Introduction
Oocyte maturation refers to the release of meiotic arrest that allows oocytes to advance from prophase I to metaphase II (MII) of meiosis (Jamnongjit and Hammes, Reference Jamnongjit and Hammes2005). Oocyte maturation takes place from the germinal vesicle (GV) stage to the MII stage. The first indication for this process is disappearance of the GV. This change is called GV breakdown (GVBD). After GVBD, oocytes pass through metaphase I (MI) of the first meiotic division and entry into the second meiotic division. Thereafter, oocytes are arrested at MII of the second meiotic division until fertilization takes place (Masui and Clarke, Reference Masui and Clarke1979).
Establishment of cellular polarity is one of the key events during oocyte maturation (Yi et al., Reference Yi, Rubinstein and Li2013) This includes asymmetric spindle positioning and cortical polarization. The GV is first positioned near the cortex. Spindle assembly starts after GVBD. Spindle fibres then align the chromosomes along the middle of the cell nucleus. After that, the spindle–chromosomes complex migrates towards one side of the cortex and polar bodies (PB) are excluded (Yi et al., Reference Yi, Rubinstein and Li2013). This process involves asymmetric organization of several cellular components, including the plasma membrane, cytoskeleton and organelles (Kloc et al., Reference Kloc, Ghobrial, Borsuk and Kubiak2012).
Inscuteable (Insc) was first identified as a novel neural precursor gene in Drosophila (Kraut and Campos-Ortega, Reference Kraut and Campos-Ortega1996). Its expression is coincident with sites of cell shape changes or cell and tissue movement in the embryo, i.e. neuroblasts, trachea, malpighian tubules, and pupal wing epithelia (Kraut and Campos-Ortega, Reference Kraut and Campos-Ortega1996). In addition, the Insc protein shows common features with a family of putative cytoskeletal associated proteins suggesting a potential role in cytoskeleton regulation (Kraut and Campos-Ortega, Reference Kraut and Campos-Ortega1996). Further studies demonstrated that Insc is required for correct spindle orientation and segregation of other polarity proteins such as Numb and Prospero into the basal daughter cell in Drosophila (Kraut et al., Reference Kraut, Chia, Jan, Jan and Knoblich1996; Knoblich et al., Reference Knoblich, Jan and Jan1999). Later, a mammalian homologue of Insc (mInsc) was identified, but showed weak homology with the Drosophila counterpart. Despite the overall low sequence conservation, mInsc contains a conserved domain that includes the asymmetry domain (Knoblich et al., Reference Knoblich, Jan and Jan1999) and has also been shown to involve asymmetric cell division in neuroblast, retina, lung and other cell types (Lechler and Fuchs, Reference Lechler and Fuchs2005; Zigman et al., Reference Zigman, Cayouette, Charalambous, Schleiffer, Hoeller, Dunican, McCudden, Finberg, Barres, Siderovski and Knoblich2005; Postiglione et al., Reference Postiglione, Juschke, Xie, Haas, Charalambous and Knoblich2011). However, it is role in mouse meiotic maturation is not known. In this study, we analyzed the subcellular localization of mInsc during mouse oocyte maturation and investigated the effects of mInsc depletion on meiotic spindle organization and oocyte maturation.
Materials and methods
Animals
All animal studies were approved by the Animal Care and Use Committee of Wuhan University and conducted in accordance with the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals. In this study, 4–5-week-old female Kunming mice were used. The mice were housed for 1 week before the start of experiments and fed a standard diet on a 12-h light, 12-h dark schedule at a room temperature of 25°C.
Oocytes collection and culture
To collect the GV-stage oocytes, mice were stimulated with 10 IU pregnant mare serum gonadotropin (PMSG), and sacrificed after 24 h via cervical dislocation. A 28G microinjection needle under a stereomicroscope was used to puncture ovarian antral follicles and to release the GV-stage oocytes. GV oocytes were cultured for 3–4 h to collect GVBD-stage oocytes, 7–9 h to collect MI-stage oocytes and 14–16 h to collect MII-stage oocytes. The isolated oocytes were cultured in M16 medium under paraffin oil at 37°C, in 5% CO2 in air.
To collect in vivo matured oocytes at each developmental stage, the mice were treated with 10 IU PMSG for 48 h, followed by 10 IU human chorionic gonadotropin (hCG). Mice were sacrificed via cervical dislocation after 3, 8–10 or 13–15 post-hCG administration to obtain oocytes at the GVBD, MI and MII stages respectively. The isolated oocytes were cultured in M16 medium under paraffin oil at 37°C, in 5% CO2 in air.
Reverse transcription and quantitative real-time polymerase chain reaction (qRT-PCR)
Here, 300 in vivo matured oocytes at GVBD, MI and MII stages respectively were isolated, treated with 0.1% hyaluronidase to remove cumulus cells and washed three times in phosphate-buffered saline (PBS). For qRT-PCR, mRNA was extracted from 300 oocytes using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Briefly, after cell lysis using TRIzol reagent, chloroform was added to each tube. Samples were centrifuged and RNA in the upper aqueous phase was pelleted in 100% isopropanol. The RNA pellet was washed with 75% ethanol and eluted in DEPC-treated water. Any contaminating DNA was removed using the TURBO DNA-free Kit (Ambion). Complementary DNA was prepared using the Superscript III Reverse Transcriptase kit (Invitrogen) according to the manufacturer’s instructions and using oligo-dT as the primer. Following reverse transcription, 5.0 oocyte equivalents were used for PCR to amplify mInsc using the following forward primer: 5´-TGCAGAAGTTGAATTGTGTCAAGG-3´ and reverse primer, 5´-TGAGAAGAGAGGGAGCAGTATGAA-3′ (80 base pair product). RT-PCR amplification was carried out for 45 cycles using the following programme: denaturation at 94°C for 30 s, annealing at 55°C for 34 s and extension at 68°C for 10 s. The RT-PCR amplification products were examined by melting curve analysis. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene to normalize the level of target gene expression. Relative expression was estimated using the 2−ΔΔCT method (Schmittgen and Livak, Reference Schmittgen and Livak2008).
Western blotting
Protein was isolated from oocytes (500 oocytes for each developmental stage) in the lysis buffer and added into 20 μl 2× sodium dodecyle sulphate (SDS). Samples were boiled for 5 min and centrifuged for 3 min at 13,000 rpm. The supernatant containing the soluble protein was subjected to 10% SDS-PAGE. Proteins were transferred to nitrocellulose (NC) membranes, which were blocked with Tris-buffered saline containing 0.1% Tween 20 (TBST) overnight at 4°C and incubated with antibodies against Inscuteable1 (sc-243091, Santa Cruz, USA; 1:100) and GAPDH as the negative control for 1–2 h at room temperature. After five washes in TBST, membranes were incubated with horseradish peroxidase-conjugated secondary antibody (Goat IgG-HPR, 1:5000 for Inscuteable1 and mouse IgG-HRP for GAPDH). After five washes in TBST, the immunoreactive proteins were detected using an ECL Western blotting detection kit (Amersham Biosciences).
Transient transfection of Madin-Darby canine kidney (MDCK) cells
For transient transfections, MDCK cells were grown in six-well plates in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS). After 24 h, 0.5 to 1 μg of plasmid DNA mixed with 4 μl lipofectamine 2000 reagent (Life Technologies) was added to the cells in each well. After 24 h, the transfected cells were mixed with 2× SDS gel loading buffer and analyzed by western blotting as described above.
Manipulation of the cytoskeleton
To disrupt the microtubules, in vivo matured MII oocytes were obtained as described above. The oocytes were cultured in droplets of M16 medium containing 10 μg/ml colchicine in oil, in 5% CO2 in air in an humidified chamber for 1 h and then washed three times (3 min each wash) in fresh M2 medium.
To disrupt the microfilaments, GV oocytes were obtained and cultured in droplets of M16 medium containing 1 μg/ml cytochalasin B in oil, in 5% CO2 in air in a humidified chamber for 6–12 h and then washed three times (5 min each wash) in fresh M2 medium.
siRNA microinjection
Microinjection was performed under a microscope at ×10 magnification. The small interfering RNA (siRNA) of mInsc (sequence: UCCUCAAGGUUUAUAAGAATT) (GenePharma, Shanghai, China), or the siRNA control, was microinjected (25 μM) into the cytoplasm of fully grown GV oocytes using an Eppendorf microinjection instrument (Hamburg, Germany) and completed within 30 min. Oocytes were kept in M2 medium supplemented with 2.5 μM milrinone (Sigma-Aldrich, St. Louis, MO, USA) to prevent GV breakdown and to allow the mInsc-siRNA to complete its role during this period. After 24 h, oocytes were washed thoroughly to resume meiosis. Each experiment was repeated three to five times.
Immunofluorescence
For staining of mInsc, α-tubulin or nucleus, oocytes were fixed in 4% paraformaldehyde in PBS at room temperature for 30 min and then transferred to a membrane permeabilization solution (0.5% Triton X-100) overnight. After 1 h in blocking buffer (1% bovine serum albumin (BSA)-supplemented PBS), oocytes were incubated at 37°C for 2 h with rabbit anti-mInsc (1:100) and anti-α-tubulin-FITC (1:50). After three washes in wash buffer (0.1% Tween 20 and 0.01% Triton X-100 in PBS), oocytes were labelled with goat-anti-rabbit Cy5 (1:100; for mInsc) at room temperature for 1 h. After three washes in wash buffer, oocytes were stained with the DNA-specific dye DAPI (1 μg/ml) for 5 min and washed five times in the wash buffer. Samples were mounted on glass slides and examined under a confocal laser-scanning microscope.
Statistics
All data were analyzed using SPSS 16.0 software Spss16.0 (SPSS Inc., Chicago, IL, USA). Results were presented as mean ± standard deviation (SD) of at least three independent experiments. Comparisons between two groups were made using the t-test. A P-value of < 0.05 was considered to be significant.
Results
Expression and subcellular localization of mInc during oocyte meiotic maturation
We first examined the expression level of mInsc during mouse oocyte maturation. We collected 900 oocytes for each stage, corresponding to GVBD, MI and MII stages, for western blot analysis. We detected mInsc expression in all stages (Fig. 1A). The level of mInc was slightly increased in GV oocytes and remained steady in MI- and MII-stage oocytes (Fig. 1A, B).

Figure 1. Expression and subcellular location of mInc during oocyte meiotic maturation. (A) Protein levels of mInc in germinal vescicle (GV), GV breakdown (GVBD), metaphase I (MI) and MII oocytes were assessed using western blot. (B) Quantification of (A). (C) GV, GVBD, MI and MII oocytes were immunostained with mInc (red), α-tubulin (green) and DAPI (DNA, blue).
To further determine the subcellular localization of mInc during oocyte maturation, we collected oocytes at the GVBD, MI and MII stages. We performed immunohistochemical staining using the antibody against mInc. In the GV-stage oocytes, mInc was distributed uniformly throughout the cytoplasm (Fig. 1C). In GVBD-stage oocytes, mInc started to accumulate around the nuclei in the cytoplasm and associated with the spindles, as indicated by microtubule staining (Fig. 1C). At the MI stage, microtubules organized into the spindle and mInc came to accumulate in the spindle equatorial plane (Fig. 1C). At the MII stage, mInc accumulated in the cortical area overlaying the MII spindle (Fig. 1C).
Disruption of the cytoskeleton affected the subcellular location of mInsc
Microtubules and microfilaments are essential for spindle assembly and migration (Ai et al., Reference Ai, Wang, Li, Shi, Ola, Xiong, Yin, Chen and Sun2008). To determine the effect of microtubules in the subcellular localization of mInc, we cultured MII-stage cells with colchicine to disrupted the microtubules. As expected, the spindle was deformed and the chromosomes were disorganized (Fig. 2). mInsc was disturbed diffusively throughout the cytoplasm (Fig. 2). To determine the effect of microfilaments in the localization of mInc, we cultured the GV-stage oocytes with cytochalasin B to disrupt the microfilaments. Most chromosomes became arranged in the equatorial plane. Some chromosomes were disorganized. α-Tubulin staining showed that the actin filaments in the spindle were decreased but the position of the centrioles remained intact (Fig. 2). The majority of mInsc was found at the centrioles. Staining of mInsc at the microfilaments of the spindle significantly decreased (Fig. 2).
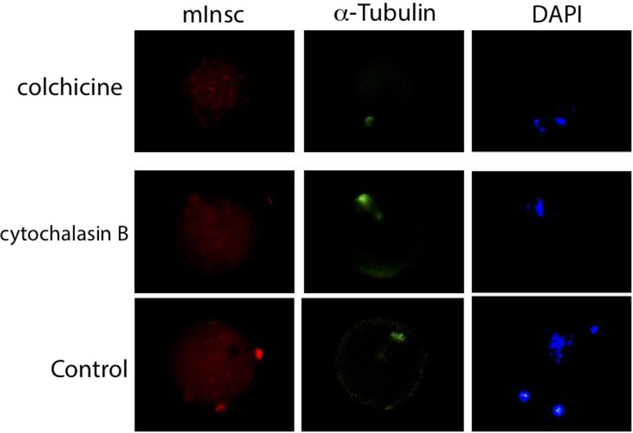
Figure 2. Effects of cytoskeleton on subcellular location of mInsc. In vivo matured metaphase II (MII) oocytes were treated with colchicine for 1 h and immunostained with mInc (red), α-tubulin (green) and DAPI (DNA, blue). Germinal vesicle (GV) oocytes were treated with cytochalasin B for 6–12 h and immunostained with mInc (red), α-tubulin (green) and DAPI (DNA, blue).
Depletion of mInsc impaired asymmetric division and affected spindle assembly, chromosome alignment and actin cap formation in oocytes
Formation of the cortical actin cap is one of the predominant features of oocyte polarization (Yi et al., Reference Yi, Rubinstein and Li2013). To determine the role of mInsc in oocyte maturation, we designed an siRNA plasmid against the mInsc gene. We first assessed whether mInsc-siRNA could effectively knock down the expression of mInc in MDCK cells. MDCK cells were transiently transfected with mInc-siRNA and control RNA, and expression of mInc was assessed by western blot. Compared with the control, the protein level of mInc in mInc-siRNA injected oocytes was significantly decreased (P < 0.05) (Fig. 3A, B). Depletion of mInsc caused an increase in symmetrical division and formation of the giant polar body (Fig. 3C). Immunofluorescence staining showed that mInsc depletion significantly impaired oocyte polarity as indicated by the disappearance of the actin cap, which is essential for asymmetric division (Fig. 3D). In contrast, spindle assembly and arrangements of chromosomes remained intact (Fig. 3D).

Figure 3. Effective knockdown of mInsc impairs asymmetric division of mouse oocytes. (A) Western blot of MDCK cells expressing siRNA against mInsc (mInsc-siRNA). (B) Quantification of (A). (C) Morphology of control siRNA (left) or mInsc-siRNA (right) injected oocytes. (D) Germinal vesicle (GV) oocytes were injected with control siRNA and mInsc-siRNA. Immunostaining showed spindle location. Green, α-tubulin; red, β-actin; blue, DAPI (DNA).
Depletion or overexpression of mInsc impaired oocyte maturation
Oocyte maturation includes nuclear maturation events characterized by germinal vesicle breakdown (GVBD) and the extrusion of the PBI (Ye et al., Reference Ye, Kawamura, Sasaki, Kawamura, Groenen, Gelpke, Rauch, Hseuh and Tanaka2009). To determine whether mInc regulates mouse oocyte maturation, mInc-siRNA or mInsc-mRNA was microinjected into the cytoplasm of GV oocytes. After culturing for 4 h, the percentage of GVBD oocytes in either the mInsc-siRNA group or the mInsc-mRNA group was significantly lower than the control group (Table 1). After culturing for 22 h, the percentages of first polar body (PBI) extrusion in both groups were remarkably lower than the control group (Table 1). The results suggest that depletion or overexpression of mInsc impaired oocyte maturation.
Table 1. mInsc-mRNA or mInsc-siRNA injection impaired oocyte maturation

Notes: GV: germinal vesicle; GVBD: GV breakdown; PBI: extrusion of first polar body.
* P < 0.05.
Discussion
Asymmetric cell division is an important process to generate cell diversity and maintain tissue homeostasis (Chartier et al., Reference Chartier, Hyenne and Labbe2010). Asymmetric cell division requires the establishment of cortical cell polarity and the orientation of the mitotic spindle along the axis of cell division, which involves the Bazooka/Par6/aPKC complex and the NuMA/LGN/Gai complex (di Pietro et al., Reference di Pietro, Echard and Morin2016). Insc is thought to link cell polarity and spindle positioning in diverse systems by binding the polarity protein Bazooka and the spindle orientation protein LGN (Mauser and Prehoda, Reference Mauser and Prehoda2012). Recently, a mammalian homologue of Insc was cloned but showed weak homology with the Drosophila counterpart. However, mammalian Insc proteins bind to Pins, the mammalian homologue of LGN and also to Par3, the mammalian homologue of Bazooka. Although LGN by itself is incapable of interacting with Par3, co-expression of mammalian Insc leads to the interaction between LGN and Par3 (Izaki et al., Reference Izaki, Kamakura, Kohjima and Sumimoto2006), indicating that mInsc might play an evolutionarily conserved role as Insc. In this paper, we investigated the role of mInsc in mouse oocyte maturation.
We first examined the expression of mInsc at four stages of oocyte maturation. We found that mInsc protein increased slightly but not significantly at the GVBD stage and remained constant during oocyte maturation (Fig. 1A, B). Next, we investigated the subcellular location of mInsc during oocyte maturation (Fig. 1C). In Drosophila neuroblasts, Insc binds to PAR3 and is localized in an apical cortical crescent (Wodarz et al., Reference Wodarz, Ramrath, Kuchinke and Knust1999). In our study, the subcellular location of mInsc was diffusely expressed throughout the cytoplasm at the GV stage. Starting from GVBD, mInsc accumulated around the nuclei in the cytoplasm and associated with α-tubulin indicating that mInsc may be required for spindle formation. At MI stage, mInsc was located at the spindle filaments and moved with spindle suggesting it may help to stabilize the spindle like other cytoskeleton adaptor proteins. For example, Berdnik and Knoblich (Reference Berdnik and Knoblich2002) found that Aurora-A is required for centrosome maturation and actin-dependent asymmetric protein localization during mitosis. For another example, mammalian diaphanous1 (mDia1), a homologue of Drosophila diaphanous, catalyzes actin nucleation and polymerization (Zhang et al., Reference Zhang, Wang, Niu, Liu, Rui, Cui, Kim and Sun2015).
As mInsc localized with α-tubulin suggesting it associates with microtubules, we asked whether disruption of microtubules could change the mInsc location. We found using colchicine that mInsc was diffusely expressed in the cytoplasm (Fig. 2). Because movement of the spindle requires microfilament activity (Sun and Schatten, Reference Sun and Schatten2006), we used cytochalasin B to disrupt the microfilaments and found that most mInsc was found at the centrioles. Staining of mInsc at the microfilaments of the spindle significantly decreased (Fig. 2). These data demonstrated that the correct subcellular localization of mInsc requires an integrated network of cytoskeletal elements.
To further investigate the function of mInsc in mouse oocyte maturation, we developed an mInsc-siRNA plasmid and verified that it significantly knocked down expression of mInsc in MDCK cells (Fig. 3A, B). Interesting, both depletion and overexpression of mInsc significantly impaired oocyte maturation (Table 1). A previous study suggested that mInsc induced apical–basal spindle orientation to facilitate intermediate progenitor generation in the developing neocortex (Postiglione et al., Reference Postiglione, Juschke, Xie, Haas, Charalambous and Knoblich2011). In our study, we found that depletion of mInsc resulted in an increase in symmetrical division and formation of giant PBs and polarity defects as indicated by the disappearance of the actin cap (Fig. 3C, D). These data suggested that mInsc might regulate oocyte maturation through regulating spindle assembly and chromosome alignments and actin cap formation (Fig. 3C, D).
To conclude, our study demonstrates that mInsc is present at each stage of mouse oocyte maturation. mInsc colocalizes with microtubules in the mitotic spindle and regulates meiotic spindle organization in mouse oocytes.
Author contributions
ZX designed research and wrote the manuscript. MTX performed the experiments and analysis the data. JY and WMX edited the manuscript.
Financial support
This work was supported by the National Natural Science Foundation of China (Grant No. 81471455 and No. 81100418).
Conflict of interest
The authors declare no conflict of interest.
Ethical standards
All animal studies were approved by the Animal Care and Use Committee of Wuhan University and conducted in accordance with the NIH guidelines for the Care and Use of Laboratory Animals.






