclodextrins (CLC; 1 mg or 2 mg/120 × 106 sperm) on post-thawing anisotropy of sperm plasma membranes
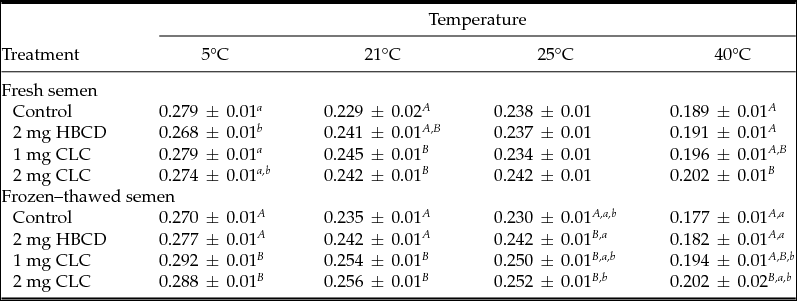
Different superscripts within the same column represent significant differences: A,BP < 0.01; a,bP < 0.05.
After freezing–thawing, spermatozoa treated with the two CLC concentrations showed the highest anisotropy (highest rigidity) at 5, 21, 25 and 40°C in relation to the control group and the spermatozoa treated with HBCD. At 25°C, the lowest anisotropy (highest fluidity) was observed in the thawed semen from the control group (Table 2). At 5, 21, and 40°C the highest fluidity (lowest anisotropy) was observed in thawed semen from control and HBCD groups (Table 2).
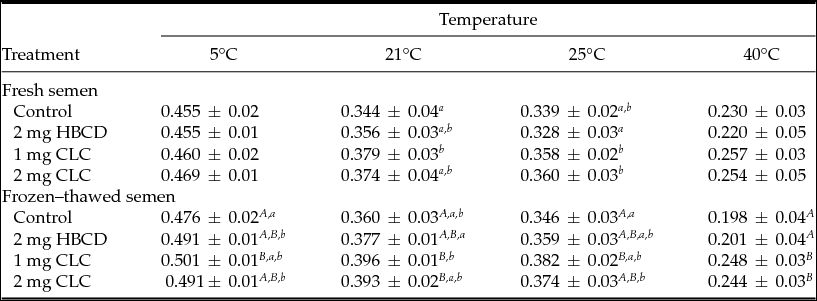
The GPs of sperm membranes measured at 5 and 40°C were similar in the fresh semen of all groups. The highest GP was detected at 21°C in fresh spermatozoa incubated with 1 mg CLC, whereas at 25°C the lowest GP value was observed in spermatozoa incubated with HBCD (Table 3).
Table 3 Influence of pre-freezing incubation of chicken spermatozoa with 2-hydroxypropyl-β-cyclodextrin (HBCD; 2 mg/120 × 106 sperm) or cholesterol-loaded cyclodextrins (CLC; 1 mg or 2 mg/120 × 106 sperm) on post-thawing general polarization of sperm plasma membranes
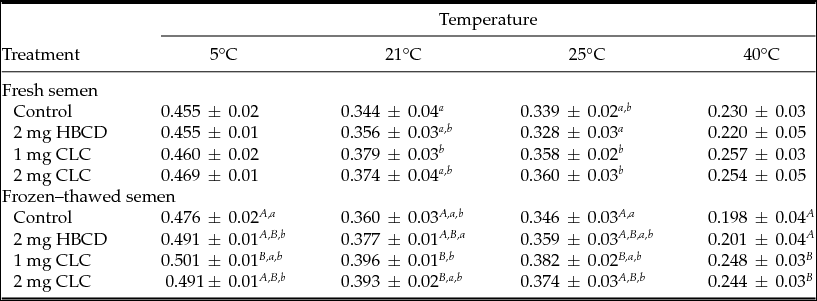
Different superscripts within the same column represent significant differences: A,BP < 0.01; a,bP < 0.05.
After freezing–thawing, samples treated with 1 mg CLC exhibited the highest value of GP measured at 5°C, which was 0.5 (P < 0.01), in relation to the control samples. Similar trends were observed at other temperatures, with the exception that at 40°C samples treated with 1 mg or 2 mg CLC showed higher GP than the control and the HBCD group (P < 0.001).
Experiment 3: Effect of CLC on thawed sperm viability and motility after 2 h storage at 5°C
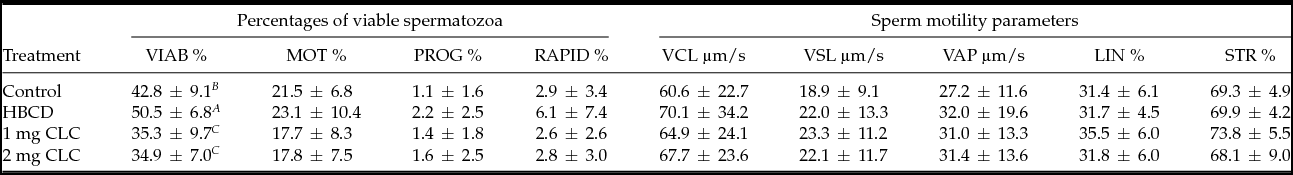
After in vitro post-thawing storage of the sperm, the highest percentage of viable spermatozoa was observed in the HBCD group in relation to the other treatments (P < 0.01) (Table 4). Exposure to 1 mg or 2 mg of CLC before freezing significantly decreased the percentage of live spermatozoa after thawing, in relation to the other groups. After semen storage for 2 h at 5°C, the lowest viability of spermatozoa (34.9%) was noted in the samples treated with 2 mg CLC (Table 4).
Table 4 Influence of pre-freezing incubation of thawed chicken spermatozoa with 2-hydroxypropyl-β-cyclodextrin (HBCD; 2 mg) or cholesterol-loaded cyclodextrins (CLC; 1 mg or 2 mg) on percentages of viable spermatozoa and sperm motility parameters after 2 h storage at 5°C

Different superscripts within the same column represent significant differences: A,B,CP < 0.01.
LIN: linearity, ratio VSL/VCL; MOT: motile sperm; PROG: progressively motile sperm; RAPID: rapid sperm; STR: straightness, ratio VSL/VAP; VAP: average path velocity; VCL: curvilinear velocity; VIAB: viable sperm; VSL: straight-line velocity.
Motility parameters of thawed spermatozoa stored for 2 h were similar for all the treatments (Table 4). The sample treated with HBCD presented numerically higher percentages of total motile, progressive and rapid spermatozoa and other parameters of motility as VCL, VSL, LIN% and STR%, than the other samples, but these differences were not significant (Table 4).
Discussion
The mechanisms of sperm cryodamage are thought to be dependent on several factors, but osmotic changes across the membrane, lipid–protein reorganizations within cell membranes and thermotrophic phase change are all accepted as causes of membrane injury (Drobnis et al., Reference Drobnis, Crowe, Berger, Anchordoguy, Overstreet and Crowe1993; Purdy & Graham, Reference Purdy and Graham2004a).
In the present study, chicken spermatozoa treated with 2 mg HBCD prior to cryopreservation exhibited the highest percentages of viable and motile cells after thawing (Table 1). These results are in contrast with those reported for semen in the ram (Mocé et al., Reference Mocé, Purdy and Graham2010b), stallion (Moore et al., Reference Moore, Squires and Graham2005; Oliveira et al., Reference Oliveira, Vasconcelos, Souza, Martins-Filho, Silva, Varago and Lagares2010), bull (Mocé & Graham, Reference Mocé and Graham2006; Purdy & Graham, Reference Purdy and Graham2004a,b) and goat (Amidi et al., Reference Amidi, Farshad and Khor2010; Konyali et al., Reference Konyali, Tomás, Blanch, Gómez, Graham and Mocé2013). However, a few reports have shown that depletion of cholesterol (using 2-hydroxypropyl-β-cyclodextrin or methyl-β-cyclodextrin) increased the quality of boar spermatozoa after freezing–thawing compared with controls (Zeng & Terada, Reference Zeng and Terada2001a,b). Our study is in agreement with these reports. Removing cholesterol from chicken sperm membranes exerted a beneficial effect on their resistance to freeze–thaw damage and improved thawed sperm cryosurvival during 2 h storage (Table 4). We detected the highest percentages of progressive and rapid spermatozoa in samples treated with HBCD. Even though some parameters of motility (MOT, VCL, VSL, LIN, STR) were not significantly different among the groups investigated, spermatozoa incubated with HBCD exhibited the highest numerical values of these parameters (Table 1). To the best our knowledge, this is the first study to describe an impact of reducing or adding cholesterol from bird spermatozoa before cryopreservation.
The sperm cells of different species have different degrees of resistance towards cryopreservation which is determined by membrane phospholipid composition and also by the membrane cholesterol to phospholipid ratio (Holt, Reference Holt2000). Spermatozoa from species that have a high ratio of polyunsaturated/saturated fatty acyl residues and cholesterol to phospholipid molar ratio tend to be more susceptible to cold shock (Darin-Bennett & White, Reference Darin-Bennett and White1977). There is general confidence that fowl spermatozoa are highly resistant to cold shock as those of rabbits and humans (Darin-Bennett & White, Reference Darin-Bennett and White1977; Parks & Graham, Reference Parks and Graham1992; Parks & Lynch, Reference Parks and Lynch1992; Mocé et al., Reference Mocé, Blanch, Tomás and Graham2010a), even though the tendency mentioned above does not extend to avian spermatozoa. The cholesterol/phospholipid ratio in chicken spermatozoa is around 0.30 (Parks & Lynch, Reference Parks and Lynch1992) to 0.26 (Blesbois et al., Reference Blesbois, Grasseau and Seigneurin2005). Moreover, sperm cell membranes in this animal group have a low protein content compared with mammalian species (Parks & Lynch, Reference Parks and Lynch1992). In contrast, the ratio of cholesterol to phospholipids for bull spermatozoa is 0.45 (Parks & Lynch, Reference Parks and Lynch1992) while for human and rabbit spermatozoa it is around 1 (reviewed by Konyali et al., Reference Konyali, Tomás, Blanch, Gómez, Graham and Mocé2013).
Purdy & Graham (Reference Purdy and Graham2004b) and Moore et al. (Reference Moore, Squires and Graham2005), after treating bull and stallion spermatozoa with CLC, detected an increase in the cholesterol/phospholipids ratio due to insertion of cholesterol into the plasma membrane. This phenomenon could help spermatozoa to exhibit a reduced membrane separation phase and subsequently a reduced leakage of cellular components (Drobnis et al., Reference Drobnis, Crowe, Berger, Anchordoguy, Overstreet and Crowe1993). However, the cholesterol/phospholipid ratio of rooster spermatozoa is lower than for bull spermatozoa and also viability after freezing–thawing of chicken spermatozoa is low (approximately 40–50%), therefore, it could be expected that addition of cholesterol to chicken sperm membranes would improve cryosurvival. Therefore, the results of the present study are surprising. Parks & Lynch (Reference Parks and Lynch1992) showed that mammalian and rooster sperm membranes exhibit differences in phase behavior. The membranes of chicken spermatozoa had greater overall fluidity at physiological temperatures because their glycolipids exhibited no phase transition between temperatures from −10°C to +70°C, in contrast to mammalian spermatozoa (Parks & Lynch, Reference Parks and Lynch1992). Moreover, in our study, we demonstrated that the highest fluidity measured at 5°C was before freezing in membranes of spermatozoa from which the cholesterol was removed. In contrast, Purdy et al. (Reference Purdy, Fox and Graham2005) showed that increasing the cholesterol content of bull spermatozoa increased the fluidity of their membranes at lower temperatures. This discrepancy could be explained by the general concept that cold tolerance is associated with membrane fluidity (Giraud et al., Reference Giraud, Motta, Boucher and Grizard2000). Because intracellular ice formation occurs during cryopreservation, membrane phase transition and osmotic pressure are increased (Mazur, Reference Mazur1984; Watson, Reference Watson2000), so increasing membrane fluidity and permeability may reduce the amount and volume of ice formation, therefore minimize freezing-thaw damage.
The results of the present study showed that after freezing and thawing, the membrane fluidity of spermatozoa treated with HBCD or two concentrations of CLCs decreased (Table 2). Likewise, similar findings of inducing an increase in sperm membrane rigidity have been reported in birds (chicken, turkey, guinea fowl) (Blesbois et al., Reference Blesbois, Grasseau and Seigneurin2005), humans (Giraud et al., Reference Giraud, Motta, Boucher and Grizard2000) and bull (Purdy et al., Reference Purdy, Fox and Graham2005). Because membrane fluidity is also considered to be closely related to membrane condensation, this decrease is likely due to reorganization of membrane components and loss of membrane lipids during the cryopreservation process in human (Giraud et al., Reference Giraud, Motta, Boucher and Grizard2000). These membrane changes are generally responsible for cryo-capacitation of thawed bovine sperm (Cormier & Bailey, Reference Cormier and Bailey2003). Therefore, the addition of cholesterol to spermatozoa (by treatment with CLCs) may prevent them from undergoing premature capacitation and may improve sperm viability after thawing. Sperm cryoinjury, including the capacitation-like changes in frozen–thawed domestic mammalian spermatozoa, leads to shortened survival after thawing (Bailey et al., Reference Bailey, Bilodeau and Cormier2000).
However, as we stated above, the existence of capacitation in bird spermatozoa is still debatable. Moreover, unlike in mammals, there is no sign of motility hyperactivation related to capacitation, to accompany preparation for the chicken sperm acrosome reaction (Lemoine et al., Reference Lemoine, Grasseau, Brillard and Blesbois2008). In our study, we observed that treatment with HBCD allowed 50% of viable spermatozoa of chicken after freeze–thawing to survive storage for 2 h at 5°C (Table 4). Therefore, additional studies are needed to determine if removing cholesterol from membranes of chicken spermatozoa alters their ability to undergo an acrosome reaction and fertilize oocytes.
Because in boar spermatozoa the capacitation process may modify the membrane bilayer polarity (Gadella et al., Reference Gadella, Lopes-Cardozo, van Golde, Colenbrander and Gadella1995), the fluorescent membrane probe Laurdan could be used to detect physico-chemical modifications in sperm plasma membranes occurring during capacitation (Palleschi & Silvestroni, Reference Palleschi and Silvestroni1996; Ambrosini et al., Reference Ambrosini, Zolese, Wozniak, Genga, Boscaro, Mantero and Balercia2003). Laurdan is sensitive to membrane polarity changes. It localizes at the hydrophobic–hydrophilic interface of the lipid bilayer and allows detection of the coexistence of lipid gel and liquid-crystalline phases (phase heterogeneity) in mammalian cell lines (Parasassi et al., Reference Parasassi, Loiero, Raimondi, Ravagnan and Gratton1993). This probe is particularly sensitive to membrane cholesterol content, which modulates membrane lipid order and dynamics and decreases membrane polarity (Parasassi et al., Reference Parasassi, Di Stefano, Loiero, Ravagnan and Gratton1994; Yu et al., Reference Yu, So, French and Gratton1996; Ambrosini et al., Reference Ambrosini, Zolese, Balercia, Bertoli, Arnaldi and Mantero2001). Ambrosini et al. (Reference Ambrosini, Zolese, Balercia, Bertoli, Arnaldi and Mantero2001, Reference Ambrosini, Zolese, Wozniak, Genga, Boscaro, Mantero and Balercia2003) showed that spermatozoa of normozoospermic patients were characterized by larger polarity (low GP value) of spermatozoa and this could be related to their cholesterol/phospholipid ratio.
This is the first study, to our knowledge, to evaluate avian sperm membrane polarity. Our data indicated that in chicken semen before and after freezing–thawing, spermatozoa from the control group and in those from which membrane cholesterol was removed exhibited lower GP than CLCs-treated spermatozoa, which suggested that the membrane polarity of these sperm cells increased (Tables 2 and 3). The physico-chemical characteristics of the lipid bilayer affect many essential physiological membrane functions and the proper activity of membrane proteins. Whereas membrane polarity is known to affect important processes associated with the achievement of fertilizing ability by spermatozoa, such as membrane fusion (increase of local polarity and fluidity of the sperm membrane) (Sinha et al., Reference Sinha, Kumar and Laloraya1994).
From previous reports, it is known that usually when lipid membranes are in the gel phase they have a high cholesterol content and high values of GP (GP > 0.5). In turn, when lipid membranes are in the liquid-crystalline phase state or have a low cholesterol content, GP values are lower (GP < 0.2 when more than 80% of the lipids are in the liquid-crystalline phase state) (Parasassi et al., Reference Parasassi, Loiero, Raimondi, Ravagnan and Gratton1993). In the present study, the highest GP value of 0.5 was detected in membranes of frozen–thawed spermatozoa incubated with 1 mg CLC and measured at 5°C (Table 3), which suggests that at this stage in the sperm membranes phase transition to gel had occurred. In the crystalline-gel state, the phospholipid fatty acyl chains straighten and lengthen, resulting in more ordered and packed membranes, in which lipid and protein movement is restricted (Hammerstedt et al., Reference Hammerstedt, Graham and Nolan1990). During this phase, many protein–lipid interactions are blocked because integral proteins are excluded from gel domains within the membrane and they are clustered into the remaining liquid lipid domains. The membrane becomes unstable and its functionality is reduced since the proteins are aggregated and lipid phase transition has occurred (Amann & Pickett, Reference Amann and Pickett1987).
Previously, Blesbois et al. (Reference Blesbois, Grasseau and Seigneurin2005) observed that cryopreservation of turkey and guinea fowl semen induced a dramatic decrease in the cholesterol:phospholipid ratio, and consistent with this, spermatozoa of these species are characterized by worse freezability than chicken spermatozoa. However, an earlier study by these authors showed such a decrease in the cholesterol–phospholipid ratio also in chicken semen (Blesbois et al., Reference Blesbois, Lessire and Hermier1997). Therefore, results obtained in our study could suggest that removing cholesterol from chicken sperm membranes before cryopreservation using cyclodextrin and then freezing them in this medium (without washing) was beneficial for several reasons. This action: (i) caused decreased cholesterol content and thus increased membrane fluidity, which had a beneficial effect on freezability; (ii) cyclodextrin as a cyclic oligosaccharide with an internal hydrophobic core encapsulated cholesterol within it; then (iii) after thawing, cyclodextrin inserted the cholesterol back into the sperm cell membranes so that it could protect them against damage. In view of our results, such a mechanism of action of HBCD in chicken spermatozoa could be possible as no decrease in cryosurvival rates for rabbit spermatozoa (Mocé et al., Reference Mocé, Blanch, Tomás and Graham2010a) treated with cyclodextrins alone prior to cryopreservation was observed. Müller et al. (Reference Müller, Müller, Pincemy, Kurz and Labbe2008) also did not find a reduction in cell viability after thawing rainbow trout spermatozoa treated with cyclodextrin alone. Moreover Chakrabarty et al. (Reference Chakrabarty, Banerjee, Pal, De, Ghosh and Majumder2007) have stated that it is possible that sperm cells shed off hydrophilic lipids from the cell membrane and absorb specific lipid components from the surrounding medium, thus improving their cryoresistance. In the case of mammalian spermatozoa, lipid components are included in diluents and are derived from egg yolk. However, egg yolk is not commonly used in cryopreservation of avian semen, therefore we can suppose that cholesterol that was initially removed from membranes of spermatozoa treated with HBCD before freezing could be subsequently added to them again during thawing, by which process spermatozoa acquired the ability to resist stress factors involved in cryodamage.
In conclusion, HBCD appears to play a role in the modification of sperm membranes, increasing their fluidity and preventing them against membrane phase transition to gel, thus minimizing freeze–thaw sperm damage. HBCD treatment enhances chicken sperm viability and motility after cryopreservation and subsequent storage. This novel procedure may be useful for improving the technology for cryopreservation of fowl spermatozoa.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This study was supported by the Ministry of Science and Higher Education, program Iuventus Plus, grant no. IP2011 040171.
Language correction was supported by Wroclaw Centre of Biotechnology, programme The Leading National Research Centre (KNOW) for years 2014–2018.
Acknowledgements
The authors would like to thank Dr Karin Müller for the advice provided during research.