Introduction
Reproductive failure is a significant animal health and productive performance concern. Successful implantation of embryo requires a good quality and endometrium receptive embryo. Interference with any of these two prerequisites leads to implantation failure. During embryo development there is production of unstable metabolites of oxygen called reactive oxygen species (ROS) from gametes, embryos and their surroundings. Like other living aerobic cells, embryos and oocytes are major sources of ROS because they use oxygen to produce energy through mitochondrial oxidative phosphorylation. Intracellular imbalance between ROS and their normal scavenger antioxidants results in oxidative stress (OS). Oxidative stress adversely affects embryo development and can jeopardize embryonic health and endometrial receptivity resulting in suboptimal outcome for in vitro embryo production (IVEP) (Agarwal et al., Reference Agarwal, Aponte-Mellado, Premkumar, Shaman and Gupta2012). Several pregnancy-related disorders, defective embryo development, pregnancy loss and infertility that are attributed to cell membrane damage, DNA damage and modulation of gene expression are OS induced (Aruoma et al., Reference Aruoma, Sunb, Fujii, Neergheen, Bahorun, Kang and Sung2006). The effect of OS on reproduction is amplified because of the lack of physiological defence mechanisms against ROS. Early embryonic development is adversely affected by ROS such as superoxide anion (O2–), hydrogen peroxide (H2O2) and hydroxyl ion (OH–) that originate from embryo metabolism and their surrounding environment (Agarwal et al., Reference Agarwal, Gupta and Sharma2005). So for better in vitro embryo development and to prevent the apoptosis of early embryos it is necessary to improve OS status in the microenvironment. Several studies have attempted to enhance in vitro embryo production (IVEP) by improving the techniques performed in different species (Sirard & Coenen, Reference Sirard and Coenen2006; Abdelrazik et al., Reference Abdelrazik, Sharma, Mahfouz and Agarwal2009; Mishra et al., Reference Mishra, Chandra and Sharma2010a, Reference Mishra, Gupta, Reddy, Sejian and Ravindra2016a). To maximize the potential application of IVEP procedures, the effects of culture conditions on the developmental potential of oocytes and quality of embryos produced in vitro were assessed. The most important factor influencing the developmental potential of embryos produced in vitro is the oxygen concentration during culture that affects both oocyte maturation and embryo development (Kang et al., Reference Kang, Atikuzzaman, Kwon, Park, Kim, Moon, Koo, Jang and Lee2012). There are multiple factors that increase OS, these affect both embryo development and gene expression in preimplantation embryos resulting in suboptimal outcome of the IVEP set up (Elamaran et al., Reference Elamaran, Singh, Singh, Singla, Chauhan, Manik and Palta2012). To improve the suboptimal outcome of IVEP, attempts have been made to develop the composition medium using free radical scavengers to improve OS status in the microenvironment for better embryo development (Mukherjee et al., Reference Mukherjee, Malik, Saha, Dubey, Singhal, Boateng, Saughika, Kumar, De, Guha and Malakar2014). Culture conditions affect transcript expression in oocytes and preimplantation embryos. The expression levels of many transcripts in oocytes and preimplantation embryos are upregulated and downregulated by ROS that even affect embryo viability and offspring survivability (Wrenzycki et al., Reference Wrenzycki, Hermann and Niemann2007; Mishra et al., Reference Mishra, Sharma and Kumar2010b).
l-Ergothioneine (l-erg) is a dietary water-soluble naturally occurring amino acid mainly found in mushrooms and is a thiourea derivative of histidine, containing a sulphur atom at position 2 in the imidazole ring. This compound is made by a few organisms, notably actinobacteria and filamentous fungi. l-Erg has antioxidant properties and is a powerful scavenger of hydroxyl radicals, but its chemistry differs from conventional sulfur-containing antioxidants such as glutathione or lipoic acid. It reduces the damaging effects of environmental ultraviolet radiation and also neutralize OS by providing a reactive oxygen and nitrogen species (RONS) scavenging capacity (Akanmu et al., Reference Akanmu, Cecchini, Aruoma and Halli1991). l-Erg inhibits hydroxyl radical formation, superoxide and singlet oxygen production and lipid peroxidation (Obayashi et al., Reference Obayashi, Kurihara, Okano, Masaki and Yarosh2005). It has already been shown that l-erg eliminates most active free radicals compared with some well known antioxidants such as glutathione, uric acid and trolox (Franzoni et al., Reference Franzoni, Galeta, Laurenza, Barsotti, Di Stefano, Bocchetti, Regoli, Carpi, Balbarini, Migliore and Santoro2006). The effects of l-erg on in vitro maturation (IVM) and embryo development of sheep and bovine have been reported previously. In the sheep model it was observed that addition of l-erg (10 mM) has a beneficial effect on in vitro maturation of oocytes and embryonic development, especially from cleavage to morulae (Ozturkler et al., Reference Ozturkler, Yildiz, Gungor, Pancarci, Kaçar and Ari2010). The bovine model showed that enrichment of in vitro culture medium with l-erg (0.1 mM) improved overall embryo quality, the most important factor affecting post-implantation development (Zullo et al., Reference Zullo, Albero, Neglia, De Canditiis, Bifulco, Campanile and Gasparrini2016). However there is no such report available investigating the effect of l-erg on the intracellular oxidative status of oocytes and its antioxidant effect on subsequent embryo development in vitro. l-Erg is cell membrane impermeable and requires an organic cation transporter (OCTN1) for transport across the membrane; the gene SLC22A4 encodes OCTN1 (Grundemann et al., Reference Grundemann, Harlfinger, Goltz, Geerts, Lazar, Berkels, Jung, Rubbert and Schömig2005). The expression pattern of the SLC22A4 gene in oocytes and embryos has not been reported with regard to l-erg-mediated change in apoptotic and antioxidant genes in oocytes and embryos. Therefore this experiment was designed in the sheep model to investigate the effect of l-erg (5 mM and 10 mM) supplementation in maturation medium on oocyte maturation and subsequent developmental potential of preimplantation embryos. The subsequent objective was to show l-erg-mediated (10 mM) change in oxidative status of oocytes and mRNA abundance of apoptotic [B-cell lymphoma protein 2 (Bcl2), Bcl2-associated protein (Bax), Caspase3 (Casp3) and proliferating cell nuclear antigen (PCNA)] and antioxidant [glutathione peroxidase (GPx), Cu/Zn cytoplasmic super oxide dismutase (SOD1), Mn mitochondrial super oxide dismutase (SOD2) and catalase (CAT)] genes as well as expression of the SLC22A4 gene in matured oocytes and developing embryos (2–4-cell and blastocyst) produced in vitro.
Materials and methods
In vitro embryo production with or without l-erg
In vitro embryos were produced as per the protocol standardized in our laboratory (Mishra et al., Reference Mishra, Gupta, Reddy, Sejian and Ravindra2016a). Briefly, oocytes were aspirated from follicles (2–6 mm) of slaughterhouse ovaries (carried to the laboratory in normal saline solution fortified with antibiotics at 37–39°C) with the help of a 20-gauge needle attached to a 5 ml syringe containing oocyte collection medium [TCM-199 + BSA (3 mg/ml) + 5% FBS + heparin (10 μg/ml)]. Cumulus–oocyte complexes (COCs) (excellent and good quality) (Singh et al., Reference Singh, Shah and Tank2012) (n = 15–20) were matured in 100 μl maturation medium [TCM-199 (glutamine added) + 10% FBS + BSA (3 mg/ml) + pyruvate (4 mM) + gentamycin (50 μg/ml) + FSH (5 μg/ml) + LH (5 μg/ml) + estradiol (1 μg/ml)] with l-erg (Sigma code no: E7521,) control (0 mM) (oocytes: n = 252) and treatment (5 mM) (oocytes: n = 268) and 10 mM (oocytes: n = 264) under paraffin oil for 27 h in a 35 mm Petri dish at 5% CO2, 38.5°C and 95% relative humidity (RH) in a CO2 in air incubator. Maturation rate was assessed by the degree of cumulus expansion and extrusion of the first polar body by an aceto-orcein staining method (Sharma et al., Reference Sharma, Majumdar and Bonde1996). In vitro fertilization was performed by fresh semen collected from the ram with the help of an electro ejaculator. Semen was washed twice with washing medium [Fert-TALP (Parrish et al., Reference Parrish, Susko-Parrish, Winer and First1988) + heparin (10 μg/ml) + pyruvate (1 mM)] by centrifuging at 400 g for 5 min. Supernatant was removed and the pellet was reconstituted in fertilization medium [Fert-TALP + fatty acid free BSA (4 mg/ml) + heparin (10 μg/ml) + pyruvate (1 mM) + MEM non-essential amino acid solution (100×) (1%) + MEM amino acids solution (50×) (1%)]. Final sperm concentration was adjusted to 2–3 × 106 sperms/ml, which was assessed using a Neubauer chamber. The sperm suspension was kept in a CO2 in air incubator until matured oocytes were washed 4–5 times in fertilization medium. Finally, matured oocytes (n = 15–20) were co-incubated with 100 μl processed spermatozoa for 18 h. Following the 18 h co-incubation, presumptive zygotes were cultured in 100 μl culture medium [(TCM-199 (glutamine added) + 20% FBS + BSA (3mg/ml) + pyruvate (4 mM) + gentamycin (50 μg/ml) + MEM non-essential amino acid solution (100×) (1%) + MEM amino acids solution (50×) (1%)] to produce embryos at different developmental stages (cleavage to blastocyst). Both fertilization and culture were carried out at the same temperature and gas conditions described for maturation. Cleavage rates were recorded on day 2 (48 hpi) of culture and stages of embryonic development were evaluated every 24 h. Blastocyst development was recorded on day 7 (day 0 = day of IVF). Every 48 h, medium was replaced with 50% of freshly prepared culture medium. Finally l-erg (10 mM) was used in maturation medium to find out the oxidative status of matured oocytes, then the antioxidant effect of l-erg on embryo development was tested by partial enrichment of culture medium and l-erg-mediated change in transcript levels of apoptotic (Bcl2, Bax, Casp3 and PCNA) and antioxidant (GPx, SOD1, SOD2 and CAT) genes in oocytes and developing embryos.
Intracellular ROS and GSH levels in matured oocytes
Matured oocytes from l-erg (10 mM) treated or non-treated (0 mM) groups were taken to measure the intracellular ROS and GSH levels. Intracellular ROS and GSH levels were estimated as per the procedure described previously (Mishra et al., Reference Mishra, Reddy, Gupta and Mondal2016b). ROS levels in in vitro matured oocytes were quantified using 2′,7′-dichlorodihydrofluorescein diacetate (DCHFDA) and detected as green fluorescence at an excitation wavelength of 495 nm and emission wavelength of 520 nm. GSH levels were quantified using 4-chloromethyl-6.8-difluoro-7-hydroxycoumarin (CMF2HC) and detected as blue fluorescence at an excitation wavelength of 350 nm and an emission wavelength of 450 nm. Briefly, oocytes were washed twice in phosphate-buffered saline (PBS) + polyvinyl pyrollidone (PVP) (0.5%) (wt/vol) and fixed with 4% paraformaldehyde (PFA) and then placed in 50 µl of DCHFDA (10 µM) and CMF2HC (10 µM) for 15 min at 5% CO2, 38.5°C and 95% RH to detect ROS and GSH respectively. Finally, the oocytes were washed three times with PBS + PVP (0.5%), and then carefully mounted on a glass slide and covered with a coverslip. The fluorescence intensity of oocytes in each group was observed under a fluorescence microscope (Euromex, The Netherlands) equipped with a digital camera. Fluorescence intensities of oocytes were analysed by grey pixel intensity using Image J software (NIH,USA) normalizing untreated control oocytes as 1.
Antioxidant effect of l-erg (10 mM) on embryo development
Interestingly, 10 µM concentrations of H2O2 significantly (P < 0.05) decreased cleavage and further development, whereas at 20 µM H2O2 there was no cleavage at all (Mishra et al., Reference Mishra, Reddy, Gupta and Mondal2016b). Therefore to assess the antioxidant effect of l-erg (10 mM), embryos (2–4-cells) were cultured with H2O2 (20 µM) for 48 h followed by replacing 50% of the medium with fresh medium without H2O2 but with or without l-erg (10 mM) on every alternate day up to blastocyst development. The control group embryos (n = 56) were cultured without H2O2 and l-erg. Group 1 embryos (n = 48) were cultured with H2O2 but without l-erg and Group 2 embryos (n = 54) were cultured with H2O2 and l-erg. Embryonic development was observed in all groups.
Blastocyst staining for total cell numbers
Blastocysts produced from l-erg (10 mM) treated or non-treated oocytes and embryos exposed to H2O2 with or without l-erg were stained with bisbenzimide (Hoechst 33258) to compare the total cell numbers between the groups (Mishra et al., Reference Mishra, Reddy, Gupta and Mondal2016c). Zona pellucida of the day 7 embryos/blastocysts were removed by 1% protease digestion, then washed three times in PBS + polyvinyl pyrrolidone (PVP) (0.5%) and were fixed in 4% paraformaldehyde for 30 min. Blastocysts were permeabilized in Triton X-100 (1%) for 10 min, washed three times in PBS + PVP (0.5%) and finally stained with bisbenzimide (Hoechst 33258) (10 µg/ml) for 20 min. Blastocysts were then mounted on slides and covered with a coverslip. The total cell numbers (TCN) of blastocysts were determined by counting the number of nuclei detected as blue fluorescence at an excitation wavelength of 350 nm and an emission wavelength of 450 nm under a fluorescence microscope (Euromex, The Netherlands) equipped with a digital camera. All exposures were carried out at room temperature.
Expression levels of apoptotic, antioxidant and SLC22A4 genes in oocytes and embryos
The transcript abundance of apoptotic genes (Bcl2, Bax, Casp3 and PCNA), antioxidant genes (GPx, SOD1, SOD2 and CAT) and SLC22A4 gene (encodes the OCTN1 the transporter of l-erg) were analyzed in oocytes and embryos by real-time quantitative PCR (qPCR). For the gene expression study, oocytes matured with l-erg (10 mM) followed by embryos cultured without l-erg were treated as the treatment group, whereas in the control group neither oocytes nor embryos were exposed to l-erg. The gene-specific primers used in this study were designed using NCBI, Primer Blast software (Table 1). The specificities of the primers were tested using BLAST analysis against the genomic NCBI database.
Table 1 Primers used for gene expression analysis

qPCR analysis
Total RNA isolation, cDNA synthesis and qPCR were carried out by the procedure discussed previously (Mishra et al., Reference Mishra, Reddy, Gupta and Mondal2017). Briefly, total RNA was isolated from equal numbers of oocytes, immature (n = 20), in vitro matured (n = 20) and embryos of zygote (n = 20), 2–4-cell (n = 20) and blastocysts (n = 10) from both the groups by TRIzol (Invitrogen, Life Technologies, USA). Genomic DNA contamination was removed using the TURBO DNA-free™ kit (Ambion, Life Technologies, USA). Total RNA isolated from both groups was used for reverse transcription (RT) as the template for first strand synthesis using the evertAid First Strand cDNA Synthesis Kit (ThermoFisher Scientific, Waltham, MA, USA) as per manufacturer's guidelines. The expression levels of specific genes in oocytes and embryos were quantified by qPCR using the Step One Plus qPCR system (Applied Biosystem, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the reference gene in this study. The qPCR reactions were performed using KAPA SYBR Fast 2× master mix (KAPA Biosystems, Wilmington, USA). Each run was performed in duplicate in a 10-μl reaction containing 5 μl qPCR master mix, 5 pM of gene-specific forward and reverse primers, 1 μl of cDNA as template and final volume was adjusted to 10 μl with nuclease-free water. The PCR conditions used to amplify all genes were initial denaturation at 95°C for 20 s with 40 cycles of denaturation at 95°C for 3 s followed by annealing and extension at 60°C for 30 s. The melting curve analysis was carried out to confirm the qPCR specificity. Ct (threshold cycle for target amplification) values were analysed using the 2–∆∆Ct (normalized expression ratio) method to determine the relative level of expression of each mRNA. ∆Ct = Ct (target gene) − Ct (housekeeping gene) and ∆∆Ct = ∆Ct (target gene sample) − ∆Ct (calibrator). qPCR was conducted three times for three different sets of matured oocytes and embryos. The qPCR amplicons were confirmed by ethidium bromide-stained (0.5 μg/ml) 2% agarose gel electrophoresis.
SLC22A4 gene expression in testes
SLC22A4 gene expression in testes was carried out to get a positive sample for the expression of SLC22A4 to confirm that the used primer is specific for the SLC22A4 gene. A sheep testes sample was taken for RNA isolation with subsequent cDNA synthesis and qPCR analysis. Before RNA isolation, the testes (10 g) was exposed to liquid nitrogen and homogenized with an homogenizer. Total RNA was isolated by the conventional TRIzol (Invitrogen, Life Technologies, USA) protocol as per manufacturer's guidelines. Briefly 500 μl of TRIzol was added to the pre-homogenized testes and homogenized again, followed by the addition of another 500 μl of TRIzol and was mixed. The mixture was incubated for 5 min at room temperature. Next, 200 µl chloroform was added to the mixture, mixed and incubated for 10 min at room temperature. The whole mixture was centrifuged at 12,000 rpm for 15 min at 4°C. The upper aqueous phase was collected without touching the interphase. 500 µl isopropanol was added to the supernatant, mixed by pipetting up and down and incubated for 30 min on ice. The tubes were centrifuged at 12,000 rpm for 10 min at 4°C after incubation and the supernatant was discarded. The pellet was washed with 1000 μl of 75% freshly prepared ethanol by centrifuging at 7500 rpm for 5 min at 4°C. The supernatant was discarded and the pellet was air dried at room temperature for 10 min. The RNA pellet was resuspended in 20 µl of diethyl pyrocarbonate (DEPC) water and mixed. The mixture was incubated at 60°C for 10 min with a little shaking in-between incubations. Genomic DNA contamination was removed using the TURBO DNA-free™ kit (Ambion, Life Technologies, USA). Subsequent cDNA synthesis, qPCR and confirmation of qPCR amplicons were carried out using same procedure described for oocytes and embryos.
Statistical analysis
The results were expressed in mean ± standard error of the mean (SEM). Statistical analyses were carried out using GraphPad Prism5 software, San Diego, USA. The means between groups for developmental stages (cleavage to blastocyst) and TCN were compared by analysis of variance (ANOVA). Embryo development data were presented in the percentage form in relation to total oocytes cultured. Percentage values were arcsine transformed before analysis. A P-value < 0.05 was considered as significant. ROS levels and GSH levels between matured oocytes of control and l-erg treated groups as well as relative gene expression level at particular developmental stage of embryo between control and l-erg-treated groups were compared by Student's t-test.
Results
Effect of l-erg (5mM and 10mM) supplementation on in vitro maturation and subsequent embryo development
The results of in vitro embryo development in the presence or absence of l-erg (5 mM and 10 mM) in maturation medium are detailed in Fig. 1. Supplementation of different concentrations of l-erg (5 mM and 10 mM) in the maturation medium did not influence the maturation percentage (82.96–84.11%). l-Erg (10 mM) supplementation during the maturation period resulted in a significantly (P < 0.05) higher percentage of cleavage (53.72 vs 38.86, 46.56%) followed by morulae (34.36 vs 20.62, 25.84%) and blastocysts (14.83 vs 6.98, 9.26%) as compared with other groups of lower concentration [0 mM (control) and 5 mM]. The cleavage percentage of the 10 mM l-erg group was not significantly more than for the 5 mM l-erg group.

Figure 1 Effect of l-erg (5 mM and 10 mM) during in vitro maturation on maturation of oocytes and embryo development. Percentage results are presented as mean + standard error of the mean (SEM). a,bDifferent superscripts in the same group indicate values that differ significantly at P < 0.05. Six experiments were performed for each group. *Cleavage was calculated from total number of oocytes cultured, whereas morula and blastocysts percentage were calculated from the number of embryos cleaved.
Effect of l-erg (10 mM) on intracellular ROS and GSH levels of matured oocytes
l-Erg-treated (10 mM) matured oocytes showed significantly (P < 0.05) lower intensities for ROS, indicating decreased intracellular ROS, and significantly (P < 0.05) higher intensities for GSH, indicating increased intracellular GSH as compared with matured oocytes in the control group without l-erg (Fig. 2).

Figure 2 Intracellular ROS and GSH level in matured oocytes with l-erg (10 mM) during in vitro maturation. A, Control oocytes; B, l-erg treated oocytes. Asterisks indicate values that differ significantly at P < 0.05. Three experiments were performed for each group.
Antioxidant effect of l-erg (10 mM) on embryo development
Embryos (2–4-cell) cultured with H2O2 (20 µM) but without l-erg (10 mM) (Fig. 3B) for 48 h showed a significant (P < 0.05) decrease in the percentage of morulae (13.14 vs 50.46%) and blastocysts (2.86 vs 17.54%) compared with the control group (embryos cultured with neither H2O2 nor l-erg) (Fig. 3A). Supplementation of l-erg (10 mM) to H2O2-induced (20 µM) culture medium, showed a significant (P < 0.05) increase in the percentage of morulae (46.58 vs 13.14%) and blastocysts (16.68 vs 2.86%), which was not significantly different to that of the control group (Fig. 3C).

Figure 3 Antioxidant effect of l-erg (10 mM) on embryo development in the presence or absence of H2O2 during the post-fertilization period. A, Embryo cultured with neither H2O2 nor l-erg. B, Embryo cultured with H2O2 (20 µM). C, Embryo cultured with H2O2 (20 µM) + l-erg (10 mM). *Percentage results are presented as mean + standard error of the mean (SEM). a,bDifferent superscripts in the same group indicate embryo stages that differ significantly at P < 0.05. Four experiments were performed for each group.
Effect of l-erg (10 mM) on total cell numbers
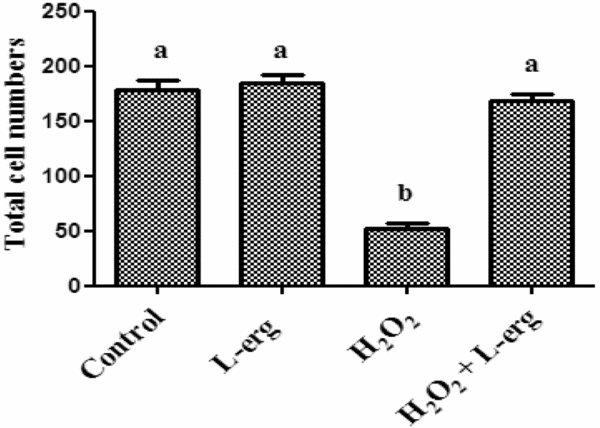
Although the percentage of blastocysts (14.83 vs 6.98%) was significantly (P < 0.05) increased due to l-erg (10 mM) supplementation in the maturation medium, TCN was not significantly different (184 ± 7.8 vs 178 ± 9.2) between l-erg-treated (10 mM) and non-treated groups. The TCN of the embryos exposed to H2O2 were significantly (P < 0.05) reduced to 52 ± 4.6. Supplementation of l-erg (10 mM) to H2O2-induced medium resulted in significantly (P < 0.05) no difference in cell numbers (168 ± 6.7) compared with that of the control (Fig. 4).

Figure 4 Effect of different culture conditions on total cell numbers of blastocyst and day 7 embryos. Results are presented as mean + standard error of the mean (SEM). a,bDifferent superscripts indicate groups that differ significantly at P < 0.05. Three experiments were performed.
Effect of l-erg (10 mM) on the relative expression levels of apoptotic and antioxidant genes in oocytes and embryos
The relative expression levels of apoptotic and antioxidant genes in matured oocytes and different developmental stages (zygote, 2–4-cell and blastocyst) of both control and l-erg-supplemented groups in relation to the expression level of immature oocytes is detailed in Fig. 5. In this study l-erg-mediated change in the relative expression level of genes in matured oocytes and developmental stages were compared with the particular stage of the control group. It was observed that l-erg (10 mM) treatment during maturation did not influence the expression of the majority of the apoptotic and antioxidant genes in matured oocytes and all the developmental stages except for antioxidant genes i.e. GPx was upregulated significantly (P < 0.05) in 2–4-cell stage embryos and SOD2 was significantly (P < 0.05) upregulated in all the developmental stages.

Figure 5 l-erg-mediated alteration in mRNA level of apoptotic and antioxidant genes in oocytes and embryos. Asterisk indicates the values that differ significantly at P < 0.05 in the same group, IMO: immature oocytes, MO: in vitro matured oocytes.
Expression profile of SLC22A4 in oocytes, developmental stages of embryos and testes
The relative expression level of SLC22A4 in oocytes (immature and matured) and developmental stages (zygotes, 2–4-cell and blastocysts) of embryos is shown in Fig. 6. It was observed that the SLC22A4 gene was not expressed in any oocytes (immature and matured) and developmental stages of embryos. In testes the SLC22A4 gene was expressed and it was confirmed that the primer used in this study was specific for SLC22A4.

Figure 6 Expression of SLC22A4 in oocytes, developmental stages of embryo and testes. (A) Expression of GAPDH (146 bp) in oocytes and developmental stages of embryo. (B) No expression of SLC22A4 (137 bp) in oocytes and developmental stages of embryo. M: Marker (DNA ladder 100 bp); 1: Immature oocytes; 2: In vitro matured oocytes; 3: Zygote; 4: 2–4-cell; 5: Blastocyst. (C) Expression of GAPDH (146 bp) and SLC22A4 (137 bp) in testes. M: Marker (DNA ladder 100 bp); 1: GAPDH; 2: SLC22A4.
Discussion
Studies are needed to minimize the OS during in vitro embryo culture to increase the developmental potential of oocytes and embryos. The present study was undertaken to ameliorate OS in the IVEP protocol in a sheep model by l-erg (5 mM and 10 mM) supplementation during the maturation period for better embryo development. Subsequent studies were conducted to find out the l-erg-mediated (10 mM) changes in expression level of apoptotic and antioxidant genes. To our current knowledge, there is no report in the literature on l-erg-mediated reduction in oxidative status of oocytes and change in the relative expression levels of apoptotic and antioxidant genes in oocytes and embryos of any species. Different concentrations of l-erg (5 mM and 10 mM) supplementation during IVM in this study showed that at 10 mM l-erg there was significant (P < 0.05) increase in the percentages of cleavage, morula and blastocyst as compared with other lower concentrations [0 mM (control) and 5 mM]. There was a beneficial effect on embryo development from zygote to blastocyst by supplementing l-erg (5 mM and 10 mM) during IVM. Supplementation of l-erg (10 mM) in maturation medium significantly (P < 0.05) increased GSH levels and decreased ROS levels in matured oocytes. To our knowledge, this report is the first to reflect l-erg-mediated change in GSH and ROS levels in matured oocytes. Improvement in developmental stages of embryos by l-erg supplementation during IVM could be due to its effects of increasing GSH and reducing intracellular ROS that protect cells from apoptosis, reported by our group previously (Mishra et al., Reference Mishra, Reddy, Gupta and Mondal2016b). The present findings of ROS and GSH levels in response to l-erg supplementation during maturation were similar to other previous reports of a reduction in ROS and an increase in GSH due to free radical scavenger supplementation during IVEP (You et al., Reference You, Lee, Hyun and Eunsong2012; Takahashi et al., Reference Takahashi, Inaba, Somfai, Kaneda, Geshi, Nagai and Manabe2013). GSH is a substrate for GSH peroxidase and it is well known that l-erg increases GSH peroxidase enzyme activity, which might influence the significant increase in GSH in oocytes (Kawano et al., Reference Kawano, Murata, Iriguchi, Mayumi and Hama1983). ROS produced from oocytes during the maturation period are neutralized due to the free radical scavenging effect of l-erg that protects cellular organelles including mitochondria, the major source of ROS for further subsequent developmental stages demonstrating l-erg as an antioxidant and anti-apoptotic compound. Subsequent l-erg supplementation (10 mM) of H2O2-induced (20 µM) culture medium resulted in a significant (P < 0.05) increase in the percentages of morulae and blastocysts and TCN compared with H2O2-induced culture medium and confirmed that l-erg neutralized ROS generated by H2O2 and supported the antioxidant properties of l-erg (Akanmu et al., Reference Akanmu, Cecchini, Aruoma and Halli1991). Therefore this study suggested that it is better to use l-erg during IVM of oocytes to achieve an improved number of developmental stage embryos.
Although there was improvement in the developmental potential of sheep embryos by the addition of l-erg in maturation medium, the maturation rate (82.96% to 84.11%) was not influenced by any experimental l-erg (5 mM and 10 mM) concentration. In contrast with our findings, another study reported that the addition of l-erg (10 mM) to IVM, IVF and IVC medium showed a beneficial effect on IVM of oocytes and embryonic development in sheep, especially from cleavage to morulae stages (Ozturkler et al., Reference Ozturkler, Yildiz, Gungor, Pancarci, Kaçar and Ari2010). Bovine presumptive zygotes when cultured with l-erg (0.5 mM) during the post-fertilization period showed a marked decrease in both cleavage and blastocyst development and evidence of a toxic effect was observed with no embryo production following culture with 1 mM l-erg. There was improvement in embryo quality, as indicated by improved cryotolerance, lower apoptotic rate and a higher percentage of blastocysts with the most physiological ICM:total cells ratio (Zullo et al., Reference Zullo, Albero, Neglia, De Canditiis, Bifulco, Campanile and Gasparrini2016). These studies were the only two that discussed the effect of l-erg on the developmental potential of oocytes and embryos. It should be noted that the concentration of l-erg that was beneficial on sheep embryos including our study was 10 times higher than the concentration used in the bovine study and was toxic to bovine embryos. Because of the limited information available, differences in the effect of l-erg on developmental potential of oocytes and embryos might be due to species-specific differences or concentration of l-erg used in the experiments. The concentration of l-erg in mammalian tissue is 1–2 mM, which may act as a non-toxic thiol buffering antioxidant (Grigat et al., Reference Grigat, Harlfinger, Pal, Striebinger, Golz, Geerts, Lazar, Schomig and Grundemann2007). The concentration of l-erg used for the bovine study was lower than the physiological range and might have not given a significant embryo developmental potential effect, whereas in the sheep study the concentration used was 10 times more and showed a significant result in terms of embryo developmental stages.
The present study is the first to determine the effects of l-erg (10 mM) on the relative expression levels of apoptotic (Bcl2, Bax, Casp3 and PCNA) and antioxidant (GPx, SOD1, SOD2 and CAT) genes in oocytes and developing embryos produced in vitro, therefore we cannot compare our results with other similar studies. The only studies available in the literature in sheep and bovine models were discussed above, but these only assessed the qualitative and quantitative effect of l-erg on in vitro embryo development, not at the transcript level. Although in our previous studies (Mishra et al., Reference Mishra, Reddy, Gupta and Mondal2016b, c) the use of l-carnitine altered the expression levels of apoptotic and antioxidant genes in oocytes and embryos, it was surprising to observe in the present study that l-erg (10 mM) treatment during maturation did not influence the expression levels of all apoptotic genes studied for the oocytes and developmental stages. Among the antioxidant genes studied, l-erg (10 mM) treatment significantly (P < 0.05) upregulated the expression of GPx in the 2–4-cell stage but not in matured oocytes and blastocyst stages, whereas expression of SOD2 was significantly (P < 0.05) upregulated both in 2–4-cell and blastocyst stages, but not in matured oocytes. The expression of other antioxidant transcripts (SOD1 and CAT) was not altered at both the matured oocytes and developmental stages by l-erg (10 mM) supplementation. Modifications in the microenvironment modulate gene expression in developmental stages of embryos (Zhou et al., Reference Zhou, Xiang, Walker, Farrar, Hwang, Findeisen, Sadeghieh, Arenivas, Abruzzese and Polejaeva2008; Mishra et al., Reference Mishra, Reddy, Gupta and Mondal2016b, c). Antioxidant gene expression is modulated by OS (Correa et al., Reference Correa, Rumpf, Mundim, Franco and Dode2008). Change in the expression in GPx and SOD2 in the 2–4-cell stage and blastocyst might be explained by the fact that ROS produced by the embryo diffuses to the microenvironment and is neutralized by l-erg, as ROS are able to diffuse through the plasma membrane (Guerin et al., Reference Guerin, El Mouatassim and Menezo2001). GPx is considered as the major antioxidant enzyme within the glutathione peroxidase family and its deficiency renders cells more sensitive to stress (Flentjar et al., Reference Flentjar, Crack, Boyd, Malin, De Haan, Hertzog, Kola and Iannello2002). GPx levels depend on the availability of reduced GSH and it acts in conjunction with GSH, a tripeptide cofactor for their enzymatic activity. GSH constitutes a vital component of the cellular antioxidant system (Mari et al., Reference Mari, Morales, Colell, Garcia-Ruiz and Fernez-Checa2009). In our study l-erg (10 mM) increased the GSH level in mature oocytes. Therefore l-erg-mediated upregulation of GPx in initial developmental stages (2–4-cell) was due to increased cofactor GSH concentration, whereas in the blastocyst stage GSH levels may be reduced compared with the initial developmental stages because of ROS production by embryos themselves. SOD2 is considered an indicator of OS in cells and embryos (He et al., Reference He, Peterson, Holmuhamedov, Terzic, Caplice, Oberley and Katusic2004). l-Erg is cell membrane impermeable and requires a specific carrier to be transported across the membrane. Its function is restricted only to cells and tissues in which the ergothioneine transporter OCTN1 is produced or expressed (Grundemann et al., Reference Grundemann, Harlfinger, Goltz, Geerts, Lazar, Berkels, Jung, Rubbert and Schömig2005). Upregulated expression of SOD2 due to l-erg (10 mM) supplementation may be due to the fact that l-erg cannot permeate across the plasma membrane of oocytes and embryos and that the oxidative status inside mitochondria is not reduced. Because of this intracellular OS, ATP synthesis is reduced, causing an increase in ROS formation that requires more SOD2 to neutralize the free radicals with an upregulated expression of the SOD2 gene. It has been reported previously that during ATP synthesis a large quantity of oxygen is utilized, causing a decrease in oxygen concentration and reduction in ROS formation (Gulcin, Reference Gulcin2006). In the present study, l-erg mediated the upregulated of GPx and SOD2 expression in the developmental stages with no effect on SOD1, similar to findings in an in vitro study of mouse liver that showed an inhibitory effect of ergothioneine on lipid peroxide formation (Kawano et al., Reference Kawano, Murata, Iriguchi, Mayumi and Hama1983). The CO2 incubator used for the present experiment had no controlled oxygen, so it was assumed that the oxygen tension inside incubator was at maximum tension of atmospheric oxygen. It has already been reported that high oxygen tension during in vitro embryo culture upregulated SOD2 expression due to more ROS production (Correa et al., Reference Correa, Rumpf, Mundim, Franco and Dode2008). In the present experiment, l-erg supplementation decreased the ROS concentration in oocytes, and this decrease was expected to be carried forward in embryos compared with the control. Therefore downregulation of SOD2 expression in developmental stages of embryos was expected due to l-erg supplementation during the maturation period, but in contrast there was upregulation in SOD2 expression. The l-erg-mediated upregulated expression of SOD2 in developmental stages might also be explained by reaction of cellular stressors with l-erg that may occur outside the cell, giving a false impression of intracellular effects as seen in many cell culture assays (Cheah & Halliwell, Reference Cheah and Halliwell2012). So a complete understanding of the action of l-erg in the body is still limited and much more remains to be learned. It can also be explained that, as SOD2 expression is culture condition dependant (Lequarre et al., Reference Lequarre, Feugang, Malhomme, Donnay, Massip, Dessy and Langendonckt2001) and OS affects mainly the mitochondria, this influences SOD2 expression. In contrast, the relative expression of SOD1 is not affected by l-erg supplementation because OS during our in vitro culture conditions might have not been influenced enough to alter SOD1 expression. l-Erg can chelate metalloenzymes and has affinity for metals with a strong affinity for Cu/Zn in the order Cu > Hg > Zn > Cd > Co > Ni. Because of the strong affinity of l-erg for Cu/Zn, deactivating Cu/Zn would have no effect on Cu–Zn SOD (SOD1), cytoplasmic SOD, but might stimulate Mn SOD (SOD2), mitochondrial SOD (Motohashi et al., Reference Motohashi, Mori, Sugiura and Tanaka1974). CAT is an enzymatic antioxidant that neutralizes H2O2 molecules by converting them into oxygen and water (Kobayashi et al. Reference Kobayashi, Miyazaki, Natori and Nozawa1991), and which is stimulated by OS (Guerin et al., Reference Guerin, El Mouatassim and Menezo2001). Use of antioxidants such as l-carnitine upregulated the expression of CAT in patients with coronary artery disease (Lee et al., Reference Lee, Lin, Lin and Lin2014). The lack of change in CAT expression due to l-erg supplementation might be due to the fact that OS during culture may not be influenced enough to alter CAT expression.
Although microenvironment modification by l-erg (10 mM) treatment created beneficial microenvironments by increasing GSH level and reducing ROS in oocytes, it is still not clear how l-erg alters the expression of the majority of apoptotic and a few antioxidant genes studied in matured oocytes and in developmental stages. l-Erg has already been proved to be anti-apoptotic for a higher percentage of blastocysts with a lower apoptotic proportion, as assessed by TUNEL staining (Zullo et al., Reference Zullo, Albero, Neglia, De Canditiis, Bifulco, Campanile and Gasparrini2016). But in the present study the expression of apoptotic genes was not altered in matured oocytes and in developmental stages. The ergothioneine transporter (ETT) has a beneficial role for l-erg activity. Cells with high expression of ETT can accumulate ergothioneine to high levels (Grundemann et al., Reference Grundemann, Harlfinger, Goltz, Geerts, Lazar, Berkels, Jung, Rubbert and Schömig2005). The gene SLC22A4 encodes an integral membrane protein, OCTN1 which is a powerful and highly specific transporter for the uptake of l-erg that facilitates pH-dependent transport of ergothioneine across the plasma membrane (Lamhonwah & Tein, Reference Lamhonwah and Tein2006). Cells lacking ETT do not accumulate ergothioneine, because the plasma membrane is virtually impermeable to ergothioneine (Cheah & Halliwell, Reference Cheah and Halliwell2012). Cellular uptake of l-erg exhibits significant temperature and Na+ dependence and is saturable, further supporting the involvement of carrier-mediated transport (Nakamura et al., Reference Nakamura, Sugiura, Kobayashi, Yoshida, Yabuuchi, Aizawa, Maeda and Tamai2007). Polymorphisms in the OCTN1 gene are involved in inflammatory and autoimmune diseases and show abnormal levels of ROS (Urban et al., Reference Urban, Yang, Lagpacan, Brown, Castro, Taylor, Huang, Stryke, Johns, Kawamoto, Carlson, Ferrin, Burchard and Giacomini2007). Although studies indicated the expression of the SLC22A4 gene that encodes OCTN1 for ergothioneine transport inside cells, expression of SLC22A4 on oocytes and embryos has not been reported (Lamhonwah & Tein, Reference Lamhonwah and Tein2006; Wu et al., Reference Wu, George, Huang, Wang, Conway, Leibach and Ganapathy2000; Markova et al., Reference Markova, Jurukovska, Dong, Damaghi, Smiles and Yarosh2009). In this study, SLC22A4 gene expression was not observed in matured oocytes and in any of the developmental stages of the embryo. We tried to determine SLC22A4 expression in different tissues to confirm that the primer used was specific for SLC22A4. It was observed that SLC22A4 was not expressed in all the tissues studied except in the testes (unpublished data). Therefore l-erg mediated no change in expression of the majority of the apoptotic and antioxidant genes. From this study it can be speculated that l-erg is not permeable to oocyte and embryo plasma membranes due to the absence of OCTN1, the transporter for l-erg across the membrane. The cellular uptake of l-erg is temperature and Na+ dependent and OCTN1 facilitates pH-dependent transport of l-erg across the membrane, so the in vitro culture medium and study conditions would not have influenced proper transportation of l-erg across the membrane to alter the gene expression. OCTN1 has 11 predicted trans-membrane domains and can variously function as an organic cation/proton exchanger, or a Na+-dependent or Na+-independent zwitterion transporter. Its physiological substrates are carnitine and ergothioneine, but ergothioneine is a superior substrate. OCTN1 specifically and very efficiently transports ergothioneine and OCTN2 is responsible for carnitine transport. Although transport of l-carnitine by OCTN1 is measurable, it is negligible compared with transport of l-erg (Grundemann et al., Reference Grundemann, Harlfinger, Goltz, Geerts, Lazar, Berkels, Jung, Rubbert and Schömig2005). OCTN2 is predominantly involved in l-carnitine transport and OCTN1 has poor l-carnitine transport activity, suggesting that there may be another physiological role for transport (Lamhonwah and Tein, Reference Lamhonwah and Tein2006). There are reports of OCTN1 localization in mitochondria, and significant species differences in both localization and transport mechanisms among species (Wu et al., Reference Wu, George, Huang, Wang, Conway, Leibach and Ganapathy2000). Therefore further investigation of l-erg transport and action is required. The differences in the role of both l-erg and l-carnitine towards altering gene expression were due to absence of OCTN1 (responsible for l-erg transport) across the membrane of oocytes and embryos, whereas OCTN2 (responsible for carnitine transport) might be present in oocytes and embryos to help transportation of carnitine across the membrane to alter embryonic genes expression. It was concluded from the study that l-erg supplementation during in vitro maturation reduces OS-induced embryo toxicity by decreasing intracellular ROS and increasing intracellular GSH, that in turn improved the developmental potential of oocytes and embryos. Although l-erg altered the mRNA abundance of a few antioxidant genes, the mRNA abundance of the majority of the apoptotic and antioxidant genes studied in oocytes and embryos was not altered, due to the absence of the SLC22A4 gene in oocytes and embryos that encodes OCTN1, an integral membrane protein and highly specific transporter for the uptake of l-erg across the membrane to alter gene expression. It can also be speculated that the change in the relative expression of only a few antioxidant genes in this study was not l-erg mediated, but rather microenvironment dependant and cell function mediated.
Conflict of interest
None of the authors have any conflict of interest to declare.
Acknowledgements
The authors are thankful to the Director, ICAR-National Institute of Animal Nutrition and Physiology, Bangalore, India to provide the necessary facilities to conduct the research.