Introduction
It is known that oocytes from small antral follicles (<3.0 mm) have low competence to be matured in vitro and to assure early embryo development. Recently, Labrecque et al. (Reference Labrecque, Fournier and Sirard2016) reported that the transcriptome of bovine oocytes from small follicles is very different from oocytes from large follicles (5–8 mm). As a consequence, only fully grown oocytes are efficiently matured in vitro, whereas oocytes from small antral follicles are discarded because of their limitations to be successfully matured in vitro (Dang-Nguyen et al., Reference Dang-Nguyen, Appeltant, Somfai, Ishihara, Men, Santos, Noguchi, Kaneko and Kikuchi2017). Therefore, in vitro culture of these oocytes, prior to in vitro maturation, could allow the synthesis and storage of transcripts and proteins that are involved with resumption of meiosis. To avoid meiosis resumption during this pre-maturation period, Bezerra et al. (Reference Bezerra, Silva, Rissi, Rosa, Cesaro, Costa, Gonçalves and Silva2016) reported that most of the oocytes, from bovine antral follicles of 3 to 8 mm, cultured in the presence of cilostamide remained at germinal vesicle (GV) stage. In addition, swine oocytes from antral follicles<3 mm increased their diameter and reach around 120 µm after culture in the presence of cilostamide (Lee et al., Reference Lee, Elahi, Lee, Lee, Hyun and Lee2017). Therefore, it has been hypothesized that inhibition or delay of spontaneous nuclear maturation in vitro would allow more time for oocyte accumulation of molecules that are important for early embryo development, and could potentially improve the efficiency of in vitro embryo production (Bilodeau-Goeseels, Reference Bilodeau-Goeseels2012).
Previous studies have shown that transcripts, such as GDF9, H1foo, Cyclin B1 and c-Mos, are stored in the oocyte and have important roles during oocyte maturation and early embryo development. It has been reported that GDF9 appears to be a key regulator involved in the development of oocytes at all stages and at ovulation (Castro, Cruz and Leal, Reference Castro, Cruz and Leal2016). In cattle, the overexpression of oocyte-specific linker histone (H1foo) stimulates the maturation process, showing that it is essential for oocyte maturation (Yun et al. Reference Yun, An, Ning, Zhao, Yang and Lei2014). Reduction of cyclin B levels might cause maturation promoting factor (MPF) destabilization (Tiwari and Chaube, Reference Tiwari and Chaube2017). c-Mos has a role in regulating the assembly of the spindle during meiotic oocyte division in murine species (Zhao et al., Reference Zhao, Singh and Batten1991). Therefore, evaluation of the levels of these transcripts after pre-maturation may be an indicator of oocyte competence in the bovine species. Moreover, there is a progressive increase in mitochondria during oogenesis, which is essential for the production of ATP to assure cytoskeletal and cytoplasmic organization during resumption of meiosis and oocyte competence (Cotterill et al., Reference Cotterill, Harris, Fernandez, Lu, Huntriss, Campbell and Picton2013). Furthermore, a slight change in homogeneous mitochondrial distribution in GV to an aggregate of mitochondria in MII was reported in mice oocytes that had been matured in vitro (Nazmara et al., Reference Nazmara, Salehnia and Hossein Khani2014). This indicates that a chosen culture environment might have an influence on the location of mitochondria.
Various substances, like IL1β and TNFα, can influence oocyte growth and transcript storage in vitro. It is known that IL-1 and its receptors are expressed in different compartments within the oocyte and granulosa cells of bovine antral follicles and that 10 ng/ml IL1β stimulates the activation of primordial follicles during in vitro culture (Passos et al., Reference Passos, Costa, Cunha, Silva, Ribeiro, Souza, Barroso, Dau, Saraiva, Gonçalves, Van Den Hurk and Silva2016). IL1β also modulates the in vitro proliferation of granulosa cells from antral follicles (murine: Karakji and Tsang, Reference Karakji and Tsang1995, bovine: Baratta et al., Reference Baratta, Basini, Bussolati and Tamanini1996). Furthermore, Martoriati et al. (Reference Martoriati, Caillaud, Goudet and Gérard2003) reported that IL1β is probably involved in the regulation of equine oocyte meiotic resumption. TNFα and its receptors are also expressed in bovine antral follicles and TNFα maintained the ultrastructure of cumulus cells during in vitro culture of bovine cumulus–oocyte complexes (COCs) (Silva et al., Reference Silva, Bezerra, Glanzner, Santos, Dau, Rovani, Ilha, Costa, Cunha, Donato, Peixoto, Gonçalves, Bordignon and Silva2017a). In human, TNFα and its type II receptor are both transcribed and translated in the oocyte and cumulus cells (Naz et al., Reference Naz, Zhu and Menge1997). Yan et al. (Reference Yan, Hunter, Weed, Hutchison, Lyles and Terranova1993) reported that 10 ng/ml TNFα stimulates human granulosa cell proliferation and steroidogenesis. TNFα and IL1β bind to different cell membrane receptors, but they have a great number of activities in common, especially during remodelling of bone and cartilage and in the regulation of fever, inflammation, fibroplasia, and angiogenesis (reviewed by Oppenheim et al., Reference Oppenheim, Matsushima, Yoshimura, Leonard and Neta1989). These authors have reported that TNFα and IL1β have synergistic in vivo radioprotective effects and in vitro terminal differentiation effects on tumour cell lines. However, it is still unknown if IL1β, TNFα or both interact and influence oocyte growth and gene expression during the culture of bovine oocytes from small antral follicles. Thus, the objective of this study was to evaluate the effect of IL1β and TNFα on growth, maturation, gene expression and mitochondrial distribution in cultured bovine oocytes, isolated from small bovine antral follicles.
Materials and methods
Collection and transport of bovine ovaries
Bovine ovaries (n=160) were obtained from a slaughterhouse and transported to the laboratory in saline solution (0.9% NaCl) containing antibiotics (100 IU/ml penicillin and 50 μg/ml streptomycin sulfate) at 30°C, within a maximum period of 1 h.
Oocyte collection
In the laboratory, the ovaries were washed in saline solution and COCs were aspirated from small antral follicles (1–3 mm in diameter) using a 21 gauge needle connected to a sterile syringe. Under a stereomicroscope, COCs were recovered and selected according to Leibfried and First (Reference Leibfried and First1980). After morphological evaluation, grades 1 and 2 COCs with a visible compact and intact cumulus and a dark cytoplasm were selected for culture.
Growth, pre-maturation and maturation of oocytes in vitro
For in vitro culture, the control medium was TCM-199 supplemented with 4% polyvinylpyrrolidone (PVP), 1 µg/ml estradiol, 4 mM hypoxanthine, 0.2 mM pyruvic acid, 2.2 mg/ml sodium bicarbonate, 5.0 mg/ml LH (Lutropin®-V, Bioniche, Belleville, ON, Canada), 0.5 mg/ml FSH (Follitropin®-V, Bioniche, Belleville, ON, Canada), 5% fetal bovine serum and 100 IU/ml penicillin and 50 mg/ml streptomycin sulfate. The growth medium composition was according to a protocol described previously by Huang et al. (Reference Huang, Nagano, Kang, Yanagawa and Takahashi2013), with modifications. The COCs were cultured individually in 96-well plates with 150 µl in each well for 48 h in control medium alone or supplemented with 10 ng/ml IL1β (Passos et al., Reference Passos, Costa, Cunha, Silva, Ribeiro, Souza, Barroso, Dau, Saraiva, Gonçalves, Van Den Hurk and Silva2016), 100 ng/ml TNFα (Silva et al., Reference Silva, Bezerra, Glanzner, Santos, Dau, Rovani, Ilha, Costa, Cunha, Donato, Peixoto, Gonçalves, Bordignon and Silva2017a,Reference Silva, Ribeiro, Menezes, Barberino, Passos, Dau, Costa, Melo, Bezerra, Donato, Peixoto, Matos, Gonçalves, Van Den Hurk and Silvab) or both. In each treatment, 109 to 115 oocytes were cultured in vitro. After the growth period, two perpendicular measurements were performed in the oocytes using an inverted microscope with NIS Elements 4.2 software (Nikon, Nikon Instruments Inc., Japan). Then, morphologically normal COCs from each treatment were destined to in vitro pre-maturation.
The in vitro pre-maturation medium (pre-IVM) was TCM-199 containing Earle’s salts and l-glutamine (Sigma) supplemented with 0.2 mM pyruvic acid, 5.0 µg/ml LH (Lutropin®-V, Bioniche, Belleville, ON, Canada), 0.5 µg/ml FSH (Follitropin®-V, Bioniche, Belleville, ON, Canada), 0.4% BSA, 10 μM of cilostamide and 100 IU/ml penicillin and 50 μg/ml streptomycin sulfate (Bezerra et al., Reference Bezerra, Silva, Rissi, Rosa, Cesaro, Costa, Gonçalves and Silva2016). The COCs were cultured individually in 96-well plates for 20 h at 38.5°C, with 5% CO2 in air and then, the COCs were destined for in vitro maturation or for the evaluation of meiotic progression, as described previously by Bezerra et al. (Reference Bezerra, Silva, Rissi, Rosa, Cesaro, Costa, Gonçalves and Silva2016) and Hirao et al. (Reference Hirao, Itoh, Shimizu, Iga, Aoyagi, Kobayashi, Kacchi, Hoshi and Takenouchi2004).
The maturation medium for the treatments was the same medium that was used during pre-maturation but without cilostamide. The COCs were cultured individually for 22 h (Hirao et al., Reference Hirao, Itoh, Shimizu, Iga, Aoyagi, Kobayashi, Kacchi, Hoshi and Takenouchi2004; Bezerra et al., Reference Bezerra, Silva, Rissi, Rosa, Cesaro, Costa, Gonçalves and Silva2016), and all were intended for evaluation of meiotic progression.
Evaluation of meiotic progression and mitochondrial distribution
To evaluate meiotic progression after culture, the cumulus cells were removed by vortexing and the oocytes were fixed in 4% paraformaldehyde for 15 min and transferred to 0.1% Triton X-100. The chromatin configuration was observed after addition of 10 µg/ml Hoechst 33342 under an epifluorescence inverted microscope (Leica, DMI4000B). Oocytes were classified according to the nuclear maturation stage as GV or germinal vesicle break-down (GVBD).
For analysis of the mitochondrial distribution before and after maturation, 20 oocytes per treatment were stripped and incubated in a solution containing 100 nM Mito Tracker Red (SIGMA) for 45 min at 38°C in a saturated humidity atmosphere containing 5% CO2 and 95% air. Thereafter, the oocytes were analyzed under an epifluorescence inverted microscope (Leica, DMI4000B). Oocytes collected from large antral follicles (>5 mm in diameter) were used as fresh control. The mitochondrial distribution pattern in each oocyte was classified as: (i) heterogeneous – mitochondria distributed unevenly within the oocyte cytoplasm; (ii) homogeneous – mitochondria distributed throughout the entire oocyte; and (iii) peripheral – mitochondria distributed only in the periphery of the oocyte.
RNA extraction, reverse transcription and real-time polymerase chain reaction
In total, 240 oocytes [four groups of 15 oocytes (n=60) in each treatment] were used for real-time polymerase chain reaction (PCR). Total RNA was extracted from pools of oocytes using the TRIzol reagent (Invitrogen, São Paulo, Brazil), according to manufacturer’s instruction. The RNA concentration was estimated by reading the absorbance at 260 nm and was checked for purity at 280 nm in a spectrophotometer (Amersham Biosciences, Cambridge, UK) and 1 µg of total RNA was used for reverse transcription. Before the reverse transcription reaction, samples of RNA were incubated for 5 min at 70°C and then cooled in ice. Reverse transcription was performed in a total volume of 20 µl composed of 10 µl of sample RNA, 4 µl reverse transcriptase buffer (Invitrogen), 8 units/µl RNasin, 150 units/µl of reverse transcriptase Superscript III, 0.036 U random primers, 10 mM DTT and 0.5 mM of each dNTP (Invitrogen). The mixture was incubated at 42°C for 1 h, subsequently at 80°C for 5 min and finally stored at 20°C. The negative control was prepared under the same conditions, but without addition of reverse transcriptase.
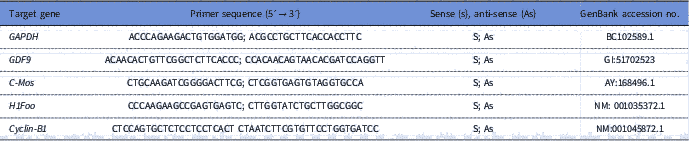
To quantify the levels of mRNA for GDF-9, Cyclin-B1, c-Mos and H1foo, each reaction in real time (20 μl) containing 10 μl of SYBR Green Master Mix (Applied Biosystems, Warrington, UK), 7.3 μl of ultra pure water, 1 μl of cDNA and 5 mM of each primer. Real-time PCR was performed in a thermocycler (Applied Biosystems, Warrington, UK). The primers designed to perform amplification of mRNA for GDF-9, Cyclin-B1, c-Mos and H1foo are shown in Table 1. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as endogenous controls for normalization of messenger RNA expression. GAPDH was successfully used as housekeeping gene in bovine oocytes (Silva et al., Reference Silva, Bezerra, Glanzner, Santos, Dau, Rovani, Ilha, Costa, Cunha, Donato, Peixoto, Gonçalves, Bordignon and Silva2017a).
Table 1 Primer pairs used to real-time PCR
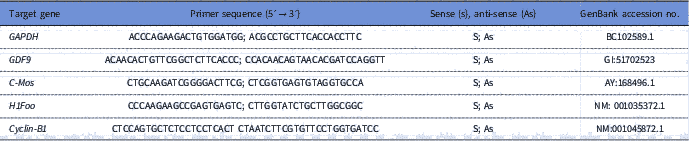
The specificity of each primer pair was confirmed by melting curve analysis of PCR products. The thermal cycling profile for the first round of PCR was: initial denaturation and activation of the polymerase for 10 min at 95°C, followed by 40 cycles of 15 s at 95°C, 30 s at 58°C, and 30 s at 72°C. The final extension was for 10 s at 72°C. Primer efficiency was determined using serial dilutions of the target cDNA. All reactions were performed in triplicate. The negative control was prepared under the same conditions but without addition of cDNA. The ΔΔCT method was used to transform CT values into normalized relative messenger RNA expression levels (Livak and Schmittgen, Reference Livak and Schmittgen2001).
Statistical analysis
Statistical analysis was performed using Graph Pad Prism 6 software. Paired T-test was used to compare oocyte diameters before and after culture. Analysis of variance (ANOVA) with Kruskal–Wallis test were used to compare the average oocyte growth, and the levels of mRNA in oocytes cultured in the different treatments. The percentages of oocytes at GV or GVBD after pre-maturation and maturation in each treatment were compared by chi-squared test. Differences were considered significant when the P-value was<0.05.
Results
Effects of IL1β and TNFα on oocyte growth during the culture of COCs
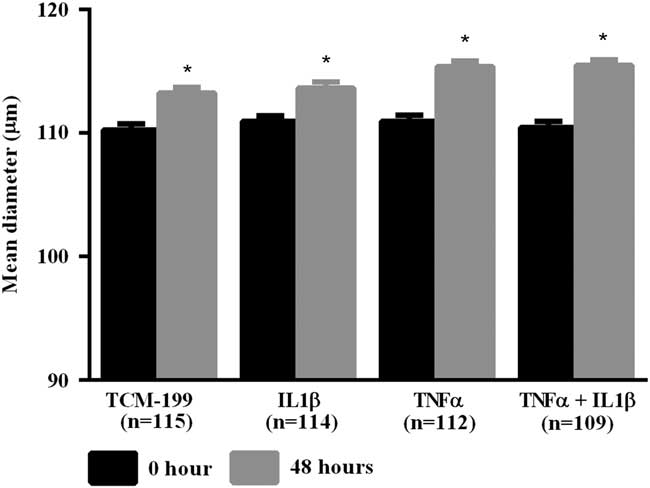
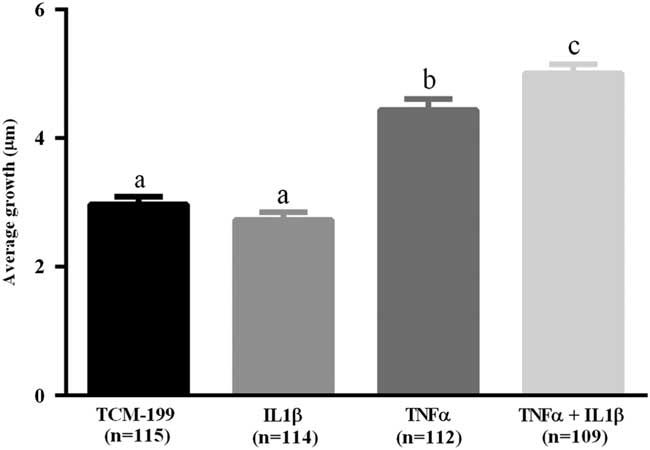
After culturing COCs for 48 h in TCM-199 alone (n=114) or supplemented with IL1β (n=113), TNFα (n=110) or both cytokines (n=108), a significant increase in diameter of oocytes was observed when compared with time zero (Fig. 1, P<0.05). Additionally, oocytes from COCs cultured in medium supplemented with TNFα alone or both TNFα and IL1β had larger average growth than those cultured in control medium alone or supplemented with IL1β (Fig. 2). A positive interaction between TNFα and IL1β was observed, as oocytes cultured in the presence of both substances had larger average growth than those cultured either with TNFα or IL1β (Fig. 2).
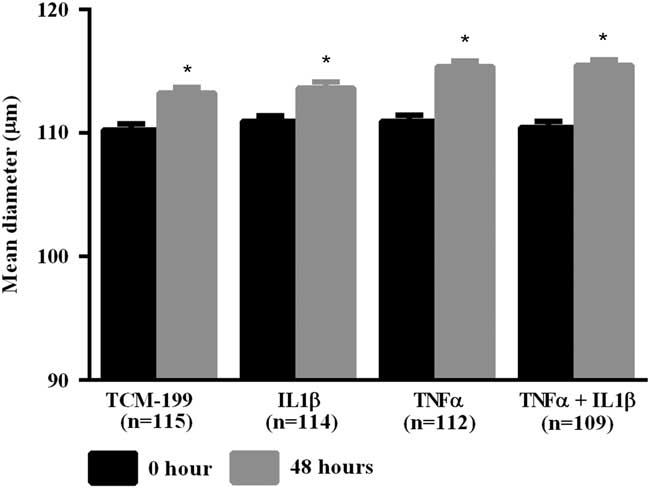
Figure 1 Mean diameter of oocytes cultured in TCM-199 alone or supplemented with IL1β, TNFα and both IL1β and TNFα for 0 and 48 h. *Significant difference between 0 and 48 h within the same treatment (P<0.05).
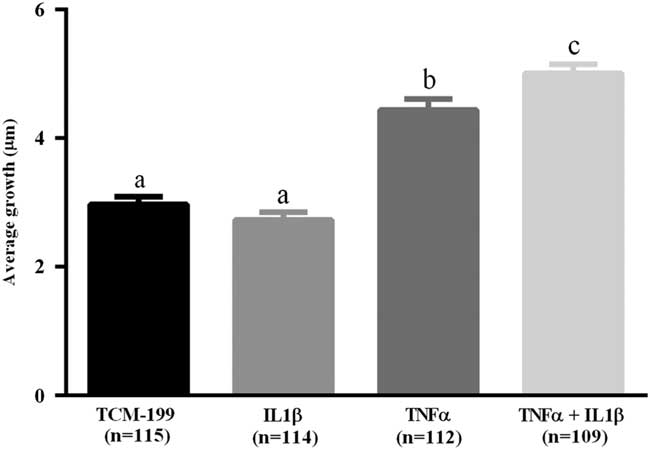
Figure 2 Average growth of oocytes cultured in TCM-199 alone or supplemented with IL1β, TNFα and both IL1β and TNFα for 48 h. a,b,cSignificant difference among treatments (P<0.05).
Pre-maturation and maturation of in vitro grown oocytes
After the growth period of oocytes, COCs were pre-matured for 20 h in medium supplemented with cilostamide. As shown in Table 2, after pre-maturation the percentage of oocytes at GV stage varied from 47.0 to 65.0%, while no significant effect of TNFα, IL1β or both TNFα and IL1β treatments on meiosis resumption was observed. After the in vitro maturation period, a significant reduction in the percentages of oocytes at GV was observed, which was accompanied by a significant increase in the percentages of oocytes showing meiosis resumption, but no significant differences among treatments were seen (Table 2).
Table 2 Percentage of oocytes in germinal vesicle (GV) and resumption of meiosis after pre-maturation and in vitro maturation (IVM)
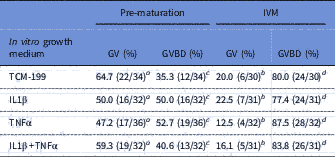
a,b Significant difference between the percentages of oocytes at GV after pre-maturation and maturation (P<0.05).
c,d Significant difference between the percentages of oocytes at GVBD after pre-maturation and maturation (P<0.05).
Expression of mRNA for GDF-9, c-Mos, H1foo and Cyclin-B1
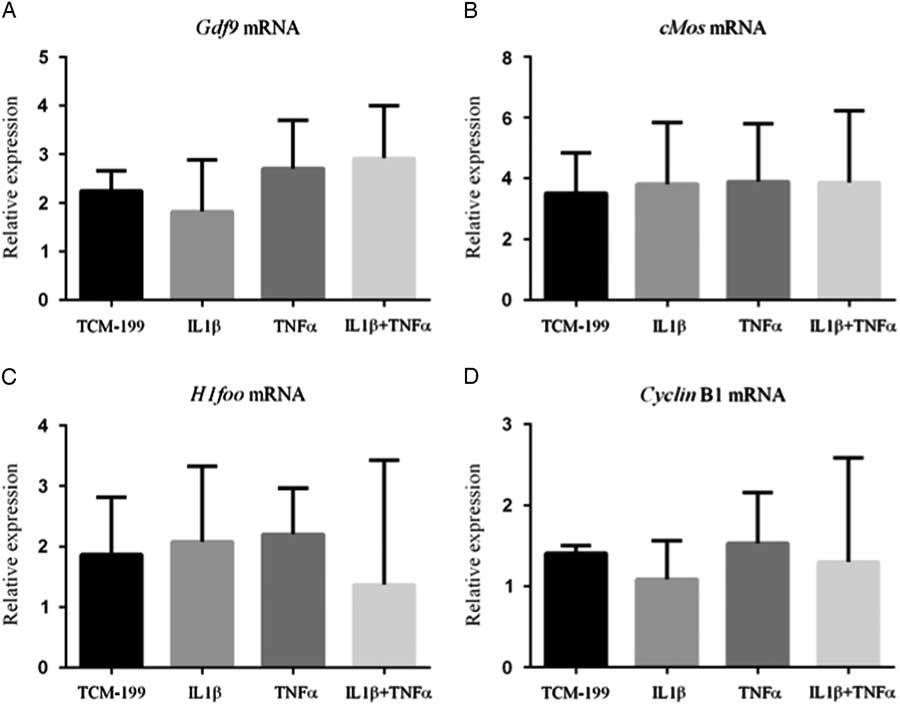
Figure 3 shows that TNFα, IL1β and both TNFα and IL1β did not influence the mRNA expression for GDF-9, Cyclin-B1, c-Mos and H1foo after culture of COCs.
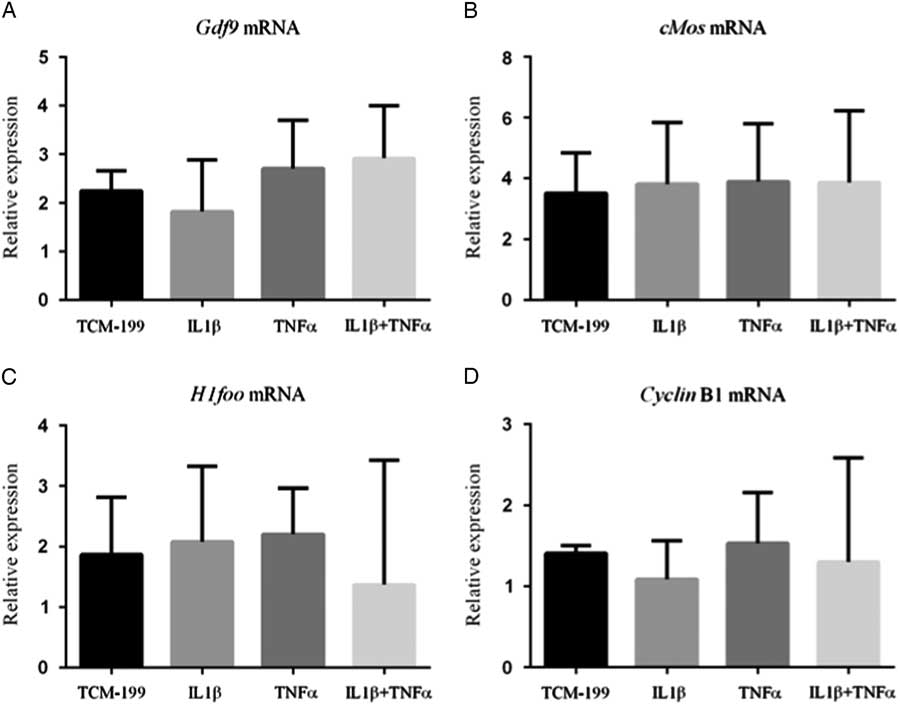
Figure 3 Expression of mRNA for (A) GDF-9; (B) c-Mos; (C) H1foo; and (D) Cyclin-B1 in bovine oocytes that had been gown in TCM-199 alone or supplemented with IL1β, TNFα and both IL1β and TNFα for 48 h and pre-matured for 20 h.
Mitochondrial distribution in oocytes before and after pre-maturation and IVM
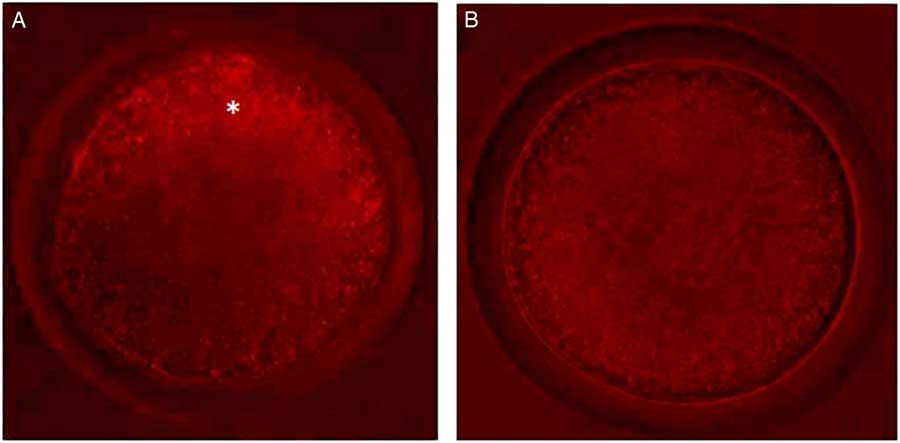
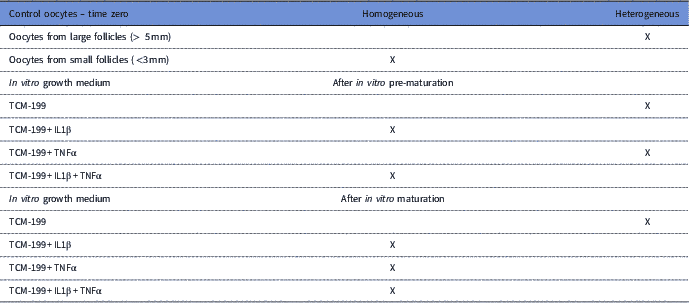
Control oocytes from large antral follicles (>5 mm) had a heterogeneous pattern of mitochondrial distribution (Fig. 4 A). However, in control oocytes from small antral follicles (1–3 mm), mitochondria were homogeneously distributed (Fig. 4 A). After pre-maturation of COCs in control medium alone or supplemented with TNFα, mitochondria were heterogeneously distributed throughout oocytes. After maturation, mitochondria were homogeneously distributed in oocytes, except for those grown in control medium, in which the mitochondria were heterogeneously distributed. Table 3 shows the mitochondrial distribution in the control oocytes or in those that underwent pre-maturation or in vitro maturation.
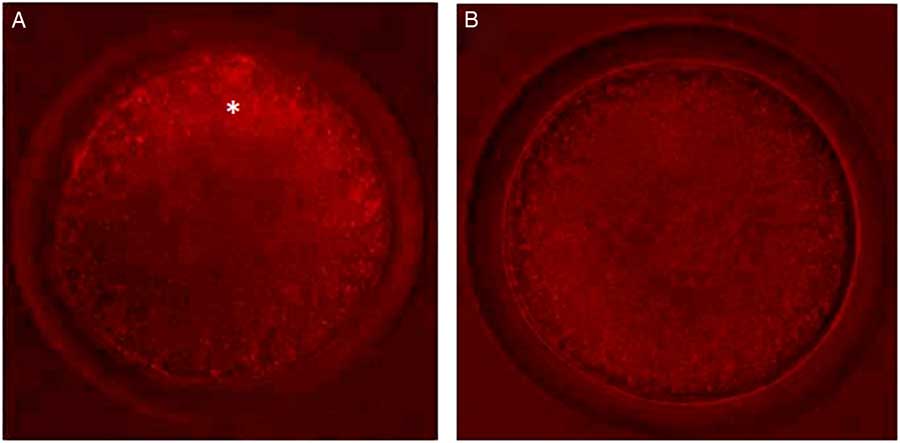
Figure 4 Oocytes with different types of mitochondrial distribution. (A) Oocytes from large antral follicles with heterogeneously distributed mitochondria. (B) Oocytes from small antral follicles with homogeneously distributed mitochondria. Original magnification ×400. *Indicates mitochondria distributed predominantly in periphery of the oocyte.
Table 3 Mitochondrial distribution of fresh oocytes at time zero and after pre-maturation and maturation in vitro of oocytes that had been previously grown in TCM-199 alone or supplemented with IL1β, TNFα and both IL1β and TNFα for 48 h
Discussion
This study shows for the first time that TNFα and both TNFα and IL1β promote oocyte growth in vitro. Recently, Silva et al. (Reference Silva, Bezerra, Glanzner, Santos, Dau, Rovani, Ilha, Costa, Cunha, Donato, Peixoto, Gonçalves, Bordignon and Silva2017a) have shown that, in cattle, TNFα and its receptors (TNFR1 and TNFR2) are expressed in bovine oocytes. In humans, TNFα expression increases gradually during follicular phases (Zolti et al., Reference Zolti, Bider, Seidman, Mashiach and Ben-Rafael1992). Although IL1β did not promote significant bovine oocyte growth in our study, Takehara et al. (Reference Takehara, Dharmarajan, Faufman and Wallach1994) reported that this substance is involved in the regulation of rabbit oocyte meiotic resumption. In addition, IL1β is known to act as a paracrine factor, which is involved in the cascade of events that lead to ovulation (Ben-Shlomo and Adashi, Reference Ben-Shlomo and Adashi1994). Moreover, oocytes and granulosa cells from bovine follicles expressed the proteins for IL1β, IL-1 receptor antagonist (IL-1RA) and the IL-1 receptors IL-1RI and IL-1RII (Passos et al., Reference Passos, Costa, Cunha, Silva, Ribeiro, Souza, Barroso, Dau, Saraiva, Gonçalves, Van Den Hurk and Silva2016).
During pre-maturation, high percentages (47–65%) of oocytes were kept at the GV stage. A previous study, using this same medium composition and bovine oocytes from large bovine antral follicles (3–8 mm in diameter), had shown that 36% oocytes remained at the GV stage after 12 h of culture (Bezerra et al., Reference Bezerra, Silva, Rissi, Rosa, Cesaro, Costa, Gonçalves and Silva2016). The findings showed that blockade of meiosis resumption with cilostamide is more efficient in COCs of small antral follicles. Maintenance of oocytes at GV is very important, as it is during meiotic arrest that the oocytes undergo structural and molecular modifications, including redistribution of organelles, storage of RNAs and messenger proteins (Crocomo et al., Reference Crocomo, Marques Filho, Ulian, Branchini, Silva, Ackermann, Landim-Alvarenga and Bicudo2015).
After maturation of COCs that had been grown and pre-maturated in vitro, the GVBD rate ranged from 78.0 to 88.0% (Table 2). Supplementation of culture medium with TNFα, IL1β or both did not significantly influence the rate of GVBD. Regarding oocytes of large follicles, previous studies have shown that IL1β induces GVBD in rabbit oocytes (Takehara et al., Reference Takehara, Dharmarajan, Faufman and Wallach1994). In equine species, in vivo intrafollicular injection of IL1β induced ovulation, but this substance was unable to induce the cortical granule migration and remodelling of mitochondria that commonly occurs during oocyte maturation (Caillaud et al., Reference Caillaud, Duchamp and Gérard2005). In addition, in vitro studies have shown that IL1β had no positive effect on equine oocyte nuclear maturation (Martoriati et al., Reference Martoriati, Caillaud, Goudet and Gérard2003). Studies have also suggested that TNFα may stimulate oocyte maturation and may represent an intraovarian mediator of the effects of LH on the maturation of fish oocytes (Crespo et al., Reference Crespo, Mañanós, Roher, MacKenzie and Planas2012). Conversely, exposure of porcine oocytes to TNFα caused a reduction in their maturation from GV stage to MII stage and increased the proportion of oocytes with abnormal chromosome alignment and cytoskeleton structure (Ma et al., Reference Ma, Yan, Qiao, Sha, Li, Chen and Sun2010). In bovine species, TNFα had an inhibitory effect on both in vitro oocyte maturation and embryo development (Jackson et al., Reference Jackson, Farin and Whisnant2012). These data show that the effects of TNFα and IL1β depend on species and the stage of oocyte development.
Our results furthermore showed that TNFα, IL1β or both did not influence the mRNA levels for GDF9, c-Mos, H1foo and Cyclin-B1 in cultured oocytes. It has been described in the literature that around 50% of the genes are transcribed and stored in the oocyte during growth (Wang et al., Reference Wang, Piotrowska, Ciemerych, Milenkovic, Scott, Davis and Zernicka-Goetz2004), which means that many thousands of different types of mRNAs are synthesized. We have investigated the effects of TNFα and IL1β on the levels of mRNAs for only four genes. Despite the fact that TNFα and IL1β have not influenced the expression of these genes, we cannot exclude the possibility that other genes, related to oocyte growth, have their expression influenced by these substances. Regarding the genes investigated, previous studies have shown that GDF-9 plays an important role during the development of follicular cells (Hussein et al., Reference Hussein, Thompson and Gilchrist2006), whereas c-Mos is important for the regulation of meiotic spindle assembly (Zhao et al., Reference Zhao, Singh and Batten1991). Moreover, H1foo is expressed during the GV stage especially and its presence is essential for oocyte maturation (Kunitomi et al., Reference Kunitomi, Yuasa, Sugiyama, Saito, Seki, Kusumoto, Kashimura, Takei, Tohyama, Hashimoto, Egashira, Tanimoto, Mizuno, Tanaka, Okuno, Yamazawa, Watanabe, Oda, Kaneda, Matsuzaki, Nagai, Okano, Yagami, Tanaka and Fukuda2016). Finally, Cyclin-B1 is a subunit that forms the MPF, responsible for the onset of nuclear maturation (Bilodeau-Goeseels, Reference Bilodeau-Goeseels2012). The absence of a positive effect of TNFα, IL1β or both on the rates of GVBD can be due to the fact that oocytes still did reach the minimum size (120 µm) to assure their competence (Otoi et al., Reference Otoi, Yamamoto, Koyama, Tachikawa and Suzuki1997).
Control oocytes from large antral follicles (>5 mm) have a predominant heterogeneous mitochondrial distribution. This distribution was also seen in pre-matured oocytes that had been grown in vitro in TCM-199 alone or supplemented with TNFα. After IVM, mitochondrial distribution remained heterogeneous only in oocytes that were cultured in TCM-199 medium that lacked cytokines. Previous studies have recently shown that mitochondria in human oocytes at the GV stage have a heterogeneous distribution but after GVBD this configuration changed rapidly to a homogeneous distribution (Takahashi et al., Reference Takahashi, Hashimoto, Yamochi, Goto, Yamanaka, Amo, Matsumoto, Inoue, Ito, Nakaoka, Suzuki and Morimoto2016). In contrast, cat oocytes either in GV or MII predominantly have mitochondria distributed in the peripheral region (Sowińska et al., Reference Sowińska, Müller, Niżański and Jewgenow2017). In sheep, migration of mitochondria to the periphery of the oocyte was observed after 6 h of IVM in COCs from follicles with a diameter of approximately 2 mm (Máximo et al., Reference Máximo, Martins Da Silva, Mondadori, Neves and Lucci2012). In general, in mammalian oocytes at the GV stage, mitochondria are found predominantly in the periphery of the cytoplasm, and with small groups scattered more to the centre of the oocyte (Hyttel et al., Reference Hyttel, Fair, Callesen and Greve1997; Sun et al., Reference Sun, Wu, Lai, Park, Cabot, Cheong, Day, Prather and Schatten2001). In oocytes at the MII stage, the mitochondria occupy a more centralized position in the cytoplasm (Hyttel et al., Reference Hyttel, Fair, Callesen and Greve1997; Sun et al., Reference Sun, Wu, Lai, Park, Cabot, Cheong, Day, Prather and Schatten2001; Adona et al., Reference Adona, Pires, Quetglas, Schwarz and Leal2008). Our results point to a homogeneous distribution after IVM of bovine oocytes treated with IL1β, TNFα or both cytokines, suggesting an important role of these compounds in the regulation of cytoplasmic maturation, and in the distribution of mitochondria especially.
In conclusion, TNFα and both TNFα and IL1β promote the in vitro oocyte growth during a 48 h culture period. However, the oocytes still did not reach the minimum size to assure oocyte competence and, therefore, this growth was not linked with an increase in the maturation rate of oocytes from small follicles. In addition, these cytokines do not influence the expression of mRNAs for GDF-9, c-Mos, cyclin B1 and H1foo in the oocyte. TNFα has, however, an effect on the distribution of mitochondria in pre-matured oocytes.
Financial support
This research was supported by a grant from the National Council for Scientific and Technological Development (CNPq, Brazil, grant no. 552376/2011–6).
Conflicts of interest
There are no conflicts of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.