Introduction
In recent decades, the maintenance of fish biodiversity in wild populations has been affected by several factors such as pollution, dam construction and predatory fishing (Winemiller et al., Reference Winemiller, McIntyre, Castello, Fluet-Chouinard, Giarrizzo, Nam, Baird, Darwall, Lujan, Harrison, Stiassny, Silvano, Fitzgerald, Pelicice, Agostinho, Gomes, Albert, Baran, Petrere, Zarfl, Mulligan, Sullivan, Arantes, Sousa, Koning, Hoeinghaus, Sabaj, Lundberg, Armbruster, Thieme, Petry, Zuanon, Torrente Vilara, Snoeks, Ou, Rainboth, Pavanelli, Akama, Van Soesbergen and Sáenz2016; Marques et al., Reference Marques, Dias, Perbiche-Neves, Kashiwaqui and Ramos2018). Due to the increasing demand for fish protein and declines in wild fish stocks, fish farming has steadily expanded in all continents (FAO, 2018).
In terms of freshwater fish farming, Brazil is among the largest fish producers in the Americas. Most species it produces are exotic, most notably Nile tilapia, Oreochromis niloticus (De Amorim et al., Reference De Amorim, Gomes, Martins, Sato, Rizzo and Bazzoli2009; Valladão et al., Reference Valladão, Gallani and Pilarski2018). To find native species with zootechnical potential for aquaculture, basic reproductive parameters, such as protocols for induced/artificial reproduction and initial development, must be determined (Arantes et al., Reference Arantes, Sato, Sampaio, Rizzo and Bazzoli2013). In addition, the induced reproduction of native species, associated with government and/or private restocking programmes, has also helped to mitigate the declines in wild populations (Reynalte-Tataje et al., Reference Reynalte-Tataje, Lopes, Ávila-Simas, de Garcia and Zaniboni-Filho2013).
The application of hormonal therapies to induce fish spawning is based on the administration of gonadotropin-releasing hormone (GnRH; Mylonas and Zohar, Reference Mylonas and Zohar2001) through a preparation containing a synthetic GnRH analogue (Sugumar and Munuswamy, Reference Sugumar and Munuswamy2006) or by the heteroplastic pituitary method, in which a commercial extract of carp (C. carpio) pituitary gland is injected into the celomic cavity or intramuscularly (Sampaio et al., Reference Sampaio, Robaldo and Bianchini2008). In Brazilian commercial fish farming, spawning induction is generally carried out through hypophysation, which is more economical than other techniques and provides good results regarding egg production, fertility and fertilization rates (Sato et al., Reference Sato, Fenerich-Verani and Godinho2003b; Saint-Paul, Reference Saint-Paul2017). As the majority of commercially important neotropical teleosts are migrants and cannot complete reproduction in captivity or in lentic environments, knowledge on induced reproduction is essential for the development of fish farming and the conservation of wild populations (Kuradomi and Batlouni, Reference Kuradomi and Batlouni2018; Sato et al., Reference Sato, Kuradomi, Calil, Jesus Silva, Abreu, Figueiredo-Ariki, Freitas and Batlouni2020)
Salminus species are distributed in several neotropical basins, being one of the main targets of professional/amateur fishers. Additionally, increasing rates of river damming and pollution threaten populations of this genus (Bayley and Petrere Jr, Reference Bayley and Petrere1989; Sato et al., Reference Sato, Fenerechi-Verani, Nuñer, Godinho, Verani, Godinho and Godinho2003a; Freitas et al., Reference Freitas, Prado, Arantes, Santiago, Sato, Bazzoli and Rizzo2013; Araújo et al., Reference Araújo, Mello, Moreira, Hilsdorf, Marques and Honji2020). Studies carried out in recent years have increased knowledge about farming of Salminus species. For example, the stress response of S. brasiliensis in handling and different stocking densities (Braun et al., Reference Braun, de Lima, Baldisserotto, Dafre and de Oliveira Nuñer2010); the influence of different food and photoperiod in post-larvae of S. brasiliensis (Schütz and Nuñer, Reference Schütz and de Oliveira2007); and embryonic development in S. hilarii (Araújo et al., Reference Araújo, Mello, Moreira, Hilsdorf, Marques and Honji2020).
S. franciscanus, popularly known as dourado, is an endemic species in the São Francisco River basin, southeastern Brazil that is ecologically important (top-down regulation) and commercially valuable. This species can reach more than 1.4 m in total length and 30 kg in mass, and has an essentially piscivorous habit. In the rainy season, when the water level, temperature and photoperiod increase, females spawn in a single batch of non-adhesive eggs after a upstream migration (Lima and Britski, Reference Lima and Britski2007; Freitas et al., Reference Freitas, Prado, Arantes, Santiago, Sato, Bazzoli and Rizzo2013; Honorato-Sampaio et al., Reference Honorato-Sampaio, Prado, Sato, Bazzoli and Rizzo2015). However, the population of S. franciscanus has been decreasing dramatically in the last years due to overfishing, habitat loss and pollution. Recently, this species was included in the National Plan for the Conservation of Threatened Fish Species of São Francisco River basin by Brazilian environmental authorities (Sato and Sampaio, Reference Sato and Sampaio2005; Coimbra et al., Reference Coimbra, Dantas, Luna, Lima, Sales, da Silva and Lima2020). Conversely, the aquaculture production of dourado is increasing in Brazil, reaching approximately 60 tonnes in 2019, although this number corresponding to only 0.02% of the total Brazilian fish production (Brasil Instituto Brasileiro de Geografia e Estatística, 2020)
Despite the great ecological importance and aquaculture potential of S. franciscanus, only a few studies regarding its reproductive biology are available in literature such as Freitas et al. (Reference Freitas, Prado, Arantes, Santiago, Sato, Bazzoli and Rizzo2013) and Honorato-Sampaio et al. (Reference Honorato-Sampaio, Prado, Sato, Bazzoli and Rizzo2015). However, none of these studies specifically addresses and describes the main morphological events of the embryological process of this species. In this sense, our study aimed to increase the knowledge of its embryogenesis and reproduction in captivity.
Material and methods
Sampling
Wild adult S. franciscanus females and males were captured using gill nets in two stretches of the São Francisco River, Minas Gerais state, Brazil: (1) immediately downstream of the Três Marias dam (18°11′S, 45°14′W); and (2) at the confluence with the Abaeté river (18°02′S, 45°11′W). Sampling occurred on the reproductive season of dourado (Freitas et al., Reference Freitas, Prado, Arantes, Santiago, Sato, Bazzoli and Rizzo2013) from October to December 2016 and captured 28 specimens (16 female, 12 male). These fish were transported and kept in 200 m2 tanks, with an average depth of 1 m and under a natural photoperiod at the Integrated Fisheries and Aquaculture Center (CODEVASF), Três Marias, Minas Gerais, Brazil (18°11′58′′S, 45°15′07′′W). All specimens were fed with commercial diet for carnivorous fish containing 40% of crude protein. This work was approved by the Ethics Committee on the Use of Animals (CEUA PUC Minas, protocol no. 021/2015).
Broodstock determination and induced reproduction
The hypophysation protocol was performed according to Sato et al. (Reference Sato, Fenerich-Verani, Verani, Godinho and Vieira1997). Eight mature females and eight mature males of S. franciscanus, with an average total length of 66.4 ± 11.1 cm for females and 58.3 ± 10.2 cm for males; and an average body weight of 4.04 ± 2.32 kg for females and 3.62 ± 1.12 kg for males, were transferred to four hypophysation tanks (3.0 m2), two for males and two for females. The selected females presented bulging bellies and dilated and vascularized urogenital papilla. The selected males released semen after light pressure on the ventral region. The water temperature of the hypophysation tanks was maintained at 26.1 ± 0.6°C throughout the procedure (Table 1).
Table 1. Mean ± standard deviation of water parameters recorded during the hypophysation protocol and egg incubation of S. franciscanus

In females, two doses (0.8 and 5.6 mg/kg body weight) of crude pituitary extract of common carp (C. carpio) were administered with a 14 h interval. For males, a single dose (2.7 mg/kg body weight) of crude pituitary extract was applied at the same time as the females’ second dose. The injections were administered in the coelomic cavity and the doses were diluted in 0.9% sodium chloride solution (considering 1.0 ml in each dose). The gametes were manually stripped 8 h after the second female dose. Only five females and five males underwent gamete extrusion. Fertilization was carried out dry, in which stripped oocytes and sperm were gently mixed in a previously sterilized plastic container. After mixing, water was added to allow the fertilization and eggs hydration. After all procedures, males and females were transferred back into the living tank (200 m2). No mortalities were registered after the hypophysation protocol.
The fertilized eggs were transferred to a funnel-type incubator of 60 L capacity and constant water circulation at 24.3 ± 0.3°C. To estimate the number of total eggs, the egg mass was weighed after extrusion and then the eggs from the subsamples were counted. The eggs were considered fertilized, i.e. viable, at blastopore closure. For that, samples of eggs were collected in the incubators to check the closure of the blastospore 8 hour after the fertilization. To calculate the fertilization rate, the following formula was used: fertilization rate (FR) = (number of viable eggs/total number eggs) × 100.
Final maturation assessment
For histological confirmation of the final maturation of the broodstock, gonad samples from three females and three males were collected 1 h after the females’ second hormonal dose. The selected specimens were anaesthetised with benzocaine solution (0.1 g/l) and euthanized by cervical spinal cord cross-section following the ethical principles established by the National Animal Experimentation Control Council (CONCEA). Fragments of ovaries and testicles were collected from each specimen and fixed in Bouin’s solution for 4 h. Then, they were embedded in paraffin, cut into 5-µm-thick sections with a microtome, and stained with haematoxylin–eosin (H&E) and Masson’s trichrome (MT).
Embryogenesis
Analysis of embryonic development started immediately after transfer of the fertilized eggs to the incubators. Egg samples were collected every 10 min and photographed under a stereomicroscope until the larvae hatched. The embryonic stage was determined when more than 50% of the eggs of subsample (10 eggs per subsample) were at the same stage.
Biometric data and water quality analysis
The total length and body weight of broodstock, water parameters and egg weight are presented as mean ± standard deviation (SD).
During the hypophysation protocol and embryogenesis, the pH, temperature (ºC) and dissolved oxygen (mg/l) of the hypophysation tanks and incubators were monitored (Table 1), every 12 h (n = 4), using a Horiba U-53 multi-probe (Horiba Advanced Technology Center Ltd, Kyoto, Japan).
Results
Final follicle maturation was identified in histological slides by the presence of vitellogenic oocytes with the nucleus displaced towards the animal pole in which the micropyle is located, 1 h after the second hormonal dose in females (Fig. 1 A, B). In males, final maturation of the testicles was indicated by the lumen of the seminiferous tubules being filled with spermatozoa embedded in abundant acidophilic secretions (Fig. 1 C, D).
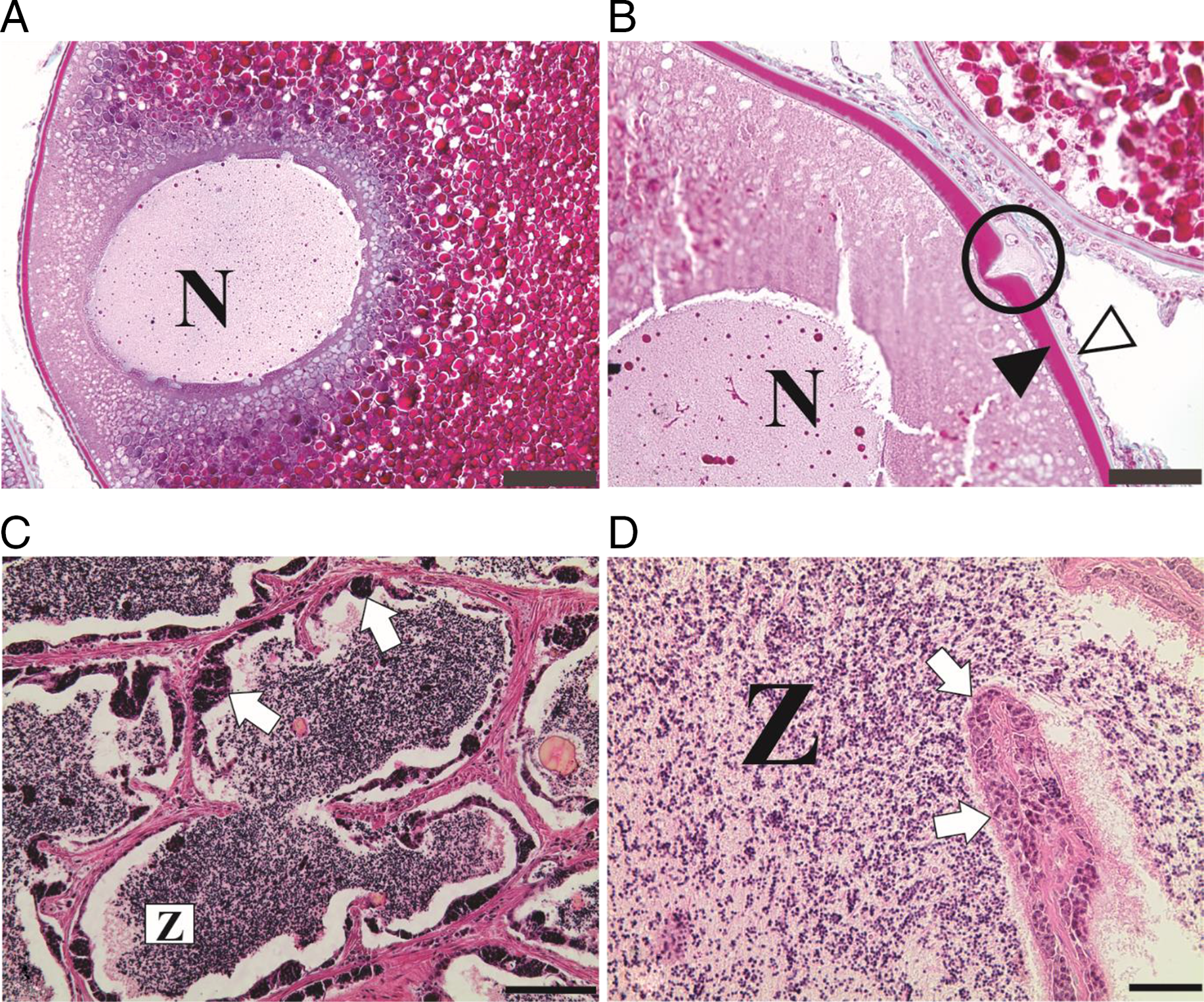
Figure 1. Cross-sections of ovaries and testicles of S. franciscanus stained in MT (A, B) and HE (C, D). (A) Nucleus (N) displaced towards the animal pole in which the micropyle is located. (B) Micropyle (circle) of vitellogenic oocyte with squamous follicular cells (white arrowhead) and thick zona radiata (black arrowhead). (C, D) Advanced maturation of testis showing a few spermatogenic cysts (white arrows) on the wall of a seminiferous tubule and lumen filled with spermatozoa (Z) embedded in acidophilic secretions. Scale bars: (A) 100 µm; (B, C) 50 µm; (D) 20 µm.
Regarding induced reproduction, three females (60%) and all males responded positively to the hypophysation protocol. The females released non-adhesive oocytes with an average mass of 385.2 ± 78.4 g of oocytes. The fertilization rate observed after the protocol was 50.4 ± 12.3%.
After dry fertilization and eggs transferred to incubators, the first cleavage was observed 40 min after fertilization, resulting in two cells (blastomeres). After that, successive divisions of the blastodisc occurred, generating 4, 8, 16 and 32 cells in 50 min, 1 h 10 min, 1 h 20 min and 1 h 40 min, respectively. Successive divisions of the blastodisc generated a high cell mass, i.e. high blastula, 4 h after fertilization. In 5 h 30 min, the flattening of blastodisc occurred, beginning the gastrula stage, characterized by the start of the morphogenetic movements called ‘epiboly’, in which the cells undergo rapid mitotic divisions. The end of the gastrula stage was determined at the blastopore closure and occurred in 7 h 30 min after fertilization. This morphological event was characterized by the end of the epiboly movement in which the yolk sac is completely surrounded by blastoderm cells.
The organogenesis stage started right after the blastopore closure and was typified by the origin of rudimentary organs and systems from embryonic layers. After 10 h the differentiation of endoderm, mesoderm and ectoderm layers was detected and early formation of the optic vesicle was observed. A distinction between cranial and caudal regions was observed after 12 h 20 min. At this time, it was also possible to observe the somites and yolk sac.
After 15 h of incubation, the beginning of the pre-larval stage was noted, this is characterized by the release of the tail from the yolk sac and the embryonic fin was detected. In this phase, spasmodic movements were observed that increased as long as the embryo developed. The hatching, characterized by chorion rupture and presence of free-swimming larvae, occurred 20 h after fertilization at 24.3 ± 0.3°C. The main stages of embryogenesis and the respective morphological events are detailed in Table 2 and Fig. 2.
Table 2. Embryonic phases and the respective morphological events recorded during the embryogenesis of S. franciscanus at 24°C

TPF, Time post-fertilization.

Figure 2. Timeline of the main morphological events recorded during the embryonic development of S. franciscanus after induced reproduction at the Integrated Fisheries and Aquaculture Center (CODEVASF), Três Marias, Minas Gerais, Brazil. HPF, hours post-fertilization.
Discussion
The data obtained in the present study describe the main morphological events of the embryogenesis process and show results after using a hypophysation protocol for artificial reproduction in the dourado fish, S. franciscanus.
As observed in our data, the displacement of the nucleus towards the micropyle at the animal pole, 1 h after the females’ second dose, i.e. 15 h after the females’ first dose, was consistent with the study by Arantes et al. (Reference Arantes, Santos, Rizzo, Sato and Bazzoli2011), who observed the displacement of the nucleus towards the micropyle 14 h after a females’ first dose in other migratory Characid fish, Prochilodus argenteus. This nuclear migration towards the micropyle before fertilization is a typical event in the final oocyte maturation process of female teleosts (Lubzens et al., Reference Lubzens, Young, Bobe and Cerdà2010).
The acidophilic secretion detected in the lumen of the seminiferous tubules of the mature testicles was also observed in other Salminus species, such S. franciscanus (Freitas et al., Reference Freitas, Prado, Arantes, Santiago, Sato, Bazzoli and Rizzo2013), S. brasiliensis (Machado, Reference Machado2003) and S. hilarii (Honji et al., Reference Honji, Amaral, Borella, Batlouni and Moreira2020). This secretion has been also detected in the testicles of other neotropical Characiforms, including Brycon orthotaenia (Gonçalves et al., Reference Gonçalves, Bazzoli and Brito2006), and Astyanax bimaculatus and Astyanax fasciatus (Martins et al., Reference Martins, Arantes, Sato, dos Santos, Rizzo and Bazzoli2012). Isaac-Junior (Reference Isaac-Junior1999) analyzing the acidophilic secretion in S. brasiliensis detected the presence of neutral glycoproteins. In general, testicular secretions in fish are comprised of monosaccharides, polysaccharides, mucopolysaccharides, enzymes and lipids. The exact function of this secretion remains unclear, but some authors suggest that it may be related to sperm maturation and motility, and act to increase the integrity and viability of sperm (Lahnsteiner, Reference Lahnsteiner2003; Chowdhury and Joy, Reference Chowdhury and Joy2007).
The hypophysation is one of the most applied methods in freshwater fish farming and is widely used in South America. This method followed by the extrusion of the gametes through hand-stripping can be affected by several factors such as appropriate time and correct doses in hormonal administration (Dou et al., Reference Dou, Yamada, Okamura, Shinoda, Tanaka and Tsukamoto2008), stress level in broodstock handling (Gennotte et al., Reference Gennotte, Sawadogo, Milla, Kestemont, Mélard and Rougeot2012; Zanoni et al., Reference Zanoni, Costa, de Carvalho and Seiva2016) and appropriate water parameters to the biology of species (Arantes et al., Reference Arantes, Santos, Rizzo, Sato and Bazzoli2011). Honji et al. (Reference Honji, Mello, Araújo, Rodrigues-Filho, Hilsdorf and Moreira2011) reported that hypophysation-induced reproduction failure in S. hilarii may have occurred due to alterations in sperm/oocyte quality during the hand-stripping process. Conversely, it was successful for Weingartner and Zaniboni-Filho (Reference Weingartner, Zaniboni-Filho, Baldisseroto and Gomes2005) using the same protocol in S. brasiliensis.
In our study, hypophysation followed by hand-stripping gametes of S. franciscanus showed a satisfactory result and the fertilization rate. This result was similar to those recorded by Sato et al. (Reference Sato, Fenerich-Verani and Godinho2003b), and slightly lower than observed by Araújo et al. (Reference Araújo, Mello, Moreira, Hilsdorf, Marques and Honji2020) in S. hilarii. In comparative studies between hand-stripping and semi-natural methods (specialized tanks that simulate lotic conditions and without fish handling) carried out with other neotropical species, higher rates of fertilization and survival were observed with semi-natural methods (David et al., Reference David, Betina and Juan2002; Zanoni et al., Reference Zanoni, Costa, de Carvalho and Seiva2016, Reference Zanoni, Carvalho, Costa and Seiva2019; Araújo et al., Reference Araújo, Mello, Moreira, Hilsdorf, Marques and Honji2020). However, despite being more efficient in this regard, semi-natural methods are more expensive and require specialized apparatus, making them unsuitable in many situations. According to Reynalte-Tataje et al. (Reference Reynalte-Tataje, Lopes, Ávila-Simas, de Garcia and Zaniboni-Filho2013), both methods are efficient in inducing the reproduction of neotropical species in captivity, however the extrusion method may be more suitable for commercial-scale production due to its practicality and costs. It is important to emphasize that the continuity of studies aiming at new techniques and improvement of existing protocols are important for the development of fish farming and conservation.
Regarding the embryonic development of S. franciscanus, the main morphological events recorded were similar to those of the majority of teleost fish in the São Francisco River basin (Sato et al., Reference Sato, Fenerechi-Verani, Nuñer, Godinho, Verani, Godinho and Godinho2003a) and with a congeneric species S. brasiliensis (de Jesus et al., Reference de Jesus, Chehade, Costa and Borella2014) and S. hilarii (Araújo et al., Reference Araújo, Mello, Moreira, Hilsdorf, Marques and Honji2020). The initial cleavage stage is characterized by successive cell divisions, resulting in a blastomere volume increase (Marques et al., Reference Marques, Nakaghi, Faustino, Ganeco and Senhorini2008). In S. franciscanus the cleavage followed the meroblastic (partial) pattern, being restricted to the animal pole, as is commonly reported in most teleosts (Gomes et al., Reference Gomes, Sato, Rizzo and Bazzoli2013; Isaú et al., Reference Isaú, Rizzo, Amaral, Mourad and Viveiros2013; Oliveira-Almeida et al., Reference Oliveira-Almeida, Buzollo, Costa, Veríssimo-Silveira, Porto-Foresti and Ninhaus-Silveira2015). The gastrula stage begins when the first epiboly movements begins and ends when the blastopore is closed by the blastoderm. The blastopore closure is considered an important morphological event because indicates that fertilization was successful (da Rocha Perini et al., Reference da Rocha Perini, Sato, Rizzo and Bazzoli2010; Buzollo et al., Reference Buzollo, Veríssimo-Silveira, Oliveira-Almeida, Alexandre, Okuda and Ninhaus-Silveira2011), In present study it occurred at 7 h 30 min after fertilization at a water temperature of 24°C. For other migratory neotropical fish, such as Pseudoplatystoma corruscans, this stage occurred in 6 h 30 min at 25–26°C (Cardoso et al., Reference Cardoso, Alves, Ferreira and Godinho1995); and Leiarius marmoratus in which occurred in 6–7 h at 28°C (Oliveira-Almeida et al., Reference Oliveira-Almeida, Buzollo, Costa, Veríssimo-Silveira, Porto-Foresti and Ninhaus-Silveira2015). This difference is probably due to the higher water temperature as stated by Woynarovich (Reference Woynarovich1980) and Hansen and Falk-Petersen (Reference Hansen and Falk-Petersen2002), who reported that a higher water temperature accelerated the transformations that occur during early embryonic development. Conversely, even incubated at a higher temperature, S. hilarii presented the gastrula stage longer than S. franciscanus, presenting blastospore closure in 8 h 31 min at 26.2°C (Araújo et al., Reference Araújo, Mello, Moreira, Hilsdorf, Marques and Honji2020).
Organogenesis is evidenced by the differentiation of the embryonic layers, somitogenesis and the formation of the caudal cephalic corporeal axis (Morrison et al., Reference Morrison, Miyake and Wright2001). During the segmentation period, differentiation of the optical vesicle and neural tube was also detected, as noted in the present study and reported for other species (De Amorim et al., Reference De Amorim, Gomes, Martins, Sato, Rizzo and Bazzoli2009; da Rocha Perini et al., Reference da Rocha Perini, Sato, Rizzo and Bazzoli2010; Weber et al., Reference Weber, Arantes, Sato, Rizzo and Bazzoli2013; Honorato-Sampaio et al., Reference Honorato-Sampaio, Prado, Sato, Bazzoli and Rizzo2015). Finally, the hatching of newly formed larvae of S. franciscanus occurred 20 h after fertilization, while in S. brasiliensis it lasted 18 h 0 min at 23.4°C (Nakaghi et al., Reference Nakaghi, Marques, Faustino and Senhorini2006) and 21 h 17 min at 26°C in S. hilarii (Araújo et al., Reference Araújo, Mello, Moreira, Hilsdorf, Marques and Honji2020). In general, migratory fish show faster embryogenesis than non-migratory fish, for example Hoplias spp. (about 50 h post-fertilization) and Franciscodoras marmoratus (about 47 h post-fertilization) (Godinho et al., Reference Godinho, Lamas and Godinho2009; Rizzo and Bazzoli, Reference Rizzo and Bazzoli2020). The duration of S. franciscanus embryogenesis detected in the present study, i.e. 20 h at 24°C, corroborated other reports on neotropical species that have non-adhesive eggs, high fecundity and no parental care (Ninhaus-Silveira et al., Reference Ninhaus-Silveira, Foresti and De Azevedo2006; Freitas et al., Reference Freitas, Prado, Arantes, Santiago, Sato, Bazzoli and Rizzo2013; Gomes et al., Reference Gomes, Sato, Rizzo and Bazzoli2013; Isaú et al., Reference Isaú, Rizzo, Amaral, Mourad and Viveiros2013; Honorato-Sampaio et al., Reference Honorato-Sampaio, Prado, Sato, Bazzoli and Rizzo2015).
In conclusion, the present study shows that hypophysation can be used effectively to induce the final maturation of S. franciscanus in captivity. The broodstock responded positively to the induction, resulting in a good fertilization rate and normal embryonic development. In general, the results of this study improve the understanding of the reproductive biology of dourado and are useful to increase knowledge regarding fish farming of native species in the neotropical region.
As stated before, populations of the genus Salminus are negatively impacted by exploratory fishing and river pollution throughout South America (Barzotto and Mateus, Reference Barzotto and Mateus2017; Araújo et al., Reference Araújo, Mello, Moreira, Hilsdorf, Marques and Honji2020), and the use of induced reproduction techniques can be considered an alternative and sustainable way to meet commercial demands and, consequently, reduce the exploitation of wild populations. The continued development of induced reproduction techniques, especially for native species, is essential for fish farming expansion and conservation (Reynalte-Tataje et al., Reference Reynalte-Tataje, Lopes, Ávila-Simas, de Garcia and Zaniboni-Filho2013). Synthetic hormones and other types of artificial induction methods must continue to be tested in different species to reduce the stress of fish and operational costs and, finally, increase egg production and fertilization rates.
Acknowledgements
The authors would like to thank Rogério Matos for mounting the histological slides and CODEVASF and PUC Minas for their logistical support.
Financial support
This work had the financial support of the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) under grant finance code-001; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) under grants 306946/2016-5 and 407719/2016-4; and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) under grant APQ03232-15.
Statement of interest
None
Ethical standards
Not applicable
Data availability statement
Data will be made available on request






