Introduction
Donkey is experiencing a period of increasing interest. Initially appreciated for its attitude to hard and multitasking work supporting many human activities, its role has been progressively lost with industrialization and its numbers greatly reduced in industrialized countries (Starkey and Starkey, Reference Starkey and Starkey2000). This decreasing trend has now slightly changed due to new roles in milk production or for rehabilitating people with nervous or behavioural disorders. The use of the asinine germplasm has also assumed an important relevance for both conservation of endangered breeds and production of mules (Camillo et al., Reference Camillo, Rota, Biagini, Tesi, Fanelli and Panzani2018).
The use of refrigerated semen is a consolidated practice in artificial insemination (AI) with considerable positive effects. However, in horse, semen processing involves factors that may affect sperm functionality, such as the choice of semen extender, centrifugation, cooling and, in turn, resulting oxidative cell damage (Cochran et al., Reference Cochran, Amann, Froman and Pickett1984; Aurich, Reference Aurich2005). Moreover, temperature reduction is associated with an increase in the production of mitochondrial reactive oxygen species (ROS) and modifications in mitochondrial membrane potential (MMP) (Ali et al., Reference Ali, Marcondes, Bajova, Dugan and Conti2010). Individual variability may further represent a limit to sperm conservation, as donkey stallions may be distinguished as ‘good coolers’ or ‘poor coolers’ (Dorado et al., Reference Dorado, Acha, Ortiz, Gálvez, Carrasco, Díaz, Gómez-Arrones, Calero-Carretero and Hidalgo2013).
Several authors have evaluated the effects of refrigeration on asinine semen (Santos et al., Reference Santos, Henry, Sampaio and Gastal1995; Cottorello et al., Reference Cottorello, Amancio, Henry and Borges2002; Serres et al., Reference Serres, Rodriguez, Alvarez, Santiago, Gabriel, Gómez-Cuétara and Mateos2002; Rota et al., Reference Rota, Magelli, Impeduglia, Panzani and Camillo2005, Reference Rota, Magelli, Panzani and Camillo2008). Significant differences were observed between extenders in preserving cooled donkey sperm functionality (Rota et al., Reference Rota, Magelli, Panzani and Camillo2008; Alonso et al., Reference Alonso, Castañeira, Castex, Ferrante, Arraztoa, Flores, Gambarotta, Miragaya and Losinno2017). Discordant results for horse and donkey sperm have emerged about the removal of seminal plasma before cooling (Brinsko et al., Reference Brinsko, Crockett and Squires2000; Alonso et al., Reference Alonso, Castañeira, Castex, Ferrante, Arraztoa, Flores, Gambarotta, Miragaya and Losinno2017). Very little information is available on fertility of cooled-extended donkey semen after AI. Sperm stored at +15°C after dilution with skimmed milk, with or without seminal plasma, provided in jennies inseminated on same day of collection, a 21% pregnancy rate when seminal plasma was removed (n = 28) and 27% when it was not removed (n = 85) (Alvarez et al., Reference Alvarez, Serres, Crespo, Santiago, Mateos and Gómez-Cuétara2004). Following AI of jennies and mares with donkey semen diluted with milk, stored at +4°C for up to 8 h, a per-cycle pregnancy rate of 45% was found in both female groups (Vidament et al., Reference Vidament, Vincent, Martin, Magistrini and Blesbois2009). A deeper knowledge of sperm functionality during storage at low temperatures would have inevitable advantages for donkey sperm for future applications in reproductive technologies and germplasm conservation. In particular, the poor or completely absent information on mitochondrial activity and ROS production in donkey sperm needs special attention.
For this reason, this study aimed to monitor, in refrigerated donkey spermatozoa, the effects of sperm extender and concentration over time through a panel of parameters related to sperm kinetics, bioenergetics and oxidative status. This knowledge will be helpful in identifying reference criteria for storing donkey spermatozoa at +4°C and contribute to the development of AI practices in this species.
Materials and methods
Reagents
Polyvinyl alcohol (PVA), potassium hydroxide (KOH), N-acetyl cysteine (NAC), CuSO4, dimethyl sulfoxide (DMSO), menadione, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide (JC-1) and water were purchased from Sigma Chemical Company (Milan, Italy) and cell culture tested. Carbonyl cyanide 3-chlorophenylhydrazone (CCCP), 4,4-difluoro-5-(4-phenyl-1,3,butadienyl)4-bora-3a,4a-diaza-s-indacene-33-undecanoic acid (C11-BODIPY581/591) were obtained from Life Technologies (Milan, Italy). Equiplus, INRA-96 and Hyppex were purchased from Minitube (Minitub, Tiefenbach, Germany), IMV Technologies (L’Aigle, France) and Barex Biochemical Products (Enkhuizen, The Netherlands), respectively. Hydrogen peroxide solution 30% was purchased from Biochem (Chemopharma, Cosne sur Loire, France). Phosphate-buffered saline pH 7.4 designed and manufactured for mammalian cell culture was purchased from Gibco (Life Technologies, Grand Island, NY, USA).
Animals
Two adult and reproductively mature Martina Franca (MF) jackasses (J1 and J2), 7 and 9 years old and weighing 340 and 320 kg, respectively, and one adult and reproductively mature Romanian jackass (J3), 11 years old and weighing 185 kg, were enrolled in this study. These stallions were previously trained for semen collection and routinely used for AI programmes. They were housed in box stalls with an open paddock, under natural light conditions, at a private farm.
Semen collection
Semen collection was carried out from February to April 2019. Jackasses were submitted to at least three preliminary semen collections scheduled twice weekly up to 3–4 days before the beginning of the experiment to empty the sperm epididymal reservoir. Later, using a Missouri artificial vagina, semen was collected twice weekly on Tuesday and Friday, but only the semen collected on Tuesday was used for experimental purposes. After collection, the semen was filtered through a sterile gauze. Within 5 min of collection, the semen was divided equally into four parts and added to four semen extenders that were kept at the same temperature (about 25°C) as the semen was collected. Semen was diluted preliminary with each extender at a ratio of 1:2 (volume of semen:volume of extender). To define the temperature of the semen at the time of dilution, preliminary simulations were performed in which thermal lowering was evaluated from collection up to distribution into the four aliquots. This procedure allowed us to set the temperature of the extender at values close to those of the collected semen, preventing thermal stress at the time of extender addition. Then, semen samples were stored in a polystyrene ice-box with refrigeration units and kept separated from samples by polystyrene walls, allowing a temperature decrease of ~0.3–0.5°C/min to +4°C. Once in the laboratory, samples were kept at +4°C, and evaluated for sperm concentration and motility within 3–4 h from collection.
Semen extenders
Four sperm extenders used successfully for horse sperm dilution and preservation were compared.
The Kenney (K) extender (data measured: pH 6.9 ± 0.2 and osmolarity 308 ± 18 mOsm/kg) due to its known composition was used as the reference extender. The extender was prepared by adding 272 mM glucose, 24 mg/ml skimmed powder milk, 1500 IU/ml penicillin, and 1.5 mg streptomycin/ml to ultrapure tissue culture-tested water (Kenney, Reference Kenney1975).
The Equiplus (E) extender (data provided: pH 6.8 ± 0.2, osmolarity 320 ± 20 mOsm/kg) (data measured pH 7.0 ± 0.1, osmolarity 301 ± 13 mOsm/kg) has an undefined composition containing caseinates, glucose, sucrose, lincomycin and spectinomycin.
The INRA 96 (I) extender (data provided: pH 7.0 ± 0.1 and osmolarity 310 ± 20 mOsm/kg) (data measured: pH 7.1 ± 0.1, osmolarity 309 ± 10 mOsm/kg) has an undefined composition containing caseins, buffers, sugars, ultrapure water, sodium penicillin, gentamicin sulfate and amphotericin B.
The Hippex (H) extender (data measured: pH 6.7 ± 0.1 and osmolarity 332 ± 20 mOsm/kg) has an undefined composition containing Pronexcell, Lipex and gentamicin as the antibiotic. Pronexcell is a mixture of purified proteins with protective functions against components present in the seminal plasma. Lipex is a mixture of low-molecular-weight lipids with antioxidant and membrane preservation functions.
Experimental design
After evaluating sperm concentration, all samples were set at 70 × 106 spermatozoa/ml by adding or removing each specific extender. In the latter case, semen was centrifuged at 230 g for 10 min and the supernatant removed up to setting the fixed concentration. Semen was aspirated in 10 ml syringes (Becton, Dickinson and Co, Franklin Lakes, NJ, USA) to obtain anaerobic conditions. Sperm concentrations of 30, 50 and 70 × 106/ml were set by loading fixed amounts of each extender into syringes that were then stored at +4°C. At each time point, 1 ml of air was aspirated into each syringe that was vigorously shaken and approximately 1.5 ml of semen sample was collected within a 2 ml tube, taking care to remove all air bubbles inside the syringe.
Spermatozoa were evaluated at 0, 3, 24, 48 and 72 h storage for sperm kinetics, MMP, lipid peroxidation (LPO), anti-LPO potential and nitroblue tetrazolium (NBT) test. All these procedures were rigorously carried out away from light to avoid either fluorochrome bleaching or sperm oxidative stress.
Sperm kinetics
The assessment of sperm kinetics was carried out using the SCA 5.0 system (Microptic, Barcelona, Spain) as previously described (Boni et al., Reference Boni, Gallo and Cecchini2017). Sperm concentration was adjusted with respective medium to 20 × 106 spermatozoa/ml and samples were equilibrated for 2 min at 37°C. Spermatozoa with an average velocity of less than 10 µm/s were considered immotile. Sperm kinetics included: the percentage of motile spermatozoa (Tot Mot); the percentage of progressive spermatozoa (Prog Mot, average path velocity higher than 30 µm/s and straightness of track higher than 80%); the curvilinear velocity (VCL, µm/s); the straight-line velocity (VSL, µm/s); the average path velocity (VAP, µm/s); the amplitude of head displacement (ALH, µm); and the frequency of the sperm head crossing the sperm average path (BCF, Hertz).
Evaluation of MMP
An estimated number of 1 × 106 spermatozoa for each extender and sperm concentration was centrifuged at 230 g for 10 min and resuspended in 200 µl phosphate-buffered saline supplemented with 0.1% polyvinyl alcohol (PBS-PVA) and incubated with 1.5 µM JC-1 at 37°C for 30 min. JC-1 is a dye exhibiting potential-dependent accumulation in mitochondria. The potential-sensitive colour shift follows mitochondrial membrane depolarization (Boni et al., Reference Boni, Gallo and Cecchini2017). After incubation, sperm suspensions were centrifuged at 230 g for 10 min, the supernatant removed and the pellet resuspended in PBS-PVA.
Fluorescence spectra were recorded in duplicate: each sample was placed in a quartz microtube and read using a spectrofluorometer (Cary Eclipse, Agilent Technologies, Rome, Italy) (excitation wavelength, 488 nm; emission spectrum, 500–620 nm). MMP was measured by relating fluorescence peak values at ~595 nm and ~535 nm.
Evaluation of lipid peroxidation (LPO)
Lipid peroxidation was determined by C11-BODIPY581/591. Oxidation of the polyunsaturated butadienyl portion of the dye resulted in a shift in the fluorescence emission peak from ~590 nm to ~515 nm (Boni et al., Reference Boni, Gallo and Cecchini2017). A 1 mM C11-BODIPY581/591 stock solution was prepared by dilution in DMSO and stored at −80°C.
Sperm aliquots (1 × 106 spermatozoa/ml) were incubated with 2 µM C11-BODIPY581/591 for 30 min at 37°C, centrifuged at 230 g for 10 min, resuspended in 1 ml of PBS-PVA and incubated at 37°C for 30 min.
After incubation, each sample was read using a spectrofluorometer, as above. Lipid peroxidation was evaluated by relating the fluorescence peak values at ~515 nm to the sum of the fluorescence peak values at ~515 and ~590 nm.
Anti-LPO potential
Anti-LPO potential was evaluated by incubating spermatozoa with C11-BODIPY581/591 together with a mild oxidant stimulus of 2 mM menadione and 1.8/0.25 mM CuSO4/H2O2 (i.e. OxMix solution) (Orhan et al., Reference Orhan, Gurer-Orhan, Vriese, Vermeulen and Meerman2006). By exposing sperm suspensions to 0.5% OxMix, we intended to test the ability of sperm defences to counteract oxidative stress.
Sperm aliquots (1 × 106 spermatozoa/ml) were incubated with 2 µM C11-BODIPY581/591 and 0.5% OxMix in the dark for 30 min at 37°C. Spermatozoa were then centrifuged at 230 g for 10 min, resuspended in 1 ml of PBS-PVA and incubated for 30 min at 37°C.
After incubation, each sample was read using a spectrofluorometer, as above. Anti-LPO potential was evaluated by relating the fluorescence peak values at ~590 nm to the sum of the fluorescence peak values at ~515 and ~590 nm.
Intracellular fluorescent dye localization and sensitivity
JC1 and C11-BODIPY581/591 were evaluated using confocal laser scanning microscopy (Zeiss LSM 510, Zeiss Italia, Varese, Italy) to localize the stain within a specific cell compartment. MMP negative controls were prepared by adding 2 µM CCCP to extra samples loaded with JC-1, whereas LPO positive controls were prepared by incubating extra samples loaded with C11-BODIPY581/591 with 1% OxMix for 1 h. In addition, to evaluate the potential of C11-BODIPY581/591 to detect LPO occurring before dye loading, this procedure was carried out either before or at the same time as exposure to 1% OxMix. To further verify if variations of fluorescence intensity were effectively related to oxidative stress, samples were exposed simultaneously to 1% OxMix and 2 mM NAC, a powerful antioxidant compound, at the time of C11-BODIPY581/591 loading (Gallo et al., Reference Gallo, Menezo, Dale, Coppola, Dattilo, Tosti and Boni2018).
Nitroblue tetrazolium (NBT) assay
NBT assay is based on the capability of the cells incubated in a tetrazolium salt (NBT) solution to take up NBT into the cytoplasm, where it is converted by superoxide anions to water-insoluble blue formazan crystals (Baehner et al., Reference Baehner, Boxer and Davis1976). These crystals are trapped within the cell but can be released by solubilization in a solvent solution and then quantified by measuring the absorbance of the resulting purple-blue solution (Rook et al., Reference Rook, Steele, Umar and Dockrell1985; Sim Choi et al., Reference Sim Choi, Woo Kim, Cha and Kim2006).
A 0.1% NBT-PBS-PVA solution was stirred at room temperature for 1 h and then filtered. Sperm suspensions (15 × 106 spermatozoa) were centrifuged at 230 g for 5 min, the pellets were incubated with NBT solution for 45 min at 38°C (Tunc et al., Reference Tunc, Thompson and Tremellen2010). Then, samples were washed with PBS-PVA. To quantify the production of superoxide anions, intracellular formazan was solubilized in 2 M KOH:DMSO solution and read using a microplate spectrophotometer (Bio-Rad 550, Hercules, CA, USA) at 630 nm. Superoxide anion production was expressed as µg of formazan per 30 × 106 spermatozoa and related to a standard curve of absorbance values.
Statistical analysis
Data were obtained from three replicates (3 ejaculates × 3 jackasses) and entered into a spreadsheet, as follows: the experimental date, the replication number, the time of incubation (0, 3, 24, 48 and 72 h), the extenders (n = 4), the sperm concentrations (n = 3) and the 10 experimental endpoints (i.e. Tot and Prog Mot, VCL, VSL, VAP, ALH, MMP, LPO, anti-LPO potential and NBT values).
Repeated measures analysis of variance (ANOVA) (Systat 11.0 release, Systat Software GmbH, Erkrath, Germany) was used to evaluate the effects of time of incubation, the extender and the sperm concentration for each sperm parameter. The Shapiro–Wilks test was applied to evaluate normal data distribution and Leveneʼs test was used to evaluate the homogeneity assumption needed for ANOVA. Variables measured in percentages were transformed to angles corresponding to arcsine of the square root of percentage for variance analyses. Tabular data are presented as non-transformed values, for ease of interpretation. Covariate multiple range test was used to separate main effect means. Pairwise comparisons of the means were performed with the least significant difference (LSD) test. Coefficients of correlation (R) were calculated using a linear regression procedure (Systat 11.0). The minimum level of statistical significance was P < 0.05. Values are presented as means ± standard error (SE).
Results
The semen volume and the total amount of spermatozoa/ejaculate significantly differed between J1 and J2 stallions (30 ± 12 vs 70 ± 11 ml; P < 0.01 and 4.5 ± 2.7 vs 12.7 ± 2.5 × 109 spermatozoa; P < 0.05) whereas J3 yielded intermediate quantities.
Before use for spectrofluorometer assessment, either JC1 or C11-BODIPY581/591 was evaluated by confocal laser scanning microscopy to localize the stain within a specific cell compartment (Figs 1A and 2A). In Fig. 1(A), the sperm intermediate tract, corresponding to the mitochondrial location, is marked by JC-1 with green and red fluorescence emissions (Fig. 1A2, A3). In Fig. 2(A), C11-BODIPY marks all the sperm plasma membrane although with higher fluorescence intensity at the intermediate tract, the higher ROS production sperm site. In the spectrofluorometer, both fluorescent probes showed emission spectra characterized by two peaks. For JC-1, fluorescence emission peaks occurred at ~535 and ~595 nm wavelengths (Fig. 1B). The latter peak significantly decreased after 1 h incubation with 2 µM CCCP (negative controls) (Fig. 1B, C). For C11-BODIPY581/591, fluorescence emission peaks occurred at ~515 and ~590 nm wavelengths. After 1 h incubation with an oxidative stimulus, 1% OxMix (see above), the fluorescence peak at ~515 nm increased (Fig. 2B) with a consequent increase in LPO value (Fig. 2C), independently if OxMix exposure occurred before or after dye loading. However, when sperm samples were simultaneously exposed to 1% OxMix and 2 mM NAC, the LPO values did not differ from the control but were significantly lower than in the OxMix-exposed samples (Fig. 2C).

Figure 1. Laser confocal microphotographs of donkey spermatozoa after incubation with JC-1 and bisbenzimide (for nucleus staining, blue) (A1–A3). In the spectrofluometer, the fluorescence spectrum (B) showed two emission peaks (F0A and F0B) at about 535 nm (green) (A2) and 595 nm (red) (A3). MMP was calculated by F0B/F0A ratio. The addition of a substance capable of depolarizing the mitochondrial membrane (2 µM CCCP) significantly (P < 0.01) reduced the emission peak at 595 nm (C). Number of replicates, 3.
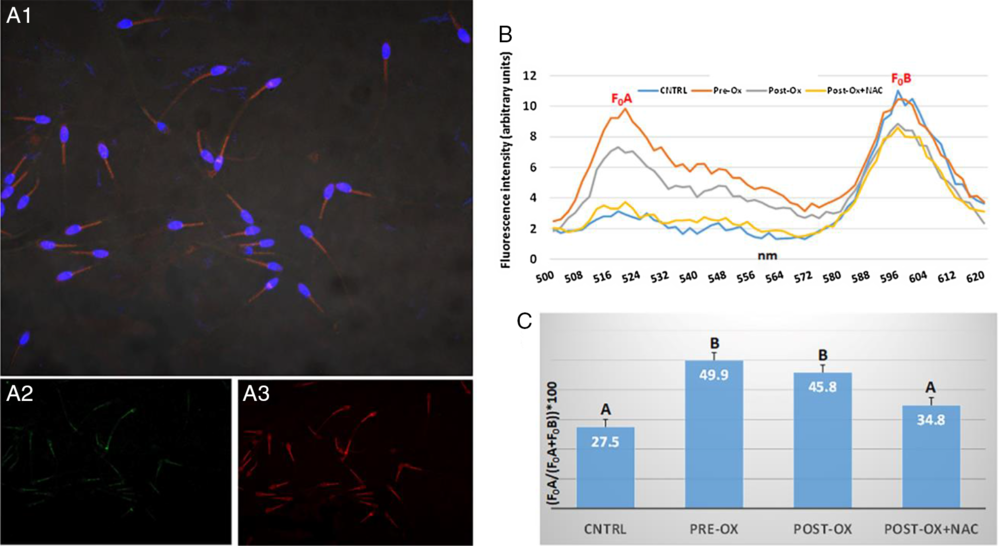
Figure 2. Evaluation of C11-BODIPY581/591 localization within donkey sperm membranes and its ability to report lipid peroxidation (LPO) occurrence. (A) Laser confocal microphotos of donkey spermatozoa after incubation with C11-BODIPY and bisbenzimide (for nucleus staining, blue). C11-BODIPY581/591 dye is localized at the membrane level showing a higher fluorescence intensity at the intermediate sperm tract, the site of mitochondrial localization with prevalent ROS production. The fluorescence spectrum (B) shows two emission peaks (F0A and F0B) at about 520 nm (green) (A2) and 595 nm (red) (A3). LPO was calculated by (F0A/(F0A+F0B))*100. It was evoked by an oxidizing solution (1% OxMix for 30 min) either before (Pre-Ox) or at the same time (Post-Ox) of sperm incubation with C11-BODIPY581/591, and counteracted by the addition of a powerful antioxidant (2 mM N-acetylcysteine, NAC). Number of replicates, 3.
The time of incubation affected all sperm kinematic, bioenergetic and oxidative parameters (Table 1). All kinematic parameters showed a progressive decline (P < 0.01) during storage. However, BCF did not show significant differences up to 72 h incubation. Also MMP showed a decreasing trend with statistically significant differences between 0 vs 3 and 24 h (P < 0.01), 3 and 24 vs 48 h (P < 0.05), and the lowest value (P < 0.01) at 72 h incubation. LPO showed a significant increase at 48 and 72 h incubation. Conversely, anti-LPO potential showed a significant (P < 0.01) decrease at 48 and 72 h incubation. NBT values were stable between 0 and 3 h incubation, but underwent a significant (P < 0.01) increase at 24, 48 and 72 h incubation.
Table 1. Effect of the time of incubation at +4°C on donkey sperm kinetics, bioenergetics, and oxidative status. Data (mean ± SE) have been covariated by stallion, extender and sperm concentration. Different capital and small letters on the same row indicate statistically significant differences (P < 0.01 and P < 0.05, respectively)

ALH, amplitude of head displacement; anti-LPO, anti-LPO potential; BCF, beat cross frequency; LPO, lipid peroxidation; MMP, mitochondrial membrane potential; NBT, nitroblue tetrazolium; Prog Mot, progressive motility; Tot Mot, total motility; VAP, average path velocity; VCL, curvilinear velocity; VSL, straight-line velocity.
The extender effects on sperm kinetics, bioenergetics, and oxidative status are solid, although not all parameters have proved to be capable of discriminating between extenders (Table 2). The highest sperm kinetics was found using the H extender, whereas I, E, and K extenders showed progressively lower values. MMP and LPO did not differ among extenders. Anti-LPO potential showed lower values with H and K compared with E and I extenders. NBT showed the highest values with the extender E; these values gradually decreased with K, H, and I extenders. This trend is, practically, the opposite of sperm kinetics.
Table 2. Effects of the extender on refrigerated (+4°C) donkey sperm kinetics, bioenergetics, and oxidative status. Data (mean ± SE) have been covariated by time of incubation, stallion and sperm concentration. Different capital and small letters on the same row indicate statistically significant differences (P < 0.01 and P < 0.05, respectively)

Sperm concentration showed univocal results in most of the parameters analyzed (Table 3). Sperm kinetics showed a progressive decrease with increasing sperm concentration. MMP and LPO did not significantly change between sperm concentrations, whereas anti-LPO potential significantly decreased with increasing sperm concentration. NBT values increased (P < 0.01) from 30 to 50 × 106 spermatozoa/ml but then decreased at 70 × 106 spermatozoa/ml.
Table 3. Effects of the sperm concentration on refrigerated (+4°C) donkey sperm kinetics, bioenergetics and oxidative status. Data (mean ± SE) have been covariated by time of incubation, stallion and extender. Different capital and small letters on the same row indicate statistically significant differences (P < 0.01 and P < 0.05, respectively)

A large variability between stallions emerged for all parameters (Table S1). J1 showed a total and progressive motility significantly (P < 0.01) higher than J2, who showed higher (P < 0.01) values than J3. VCL, VSL, VAP and ALH highlighted a superiority in J1 compared with the other two stallions that did not show significant differences between them. Only BCF showed higher (P < 0.01) values in J1 than the other two stallions and in J2 vs J3 (P < 0.01). Unexpectedly, the lowest and the highest MMP values were found in J1 and J3, respectively. Also, LPO and anti-LPO values were lower (P < 0.01) in J1 than in J2 and J3, whereas J1 showed NBT values significantly (P < 0.05) higher than J2.
Correlation coefficients (R) with relative statistical significance are shown in Table S2. Most parameters were significantly correlated with each other. Sperm kinematic parameters showed very high correlation coefficients. Lower correlation coefficients were found by crossing bioenergetic and oxidative parameters. MMP was not significantly correlated with Prog Mot, whereas NBT values were not significantly correlated with Tot Mot, ALH, BCF, and anti-LPO potential.
Discussion
A panel of indicators evaluated in real-time the dynamic changes in sperm kinetics, bioenergetics and oxidative status efficiently discriminating the effects of sperm extenders and concentrations for storing donkey sperm at +4°C. The effect of storage provided useful information to validate this analytical procedure. Experimental design based on comparison of four extenders, three sperm concentrations, five time points, three stallions and three replicates for each stallion provided solid indications supported by independent results.
The semen volume and the sperm concentration produced by the three stallions fell within a range of variability reported by other authors (Contri et al., Reference Contri, De Amicis, Veronesi, Faustini, Robbe and Carluccio2010; Quartuccio et al., Reference Quartuccio, Marino, Zanghì, Garufi and Cristarella2011; Dorado et al., Reference Dorado, Acha, Ortiz, Gálvez, Carrasco, Díaz, Gómez-Arrones, Calero-Carretero and Hidalgo2013). Total and progressive motility were very high at the time of arrival in the laboratory but decreased to around 30% on average after just 3 h storage at +4°C. A dramatic decline (from 33 to 50%) in total and progressive motility within the first 24 h storage at +4°C was reported by Cottorello et al. (Reference Cottorello, Amancio, Henry and Borges2002). Conversely, Rota et al. (Reference Rota, Magelli, Panzani and Camillo2008) comparing three extenders in donkey sperm found a mild decline in total motility after 72 h incubation. Later studies, however, showed a rapid decline in donkey sperm kinetics already after 24 h storage at +4°C (Rota et al., Reference Rota, Sgorbini, Panzani, Bonelli, Baragli, Ille, Gatta, Sighieri, Casini and Maggiorelli2018). Contri et al. (Reference Contri, De Amicis, Veronesi, Faustini, Robbe and Carluccio2010) reported an extender- and season-dependent sperm activity decline during storage (in autumn faster than in spring). The rapid decline of sperm function found in our study may be related to season, as well as to stallion, variability or even to the non-use of an electronic temperature lowering device, such as the Equitanier® (Demyda-Peyras et al., Reference Demyda-Peyras, Bottrel, Acha, Ortiz, Hidalgo, Carrasco, Gómez-Arrones, Gosalvez and Dorado2018).
Decline in MMP with increasing incubation time would suggest a good relationship between this parameter and sperm kinetics. However, this indicator did not discriminate between extenders and sperm concentrations. Furthermore, an inverse trend was found between MMP and the merit attribution of the stallions in relation to their sperm functionality. MMP information on donkey sperm is very scarce. Comparing ejaculated and epididymal donkey spermatozoa, lower MMP values were found in the former (Gloria et al., Reference Gloria, Contri, De Amicis, Robbe and Carluccio2011). Other authors (Johannisson et al., Reference Johannisson, Figueiredo, Al-Kass and Morrell2018) did not found any significant correlation between mitochondrial and oxidative indicators with established markers of sperm quality such as sperm motility, plasma membrane and chromatin integrity. Conversely, Darr et al. (Reference Darr, Cortopassi, Datta, Varner and Meyers2016) found that mitochondrial oxygen consumption of equine sperm was positively correlated with traditional measures of sperm function including motility and viability (r = 0.62 and r = 0.49, respectively, P < 0.05). MMP was used to evaluate mitochondrial functionality, implicating mitochondrial dysfunction as a major contributor to sperm ageing and cryopreservation damages (Darr et al., Reference Darr, Moraes, Scanlan, Baumber-Skaife, Loomis, Cortopassi and Meyers2017). In bovine, MMP was significantly correlated with sperm kinematic parameters, such as VCL, VSL and VAP, whereas it was not significantly correlated with total and progressive sperm motility (Boni et al., Reference Boni, Gallo and Cecchini2017). Hence, MMP does not seem to provide solid indications of sperm motility, as also demonstrated in freeze-dried spermatozoa that, even if immotile, showed high MMP values (Restrepo et al., Reference Restrepo, Varela, Duque, Gómez and Rojas2019). However, it provides additional information on sperm quality that was not obtainable by other assays.
Sperm mitochondria produce by-products, such as ROS, whereas sperm production of antioxidant compounds is small and the sperm plasma membrane is very sensitive to oxidative damage (Ball, Reference Ball2008). In donkey sperm, ROS production was only indirectly evaluated by DNA fragmentation following the freezing/thawing procedure (Cortés-Gutiérrez et al., Reference Cortés-Gutiérrez, Crespo, Gosalvez, Davila-Rodriguez, López-Fernández and Gosalvez2008). For horse sperm, many studies have evaluated the effects of ROS on physiological and pathological events (Ball, Reference Ball2008), content of ROS scavengers in seminal plasma (Baumber and Ball, Reference Baumber and Ball2005) and the possibility of conditioning sperm function by treatment with antioxidant compounds both in vivo (Del Prete et al., Reference Del Prete, Tafuri, Ciani, Pasolini, Ciotola, Albarella, Carotenuto, Peretti and Cocchia2018; Contri et al., Reference Contri, De Amicis, Molinari, Faustini, Gramenzi, Robbe and Carluccio2011) and in vitro (Ball et al., Reference Ball, Medina, Gravance and Baumber2001; Cocchia et al., Reference Cocchia, Pasolini, Mancini, Petrazzuolo, Cristofaro, Rosapane, Sica, Tortora, Lorizio and Paraggio2011). However, oxidative stress is related to multifactorial effects and critical speculation has been raised when its relevance was established in most problems related to sperm function. For example, Baumber et al. (Reference Baumber, Ball, Gravace, Medina and Davies-Morel2000) demonstrated that equine sperm MMP as well as sperm viability, acrosome integrity, and any detectable increase in lipid peroxidation were not related to the decline in sperm motility associated with ROS increase. Gibb et al. (Reference Gibb, Lambourne and Aitken2014) defined as paradoxical, the positive relationship found in horse sperm between fertility and oxidative stress, assuming higher levels of oxidative phosphorylation in horse than in other mammalian spermatozoa. This was further supported, considering that mitochondrial inhibition significantly reduced the kinetics and ATP content of equine, but not human, spermatozoa (Gibb et al., Reference Gibb, Lambourne and Aitken2014). In donkey sperm, however, we found that mitochondrial activity was positively correlated with sperm kinetics and negatively correlated with some ROS indicators, such as LPO and NBT. In addition, in a previous comparative study (Gallo et al., Reference Gallo, Menezo, Dale, Coppola, Dattilo, Tosti and Boni2018) in which human, bovine and ascidian (C. robusta) spermatozoa were incubated with compounds supporting the 1-carbon cycle, H2-DCFDA-detected ROS levels significantly differed between human and bovine sperm. In human sperm, ROS levels were negatively related to total sperm motility, whereas in bovine sperm they were positively related to total sperm motility.
In this study, for the first time, we evaluated three ROS indicators in donkey sperm. LPO and NBT assays have already been applied to horse sperm (Ball and Anthony, Reference Ball and Anthony2002; Sabeur and Ball, Reference Sabeur and Ball2006), while anti-LPO potential has been developed in this study as an alternative indicator for evaluation of cellular oxidative status. Although OxMix exposure is associated with a slight decrease (<5%) in sperm motility (data not shown), anti-LPO potential has shown an excellent sensitivity in detecting sperm oxidative status. Some concerns have arisen about the sensitivity of NBT in assessing donkey sperm function. In fact, although NBT values increased together with incubation time, they showed unexpected results between extenders (higher in the less performing extenders) and sperm concentrations. This may be associated with high ROS production in cells with either high or poor metabolic activity.
The four extenders compared in the present study, although efficiently used in equine and two of them in donkey (Cottorello et al., Reference Cottorello, Amancio, Henry and Borges2002; Rota et al., Reference Rota, Magelli, Panzani and Camillo2008; Contri et al., Reference Contri, De Amicis, Veronesi, Faustini, Robbe and Carluccio2010), showed substantial differences among them in maintaining donkey sperm functionality already after the first 3 h of storage (data not shown). Sperm kinetics allow discriminating each single extender, whereas MMP and LPO did not. Although characterized by a different functional meaning, NBT and anti-LPO showed similar results with the highest values in the E extender, which was associated with a lower sperm kinetics, and lower values in H extender, which was associated with the best sperm kinetics.
The effects of sperm concentration on sperm functionality during storage at +4°C provided solid results, attributing to the 30 × 106 spermatozoa/ml concentration the best performance in terms of sperm kinetics and anti-LPO potential. Fluctuating NBT values support the poor reliability of this assay to evaluate sperm quality (Aitken, Reference Aitken2018).
In conclusion, this study by real-time monitoring sperm kinetics, bioenergetics and oxidative status provides novel hints and reference criteria for preserving donkey spermatozoa at +4°C. Low sperm concentration and a proper extender are crucial requirements for optimum sperm cryopreservation efficiency. Field trials are requested to validate these findings, making them operational in practice.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S096719942000012X
Acknowledgements
The authors are grateful to Scuderia 3H (Avigliano, Potenza) and Santoroʼs farm (Castelmezzano, Potenza) for supplying and taking care of the animals and to Giovanna Perrone for her help in collecting semen. This study is dedicated to the memory of Vito Claps.
Financial support
None.
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Ethics approval
All the experimental procedures were conducted in strict accordance with European legislation regarding the protection of animals used for scientific purposes (European Directive 2010/63), as recognized and adopted by the Italian law (DL 2014/26). All jackasses used in this study were client-owned and informed owner consent was obtained.







