Study
This study was registered at the Australian New Zealand Clinical Trials Registry (ANZCTR), the registration number of the trial was ACTRN12614001139662.
Introduction
The delivery of healthy, normal-weight babies to term, resulting in healthy children and subsequently healthy adults, is the clear goal of assisted human conception (Gardner, Reference Gardner2016).
Recent advances in the cell culture medium have led to a shift in IVF practice from early cleavage embryo transfer to blastocyst stage transfer. The rationale for blastocyst culture is to improve both uterine and embryonic synchronicity and self-selection of viable embryos, therefore resulting in higher implantation rates (Glujovsky et al., Reference Glujovsky, Farquhar, Quinteiro Retamar, Alvarez Sedo and Blake2016). Extended culture has facilitated the move to the single blastocyst transfer, resulting in significant increases in implantation and live birth rate, while concomitantly reducing fetal loss during pregnancy (Gardner, Reference Gardner2016).
However, prolonged culture can be a source of stress for the human embryo. Sources of stress on the human embryo include identified factors such as pH and temperature shifts, exposure to atmospheric (20%) oxygen, and the build-up of toxins in the medium due to the static nature of the culture (Wale & Gardner, Reference Wale and Gardner2016). To fulfil the requirements for a good blastocyst culture, an IVF laboratory should have an appropriate quality control system, frequently monitored temperature, CO2 and air quality, and exposure to light should be minimized, oil overlay used and the number of incubators must be determined based on the number of patients.
Among all variables that affect embryo development, oxygen is one of the most powerful regulators of cell/embryo function (Wale & Gardner, Reference Wale and Gardner2016). Embryonic development to the blastocyst stage can also be significantly improved by modifying the atmosphere in which the embryos are cultivated for 5 days (Kovacic & Vlaisavljevic, Reference Kovacic and Vlaisavljevic2008). By lowering atmospheric oxygen (20%) to the physiological levels (5%) present in oviduct and uterus (2–8%) (Fischer & Bavister, Reference Fischer and Bavister1993), generation of highly reactive cytotoxic oxygen radicals can be significantly reduced (Fujitani et al., Reference Fujitani, Kasai, Ohtani, Nishimura, Yamada and Utsumi1997). Reactive oxygen species (ROS) can cause organelle and membrane damage (Fujitani et al., Reference Fujitani, Kasai, Ohtani, Nishimura, Yamada and Utsumi1997; Kwon et al., Reference Kwon, Yang, Hwang, Yoo, Kim, Lee, Ryu and Oh1999), direct and indirect DNA damage (Iwata et al., Reference Iwata, Minami and Imai2000) and altered gene expression (Harvey et al., Reference Harvey, Kind, Pantaleon, Armstrong and Thompson2004).
Benchtop incubators using 5% oxygen are designed to provide an optimum culture environment and reduced embryonic stress. One of the advantages of smaller volume incubators is the speed of recovering temperature and gas parameters after opening a chamber. No study has clearly demonstrated a distinct advantage of any specific incubator type in terms of human embryo development or clinical outcomes (Swain, Reference Swain2014).
Many studies have been published that have evaluated only the effect of O2 concentration on human embryos, but with conflicting results (Waldenstrom et al., Reference Waldenstrom, Engstrom, Hellberg and Nilsson2009; Kovacic et al., Reference Kovacic, Sajko and Vlaisavljevic2010; Gomes Sobrinho et al., Reference Gomes Sobrinho, Oliveira, Petersen, Mauri, Silva, Massaro, Baruffi, Cavagna and Franco2011; de los Santos et al., Reference de los Santos, Gamiz, Albert, Galan, Viloria, Perez, Romero and Remohi2013; Nastri et al., Reference Nastri, Nobrega, Teixeira, Amorim, Diniz, Barbosa, Giorgi, Pileggi and Martins2016).
The aim of our randomized prospective study was to compare the effects of two different types of incubation systems on live birth rate (LBR): a conservative system of large volume capacity without an option for reduced oxygen and a smaller volume benchtop incubator set to 5% oxygen.
Materials and methods
The trial was formulated considering the revised CONSORT statement for reporting randomized trials (Schulz et al., Reference Schulz, Altman and Moher2010).
This prospective, randomized, opened (masking not used) controlled trial was carried out on infertile patients in stimulated cycles (both IVF and intracytoplasmic sperm injection (ICSI)) in the Department for Clinical Embryology of the University Hospital Centre, Zagreb.
Sample size and power analysis were made using computer software G*Power for Windows version 3.1.9.2 according to the relevant data form (Bontekoe et al., Reference Bontekoe, Mantikou, van Wely, Seshadri, Repping and Mastenbroek2012) that LBR could improve from 30% using atmospheric oxygen concentration to a maximum value of 43% using a low oxygen concentration. Using that estimated difference, for Fisher’s exact test with a minimal sample power of 80% and significance level of 5%, it was necessary to include at least 460 participants allocated into two same-sized groups (at least 230 participants per group).
The study was approved by the Ethics Committee of the University of Zagreb, School of Medicine as well as the Ethics Committee of the University Hospital Centre Zagreb.
In total, 738 infertile patients, aged between 22 and 42, undergoing stimulated IVF and ICSI cycles, were assessed for eligibility. Patients with severe male infertility factors such as azoospermia or cryptozoospermia, those that were assigned to freeze all oocytes or embryos, and patients that declined to participate were excluded. The main investigator (embryologist) spoke with the patients that fulfilled the requirements for the study, described the study and if they had agreed to participate, assigned them for randomization. Written informed consent was signed before oocyte pick-up. In total, 393 patients were analysed. Only one couple participated twice.
Simple randomization was made using a randomisation table created by computer software – https://www.randomizer.org/. Embryologists were aware of the type of incubator used for each patient and patients were assigned according to the list (1–460). Each patient that initially participated was assigned an ordinal number from the list, and that number was allocated to the treatment at random – 5% O2 (benchtop incubator) or 20% O2 (classic incubator).
The primary outcome of the study was live birth rate (LBR) per all transfers − defined by the number of live birth deliveries expressed per embryo transfer cycles (denominator) in which a fetus is delivered with signs of life after complete expulsion or extraction from its mother, beyond 20 completed weeks of gestational age.
Secondary outcomes were fertilization rate (FR), clinical pregnancy rate (CPR) and LBR per each transfer subgroup – day 3 transfers, day 5 single embryo transfers (SET) and day 5 double embryo transfers (DET). Clinical pregnancy rate was defined as the number of pregnancies per embryo transfer cycle (denominator) assessed by serum hCG assay and clinical (fetal heartbeat) or ultrasound parameters (ultrasound visualization of a gestational sac, embryonic pole with heartbeat). Fertilization rate was defined as the number of fertilized oocytes (assessed by visualization of two pronuclei on day 1) per number of inseminated or microinjected oocytes.
Embryological parameters were included − number of embryos on day 3, blastocysts on day 5 and number of transferred embryos.
The controlled ovarian stimulation protocol was started on day 2 or 3 of the patient’s menstrual cycle with recombinant follicle stimulating hormone (FSH) (Gonal F, Merck-Serono; or Puregon, Merck Sharp & Dohme) or hMG (Menopur, Ferring) with a daily dose of 150–300 international units (IU). The daily dose was adjusted according to the patient’s history and during stimulation due to ovarian response. Gonadotropin-releasing hormone (GnRH) antagonist cetrorelix or ganirelix (Cetrotide, Serono and Orgalutran, Merck Sharp & Dohme) at a daily dose of 0.25 mg was administrated in a fixed protocol starting on day 6 of stimulation. Administration was continued until the day of hCG triggering. Oocyte retrieval was carried out 36 h after the administration of hCG (Ovitrelle, Merck-Serono) in doses of 5000 IU or 10,000 IU.
During oocyte pick-up, the cumulus–oocyte complexes (COC) were collected in Sydney IVF Gamete Buffer (Cook Medical). The collected COCs were transferred to four-well dishes containing Origio Fertilization Medium and was immediately allocated to an incubator assigned by randomization. Cumulus–oocyte complexes were preincubated for approximately 4 h prior to insemination (IVF). For ICSI procedure, COCs were denuded with hyaluronidase 2 h after oocyte pick-up and fertilized 1 h later (Sydney IVF Hyaluronidase, Cook Medical). All four-well dishes were equilibrated overnight under the oxygen concentration of interest, containing 0.5 ml of Origio Sequential Medium (fertilization, cleavage or blastocyst) under mineral oil. The oocytes were transferred to Origio Cleavage Medium after ICSI or, for standard IVF, the next morning. On day 3 the embryos were transferred separately to drops of 200 µl Origio Sequential Medium (blastocyst) under mineral oil.
During the study, two benchtop incubators with reduced oxygen concentration (MINC™ Benchtop Incubator, Cook Medical) and one classic CO2 incubator (Heracell™ 240i CO2, Thermo Scientific) were used only for the study. The MINC incubator utilised premixed gases (5% O2, 6% CO2 and 89% N2) and the Heracell incubator a standard gas atmosphere of 6% CO2 in the air (approx. 20% O2), both with 37°C and relative humidity ≥90%. Gas concentrations as well as temperature levels were measured each morning according to our laboratory quality control procedures. In a daily routine, a maximum of six Petri dishes were incubated at the same time in the classic incubator in comparison with the benchtop incubator with a maximum of four Petri dishes. The average number of Petri dishes at the same time in both types of incubator was three.
An assessment of embryo and blastocyst morphology was made by four embryologists that were not blinded to oxygen concentration assignment due to the use of different types of incubators.
Assessment was made on day 3 and day 5, early in the morning. On day 3, patients with less than three morphologically optimal embryos were assigned for transfer. On day 5, the highest quality blastocyst was assigned for single blastocyst transfer (SBT), and the remaining blastocysts were cryopreserved using the Kitazato vitrification protocol. In some cases, patients refused single embryo transfer so two blastocysts were transferred. Also, if there were no highest quality blastocyst, patients were given a recommendation for DET. Embryo transfer was performed using the Somatex Embryo Transfer Set (Somatex® Medical Technologies).
Differences between investigated groups regarding categorical variables were assessed using Fisher’s exact test, while differences in continuous variables were analysed using the Mann−Whitney U-test. All P-values below 0.05 were considered to be statistically significant. IBM SPSS Statistics version 25.0 was used in all statistical procedures.
Results
The first participant was enrolled in March 2015 and the last in October 2016, while the follow-up (LBR) ended in August 2017.
There were 738 infertile patients assessed for eligibility. We excluded 278 patients and, in total, 460 patients were randomized – 230 allocated to the 5% O2 group and 230 to the 20% O2 group. Additional exclusions were made because there was no fertilization (59).
The main investigator stopped the trial early due to a change of cultivation medium supplier, and the remaining participants (8) were cancelled.
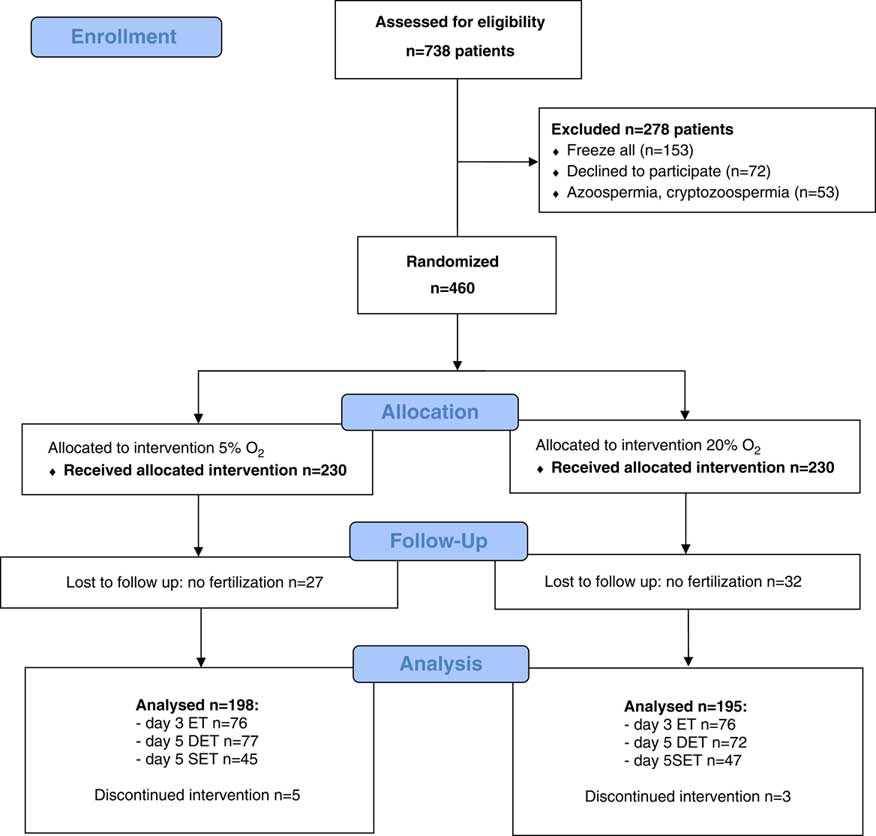
Finally, 393 patients were analysed for outcomes, 198 in the 5% O2 group and 195 in the 20% O2 group. Figure 1 shows a flow diagram of the trial with a detailed number of enrolled and randomised patients per group as well as the number of cancellations, with reasons. (Fig. 1)
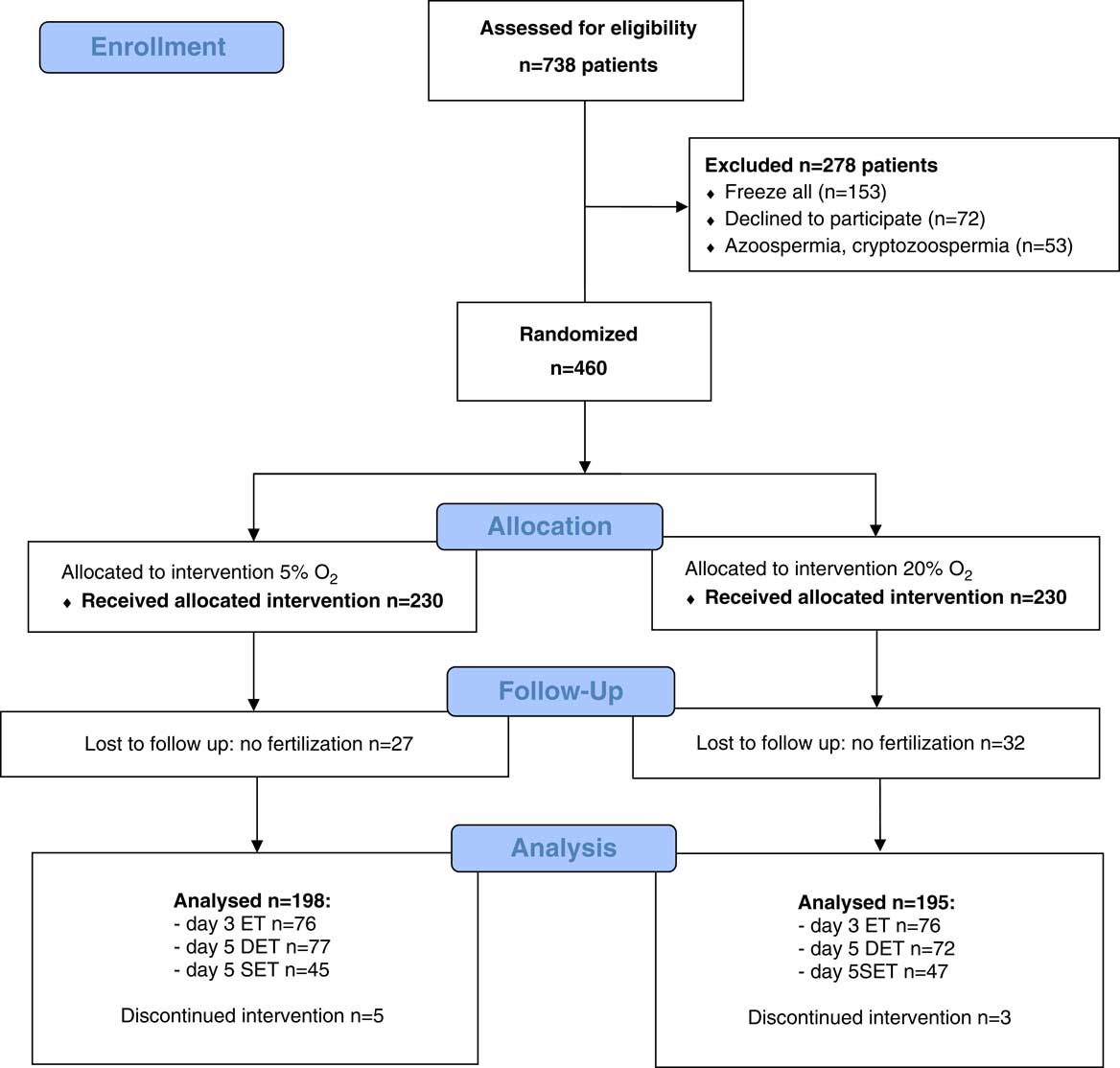
Figure 1 CONSORT flow chart.
Table 1 shows the baseline characteristics of patients in the study with the corresponding P-values between groups. Differences in the baseline characteristics between the two groups were not significant – age, body mass index (BMI), IU of gonadotrophins per cycle, oocytes in procedure per cycle as well as for fertilization method (IVF/ICSI) (Table 1).
Table 1 Baseline characteristics of patients included in the study

BMI: Body mass index; IU: International units; ICSI: Intracytoplasmic sperm injection. SD, standard deviation. P-values<0.05 are considered statistically significant.
Primary outcome – LBR per all transfers (Table 2) did not show an improvement when using the 5% oxygen group over the 20% oxygen group (25.3% versus 22.6%, P=0.531).
Table 2 Primary and secondary outcomes and comparison between the treatment groups (all transfers)

*Out of positive CPR. P-values<0.05 are considered statistically significant.
Secondary outcomes per all transfers also did not show any beneficial effect of reduced oxygen (5%) in fertilization (73.4% ± 22.4% versus 74.6% ± 24.0%, P=0.606) and clinical pregnancy rate (34.3% versus 26.7%, P=0.099). Miscarriage rate was similar (30.9% versus 28.8%, P=0.809).
Table 3 shows the comparison of the secondary outcomes between the subgroups – day 3 ET, day 5 DET and day 5 SET. Fertilization rate differed between subgroups but showed statistical significance only in the day 5 SET subgroup (85.3 ± 15.1 versus 75.1 ± 17.5; P=0.004) in favour of the 5% oxygen group. Clinical pregnancy rate showed results in favour of the 5% oxygen group in all subgroups (day 3: 23.7% versus 21.1%, P=0.701; day 5 DET: 35.0% versus 30.6%. P=0.569) but showed statistical significance only in the day 5 SET subgroup (51.1% versus 29.8%; P=0.038). Live birth rate showed results slightly in favour of the low oxygen (5%) group in all day 5 subgroups (DET: 28.6% versus 20.8%, P=0.727; SET: 28.9% versus 23.4%, P=0.550), but statistical significance was not proved. (Table 3)
Table 3 Secondary outcomes and comparison between the subgroups

DET, double embryo transfer; ET, embryo transfer; SET, single embryo transfer. P-values<0.05 are considered statistically significant.
Embryological parameters per all transfers showed that there was statistically significant higher number of blastocysts on day 5 (P=0.009) for samples placed in benchtop incubators. The number of embryos on day 3 was similar (P=0.294). (Table 4) as well as the number of transferred embryos (P=0.458).
Table 4 Embryological parameters of our study (per all transfers)

*Out of LBR. IQR- interquartile range. P-values<0.05 are considered statistically significant.
Discussion
Historically, most IVF laboratories have cultured embryos under atmospheric oxygen concentrations (20%) in large, classic incubators. Benchtop incubators with reduced oxygen (5%) require additional costs, for new equipment as well as for premixed gases. With more blastocysts and higher pregnancy rate, it is evident that costs can be justified by having surplus good embryos for additional frozen transfers, so pregnancy can be achieved from one stimulated cycle.
While existing reports indicate that smaller benchtop incubators provide faster recovery of environmental variables, there is no clear advantage of any particular incubator based on clinical outcomes (Swain, Reference Swain2014). Results of this study support such findings, while the superiority of any incubator regarding LBR, FR and CPR per all transfers was not proved.
Secondary outcomes with comparison between subgroups showed higher clinical pregnancy rates in all subgroups with statistical significance for the day 5 SET subgroup. This difference can be related to the beneficial influence of low oxygen on prolonged cultures (Bontekoe et al., Reference Bontekoe, Mantikou, van Wely, Seshadri, Repping and Mastenbroek2012; Meintjes et al., Reference Meintjes, Chantilis, Douglas, Rodriguez, Guerami, Bookout, Barnett and Madden2009; Kovacic et al., Reference Kovacic, Sajko and Vlaisavljevic2010). Also, LBRs were higher, but not significantly, in all day 5 subgroups. It is important to highlight that LBR depends not only on the embryo quality, but also on embryonic chromosomal abnormalities as well as maternal endocrine, thrombophilic and immunological disturbances that can contribute to recurrent miscarriage (Christiansen et al., Reference Christiansen, Nielsen and Kolte2006).
There was statistically a significantly higher number of blastocysts in the low oxygen group (benchtop incubator) per all transfers and this finding supports the theory that prolonged cultures under atmospheric (20%) oxygen can be a source of stress for human embryos (Wale & Gardner, Reference Wale and Gardner2016). Higher number of blastocysts give a better possibility to choose a morphologically optimal embryo for transfer, as well as to cryopreserve surplus embryos. Consequently, pregnancy rates are higher. In future studies, cumulative pregnancy rates should be included as well.
Other studies have reported different embryological outcomes regarding number and quality of embryos (Kovacic & Vlaisavljevic, Reference Kovacic and Vlaisavljevic2008; Waldenstrom et al., Reference Waldenstrom, Engstrom, Hellberg and Nilsson2009; Kovacic et al., Reference Kovacic, Sajko and Vlaisavljevic2010; de los Santos et al., Reference de los Santos, Gamiz, Albert, Galan, Viloria, Perez, Romero and Remohi2013; Nastri et al., Reference Nastri, Nobrega, Teixeira, Amorim, Diniz, Barbosa, Giorgi, Pileggi and Martins2016), but this difference was expected as designs of studies differed in their baseline parameters such as day of transfer (3/5), patient anamnesis, type and duration of infertility, cultivation medium, laboratory conditions etc.
A potential source of bias in our study could be that only participants were blinded for allocation, but embryologists were not. This caveat could be irrelevant, as our final outcome was LBR. Also, the Croatian assisted reproductive technology (ART) law does not permit fertilization of more than 12 oocytes per stimulated cycle.
Although the most important feature of the benchtop incubators was a reduced level of oxygen, other beneficial effects must be considered: rapid pH recovery, constant temperature, smaller volume with separated chambers that allowed less disturbance.
All these parameters should be strictly measured in future studies to see their direct influence on the results. It is important to define the daily patient number and workflow in order to limit overuse of any incubator. One study showed that reducing door opening from six to four times over a 6-day period could improve human blastocyst formation and good-quality blastocyst rates (Zhang et al., Reference Zhang, Li, Peng, Guo, Heng and Tong2010). Further studies should be conducted using continuous cultivation medium to avoid extra manipulation of embryos on suboptimal conditions that can influence development.
In conclusion, the use of benchtop incubators with reduced oxygen concentrations provided better results, but with no clear evidence on clinical outcomes. It is most important to optimize incubator usage in order to minimize door opening, conduct proper quality control procedures to monitor all viable parameters (temperature, CO2, O2) frequently, and preferably use low oxygen concentrations for culturing blastocysts.
Acknowledgements
The authors wish to thank all the embryologists, gynaecologists and laboratory technicians that participated in this study.
Financial support
This research received no specific grant from any funding agency, or commercial or not-for-profit sectors.
Statement of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.