Introduction
Manganese (Mn) is a trace element present in forages and cereals, and its concentration depends on soil status (Underwood & Suttle, Reference Underwood and Suttle1999). In cattle, Mn is absorbed by the small intestine and is bound to plasma transferrin (Davidsson et al. Reference Davidsson, Lonnerdal, Sandstrom, Kunz and Keen1989; Forrest Reference Forrest, Shils, Olson and Shike1993; Keen et al. Reference Keen, Ensunsa, Lönnerdal, Zidenberg-Cherr and Caballero2009). Manganese is an essential trace metal present in all tissues and mammalian cells (Aschner & Aschner, Reference Aschner and Aschner2005). This element is involved in several functions including activation of enzymes such as hydrolases, decarboxylases, transferases and kinases. Manganese is also a constituent of several metalloenzymes (Forrest, Reference Forrest, Shils, Olson and Shike1993; Keen et al., Reference Keen, Ensunsa, Lönnerdal, Zidenberg-Cherr and Caballero2009). Manganese plays an important role in protecting mammalian cells from DNA damage by preventing oxidative damage and by down-regulating apoptosis activation (Zidenberg-Cherr et al., Reference Zidenberg-Cherr, Keen, Lönnerdal and Hurley1983; Schrantz et al., Reference Schrantz, Blanchard, Mitenne, Auffredou, Vazquez and Leca1999; Holley et al., Reference Holley, Bakthavatchalu, Velez-Roman and St Clair2011; Anchordoquy et al., Reference Anchordoquy, Anchordoquy, Sirini, Mattioli, Picco and Furnus2013). It has been demonstrated that adequate dietary intake of Mn is required for normal reproductive performance in cattle (Bentley & Phillips, Reference Bentley and Phillips1951; Rojas et al., Reference Rojas, Dyer and Cassatt1965). The mode of action by which Mn deficiency may impair reproduction has not been elucidated (Hansen et al., Reference Hansen, Spears, Lloyd and Whisnant2006).
The environment wherein the cumulus–oocyte complex (COC) is exposed during in vivo and in vitro maturation (IVM) affects oocyte developmental competence (Sutton-McDowall et al., Reference Sutton-McDowall, Gilchrist and Thompson2010). During maturation, the mammalian oocyte is surrounded by numerous layers of cumulus cells (CC). Cumulus cells have distinctive transzonal cytoplasmic processes (TZP) passing through the oolemma and the zona pellucida. Gap junctions at the ends of these TZP allow the transfer of nutrients, factors, ions and amino acids, between oocyte and cumulus cells (Eppig, Reference Eppig1982; Larsen & Wert, Reference Larsen and Wert1988; Larsen, Reference Larsen, Sperelakis and Cole1989).
The communication between cumulus cells and oocyte by gap junctions are not indispensable for nuclear maturation, however they play an important role in cytoplasmic maturation and subsequent embryo development (Chian et al., Reference Chian, Niwa and Sirard1994; Kim et al., Reference Kim, Minami, Yamada and Utsumi1996). For this reason, one of the most commonly used oocyte selection criteria for IVM is COC morphology, in particular the cumulus vestment. Sutton and colleagues (Reference Sutton, Gilchrist and Thompson2003) determined that the number of cumulus layers and their degree of compaction are associated with an improvement in developmental outcome when comparing oocytes surrounded by compromised vestments and denuded oocytes (Shioya et al., Reference Shioya, Kuwayama, Fukushima and Iwasaki1988; Madison et al., Reference Madison, Avery and Greve1992; Lonergan et al., Reference Lonergan, Monaghan, Rizos, Boland and Gordon1994; Goud et al., Reference Goud, Goud, Qian, Laverge, Van der Elst, De Sutter and Dhont1998; Sutton et al., Reference Sutton, Gilchrist and Thompson2003).
In previous studies, we have demonstrated that supplementation of Mn in IVM medium improves developmental competence of cattle oocytes up to the blastocyst stage (Anchordoquy et al., Reference Anchordoquy, Anchordoquy, Sirini, Mattioli, Picco and Furnus2013). Furthermore, Mn reduces DNA damage in cumulus cells, and increases GSH content in both oocyte and cumulus cells (Anchordoquy et al., Reference Anchordoquy, Anchordoquy, Sirini, Mattioli, Picco and Furnus2013).
The aim of this study was to determine whether Mn influences bovine cumulus–oocyte complex metabolism and cytoplasmic maturation. For this purpose, experiments were designed to evaluate the effect of different Mn concentrations added to IVM medium on apoptosis, superoxide dismutase activity, and expansion of cumulus mass. The role of cumulus cells in the transport of Mn during in vitro maturation was also evaluated with regards to oocyte developmental capacity.
Materials and methods
Reagents and media
All reagents for media preparation were purchased from Sigma Chemical Co. (St. Louis, MO, USA), whereas follicle stimulating hormone (FSH) was purchased from Bioniche (Belleville, Ontario, Canada). The maturation medium was bicarbonate-buffered TCM-199 medium supplemented with 5% (v/v) fetal calf serum (FCS), 0.2 mM sodium pyruvate, 1 mM glutamine, 10 mg/ml luteinizing hormone (LH) (NIHoLH-S1), 1 mg/ml FSH, 1 mg/ml 17β-estradiol, and 50 mg/ml kanamycin (Furnus et al., Reference Furnus, de Matos and Moses1998). Standard manganese sulphate water solution was purchased from Merke (Tokyo, Japan). The fertilization medium consisted of Tyrode's albumin lactate pyruvate (TALP) supplemented with 6 mg/ml bovine serum albumin (BSA)-fatty acid free, 20 mM penicillamine, 10 mM hypotaurine, and 10 mg/ml heparin sulfate. The composition of TALP has been described previously by Parrish et al. (Reference Parrish, Susko-Parrish, Leibfried-Rutledge, Critser, Eyestone and First1986). The culture medium for embryo development consisted of modified synthetic oviduct fluid (SOFm), composed of SOF (Tervit et al., Reference Tervit, Whittingham and Rowson1972) supplemented with 1 mM glutamine, 2% (v/v) BME–essential amino acids, 1% (v/v) MEM–non-essential amino acids, and 4 mg/ml fatty acid free BSA (274–276 mOsm/kg) (Gardner et al., Reference Gardner, Lane, Spitzer and Batt1994).
Oocytes
Bovine ovaries were obtained from an abattoir and transported to the laboratory in sterile NaCl solution (9 g/L) with antibiotics (streptomycin and penicillin) at 37°C within 3 h of slaughter. Ovaries were pooled, regardless of stage of the estrus cycle of the donor. Cumulus–oocyte complexes were aspirated from 3–8 mm follicles, using an 18G needle connected to a sterile syringe. Only cumulus-intact complexes with evenly granulated cytoplasm were selected, using a low-power (×20–30 magnification) stereomicroscope, for IVM. Replicates (3–5) were performed on different days, with a separate batch of COC for each day.
In vitro maturation (IVM)
Cumulus–oocyte complexes were washed twice in TCM-199 buffered with 15 mM HEPES, and twice in IVM medium. Groups of 10 COC were transferred into 50 μl of IVM medium under mineral oil (Squibb, Princeton, NJ, USA) pre-equilibrated in a CO2 incubator. The incubations were performed at 39°C in an atmosphere of 5% CO2 in air with saturated humidity for 24 h. In a preliminary experiment, the presence of polar body + metaphase II (PB + MII) plate was evaluated in sampled oocytes from treatments and IVM medium alone with Hoechst 33342 after 24 h of in vitro maturation. In a previous study, we determined that 6 ng/ml Mn was the adequate concentration for in vitro maturation of bovine oocytes (Anchordoquy et al., Reference Anchordoquy, Anchordoquy, Sirini, Mattioli, Picco and Furnus2013).
Culture of cumulus cells
Cumulus cell monolayers (CC) were prepared by pipetting COC with a narrow bore pipette. The oocytes were discarded and cumulus mass were vigorously pipetted to allow separation. Cumulus cells were counted in a hemocytometer chamber, and aliquots of the cell suspension (0.5 ml, 1 × 106 cells/ml in IVM medium) were placed in a four-well plate under mineral oil, at 39ºC in an atmosphere of 5% CO2 in air with saturated humidity. The medium was changed every 48 h until 70–80% confluency was achieved, which was normally attained within 4–5 days. The percentage of live cells, evaluated by vital stain with trypan blue, was over 80% at the beginning of the culture.
Apoptosis by annexin V staining assay
Annexin V is a calcium-dependent phospholipid binding protein with high affinity for phosphatidylserine (PS) (Glander & Schaller Reference Glander and Schaller1999; Paasch et al., Reference Paasch, Sharma, Gupta, Grunewald, Mascha, Thomas, Glander and Agarwal2004). Early apoptosis was evaluated by membrane redistribution of PS with the annexin-V–FLUOS Staining Kit (Roche, Cat # 11–858–777–001). The assay involves simultaneous staining with both annexin-V–FLUOS (green) and the DNA stain propidium iodide (PI, red). Intact cells exclude PI and annexin-V–FLUOS. The apoptotic cells are visible as green and can be differentiated from necrotic cells by PI staining. Necrotic cells take up PI and stain orange/green, while apoptotic cells stain green only. Briefly, at the end of IVM, oocytes were stripped of surrounding cumulus cells as described above, washed twice with PBS and centrifuged at 200 g for 5 min. Then the pellet was resuspended in 100 μl of annexin-V–FLUOS labelling solution (annexin V + fluorescein, HEPES buffer and PI), and incubated in the dark 10 to 15 min at 15–25ºC. In total, 200 cells were analyzed under a fluorescence microscope per treatment.
Cumulus expansion
After IVM, cumulus expansion was measured in each COC using a computerized image-digitizing system with Image ProPlus® 3.1 which allows measurement of irregular areas. The system units were transformed to μm2 by calibration with a Maklert chamber. For comparison, each COC area was measured before IVM.
Superoxide dismutase activity
Cumulus–oocyte complexes (n = 400) were frozen and thawed twice in distilled water and then centrifuged at 10,000 g for 20 min at 4ºC. Supernatants were used to determine SOD activity with the RANSOD kit (Randox, USA). Superoxide radical produced in the incubation medium from xanthine oxidase reacts with INT [2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride] producing red formazan. This coloured compound was measured by an spectrophotometer at 505 nm. One SOD unit causes 50% inhibition in the INT reduction. The amount of chromogen inhibition was proportional to the SOD activity present in the sample.
In vitro fertilization (IVF)
Oocytes were washed twice in HEPES-TALP supplemented with 3 mg/ml BSA-fatty acid free (BSA-FAF) and placed into 50 μl drops of IVF medium under mineral oil. In all experiments, frozen semen from the same bull and batch was used. Three straws, each containing 40 × 106 spermatozoa, were thawed in a 37°C water bath. Spermatozoa were washed in a discontinuous Percoll gradient prepared by depositing 2 ml of 90% Percoll under 2 ml of 45% Percoll in a 15-ml centrifuge tube. Semen samples were deposited on the top of the Percoll gradient and centrifuged for 20 min at 500 g. The pellet was removed and resuspended in 300 μL of HEPES-TALP solution and centrifuged at 300 g for 10 min. After removal of the supernatant, spermatozoa were resuspended in IVF medium, counted in a hemocytometer chamber, and further diluted. The final sperm concentration in IVF was 2 × 106 sperm/ml. After dilution, semen viability was evaluated using Sperm VitalStain™ (Nidacon, Mölndal, Sweden). Viability was always above 79%. Incubations were conducted at 39 °C in 5% CO2 in air with saturated humidity for 24 h.
In vitro culture (IVC)
After IVF, presumptive zygotes were washed twice in HEPES-SOF, and then cultured in SOFm. Embryo culture was carried out in 40 μL drops of medium under mineral oil (10 presumptive zygotes per drop) at 39°C in an atmosphere of 7% O2, 5% CO2, and 88% N2 with saturated humidity. All embryos were cultured in the absence of glucose during the first 24 h, and further cultured for 7 days in the presence of 1.5 mM glucose. The medium was changed every 48 h, and the embryos were incubated for 8 days (day 0 = day of fertilization). At the end of incubation, the embryos were evaluated for the morphological stages of development with an inverted microscope (Diaphot, Nikon, Tokyo, Japan).
Blastocyst staining for total cell number
Day 8 blastocysts were fixed in 4% formaldehyde after washing three times in 1% polyvinylpyrrolidone (PVP) in PBS overnight. Embryos were placed in 1% Triton X-100 overnight, stained with Hoechst 33342, and mounted on slides and covered with a coverslip. The total cell numbers of blastocysts (8 days, Grade 1) from the groups of Experiment 4 were determined by counting the number of nuclei under an epifluorescence microscope. Total cell numbers of blastocysts were visualized by a Nikon Optiphot epifluorescent microscope with a ×40 magnification fluor objective (Nikon, Tokyo, Japan) equipped with a 365 nm excitation filter, a 400 nm barrier filter, and a 400 nm emission filter.
Experimental design
Effect of manganese on apoptosis of cumulus cells
In Experiment 1, the effect of adding 0 (Control) and 6 ng/ml Mn to maturation medium on apoptosis of cumulus cells was evaluated. The COC were matured for 24 h (as described above), and apoptosis were evaluated (described in section: Apoptosis by annexin V staining assay). For this purpose, 400 COC were matured in four replicates: 200 COC per treatment.
Effect of manganese on cumulus expansion
In Experiment 2, the effect of Mn on cumulus expansion following the addition of 0 (Control) or 6 ng/ml Mn to IVM medium was measured by a computerized image-digitizing system. The COC were matured individually for 24 h, and cumulus expansion were measured either before or after IVM (described in section: Cumulus expansion). For this purpose, 120 COC were matured in four replicates: 60 COC per treatment.
Manganese and superoxide dismutase activity
In Experiment 3, the addition of 0 (Control) or 6 ng/ml Mn to IVM medium was evaluated on SOD activity (section: Superoxide dismutase activity) after 24 h of in vitro maturation. For this purpose, 400 COC were matured in four replicates: 200 COC per treatment.
Role of cumulus cells in the transport of manganese during in vitro maturation
In Experiment 4, 1200 oocytes were matured in vitro in four replicates without Mn (Control) or with 6 ng/ml Mn in three maturation systems: (i) intact cumulus–oocyte complex (COC; n = 400); (ii) denuded oocytes with cumulus cell monolayer (DO+CC; n = 400); and (iii) denuded oocytes (DO, n = 400). Denuded oocytes (DO) were obtained by pipetting COC with a narrow-bore pipette when was required for the experimental design. Cleavage rates were recorded 48 h after insemination. Data reported for development to the blastocyst stage included embryos that progressed to the expanded or hatched blastocyst stages. The total cell number of blastocysts (day 8) was determined by counting the number of nuclei in the ICM cells and in the trophectoderm cells by a differential stain method under an epifluorescent microscope (Experiment 5). Total cell numbers of blastocysts were evaluated on 72 embryos, 12 embryos per treatment obtained in Experiment 4.
Statistical analysis
Completely randomized block designs were used and statistical models included the random effects of block (n = 3–5 depending on experiment) and the fixed effect of treatment Control (0 ng/ml Mn) versus 6 ng/ml Mn. Continuous response variables such as area of cumulus and SOD activity were analyzed with linear models by using the MIXED procedure of SAS (SAS Institute, Cary, NC, USA). Cumulus area before IVM (T0) was used as covariate in the analysis of cumulus expansion. Apoptosis (%) was analyzed by logistic regression using GENMOD procedure (SAS Institute, Cary, NC, USA). Cleavage and blastocysts percentages were analyzed by a randomized block design with a 2 × 3 factorial arrangement. The statistical models included the random effects of block (n = 4) and the fixed effects of treatment: Control (0 ng/ml Mn) versus 6 ng/ml Mn, maturation system (COC versus DO + CC versus DO) and their second order interaction. Cells number per embryo was analyzed by Poisson regression using the GENMOD procedure (SAS Institute, Cary, NC, USA) with Poisson distribution and log link. Data for cumulus expansion, SOD and cell number per blastocyst were expressed as least squares means (LSM) ± standard error of the mean (SEM). Apoptosis, cleavage and blastocyst rates were expressed as percentage. Statistical significance was set at P < 0.05, and at P < 0.10 for interactions.
Results
Effect of manganese on apoptosis of cumulus cells
In Experiment 1, cumulus cells (CC) from COC treated with Mn were evaluated for early apoptosis by annexin V–FLUOS (Fig. 1). The percentage of apoptotic CC was higher in COC matured without Mn (Control) than in CC matured with the addition of Mn. Early apoptosis was 7.03 and 3.67% for COC exposed to 0 (Control) and 6 ng/ml Mn respectively (P < 0.01).

Figure 1 Apoptosis evaluated by membrane redistribution of phosphatidylserine with annexin staining in cumulus cells from COC matured with different manganese concentrations. a,bBars with different letters differ significantly (P < 0.05). Bovine COCs were matured in IVM medium alone (Control: 0 ng/ml Mn) and 6 ng/ml Mn. Cumulus cells were evaluated for early apoptosis by annexin V–FLUOS.
Effect of manganese on cumulus expansion
In Experiment 2, cumulus expansion did not show significantly differences in COC treated with 0 (Control) and 6 ng/ml Mn during IVM (Table 1). No differences were found in cumulus cell number per COC either before or after IVM (before IVM: 12800 ± 1235; after IVM: 15000 ± 1100 and 15200 ± 1245 cumulus cells/COC for Control and 6 ng/ml Mn, respectively). In all experiments performed, there were no differences in percentages of nuclear maturation (89–96%) evaluated by Hoechst 33342 stain.
Table 1 Cumulus expansion with different manganese concentrations in IVM medium
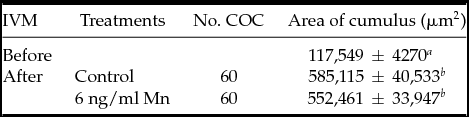
Data are expressed as least square means ± standard error of the mean (LSM ± SEM).
a ,b Values with different superscripts within each column differ (P < 0.05); 120 COC in four replicates on different days.
Effect of manganese on SOD activity
In Experiment 3, SOD activity was significantly higher in COC matured with 6 ng/ml Mn (5.5 ± 0.53 × 10−3 units/COC) than in Control (4.1 ± 0.42 × 10−3 units/COC) (P < 0.05; Fig. 2).

Figure 2 Superoxide dismutase activity in cumulus–oocyte complexes matured with or without manganese supplementation. a,bBars with different letters differ statistically (P < 0.05). SOD activity (units/COC) is expressed as least squares means (LSM) ± standard error of the mean (SEM) (400 COC in four replicates). Cumulus–oocytes cell complexes were matured in IVM medium alone (Control: 0 ng/ml Mn) or with 6 ng/ml Mn.
Effect of cumulus cells during IVM, in the presence of Mn, on the developmental capacity of oocytes and embryo quality
In Experiment 4, oocytes were in vitro matured with or without Mn supplementation to IVM medium. Cleavage and blastocyst rates were recorded after IVM in three maturation systems: (1) intact cumulus–oocyte complexes (COC); (2) denuded oocytes with cumulus cells monolayer (DO + CC); and (3) denuded oocytes (DO). No interaction was found between Mn (0 ng/ml: Control and 6 ng/ml Mn) and the maturation systems (COC, DO + CC and DO) when developmental capacity of oocytes was evaluated. Cleavage rates were significantly lower in DO maturated with or without Mn (Control: 52.9% and 6 ng/ml Mn: 55.1%; P < 0.01) than in COC (Control: 68.8% and 6 ng/ml Mn: 73.2%) and DO + CC (Control: 67.1% and 6 ng/ml Mn: 70.8%): No differences were found between COC and DO + CC (Fig. 3). In addition, blastocyst rates were significantly higher in COC (Control: 22.3% and 6 ng/ml Mn: 35%) than in DO + CC (Control: 15.8% and 6 ng/ml Mn: 25.7%; P < 0.01), and DO (Control: 6% and 6 ng/ml Mn: 12.8%; P < 0.01) (Fig. 3). Independently of the presence of cumulus cells (COC, DO + CC or DO) the blastocyst rates were higher when 6 ng/ml Mn was added to IVM medium compared to medium alone (P < 0.01) (Fig. 3).
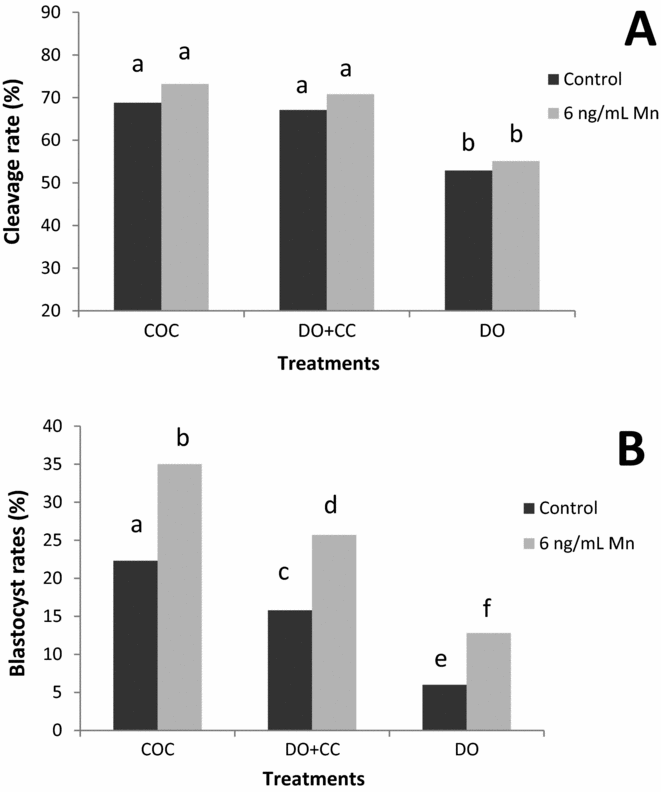
Figure 3 Role of cumulus cells during IVM on the developmental capacity of oocytes matured with or without manganese. (A) a, bIndicates significant differences (P < 0.01). (B) a–fIndicates significant differences (P < 0.01). Cleavage rates were recorded 48 h after insemination. Data reported for development to the blastocyst stage included those embryos that progressed to the expanded or hatched blastocyst stages after 8 days in culture. All values for cleavage and development rates are expressed as percentage (COC, n = 400; DO + CC, n = 400; and DO, n = 400 in four replicates on different days). COC = cumulus–oocyte complex; DO + CC = denuded oocytes cultured with cumulus cell monolayer; DO = denuded oocytes.
The number of cells per blastocyst (Experiment 5) was higher in COC compared with DO + CC and DO when Mn was added to the IVM medium (P < 0.01; Fig. 4). Interaction was found between Mn addition during IVM and the maturation systems, when mean cell number per blastocyst was evaluated (P < 0.01).
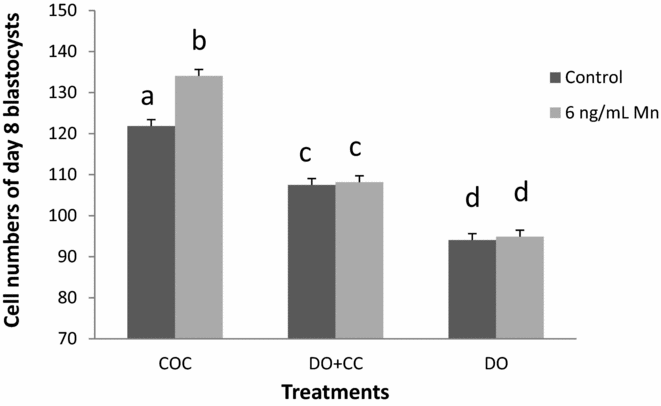
Figure 4 Effect of presence or absence of cumulus cells during IVM with or without manganese added to IVM medium on cell number per blastocyst. COC = cumulus–oocyte complex; DO + CC = denuded oocytes cultured with cumulus cell monolayer and DO = denuded oocytes. a–dValues without a common superscript differ significantly (P < 0.01). Mean cell numbers of day 8 blastocysts developed from oocytes matured with (6 ng/ml Mn) or without manganese (Control).
Discussion
The results of the present study indicate that Mn supplementation to IVM medium: (1) decreased apoptosis in cumulus cells; (2) increased SOD activity in cumulus cells; (3) did not modify cumulus expansion and cleavage rates after IVF; (4) improved subsequent embryo development up to blastocyst stage regardless of cumulus cells presence during in vitro maturation; and (5) enhanced blastocyst quality evaluated by mean cell number per blastocyst obtained from intact COC.
Gibbons and colleagues (Reference Gibbons, Dixon, Hallis, Russell, Sansom and Symonds1976) demonstrated that Mn plasma concentrations in bovine, range from 5–10 ng/ml. However, bovine Mn status were defined by Kincaid (Reference Kincaid1999) as deficient when Mn plasma concentration is lower than 6 ng/ml, and adequate when are between 6–70 ng/ml. Our studies have demonstrated that Mn concentrations in bovine are similar in plasma and follicular fluid (Anchordoquy et al., Reference Anchordoquy, Anchordoquy, Sirini, Mattioli, Picco and Furnus2013, Reference Anchordoquy, Anchordoquy, Picco, Sirini, Errecalde and Furnus2014). Adequate Mn concentration ‘protects’ cumulus cells from apoptosis (Anchordoquy et al., Reference Anchordoquy, Anchordoquy, Picco, Sirini, Errecalde and Furnus2014). The antioxidant role of Mn may be an important mechanism in preventing oxidative damage in cumulus cells. In the present study, IVM medium without Mn had a detrimental effect on the integrity of cumulus cells after in vitro maturation. The reduction of SOD activity increases DNA oxidative damage (Kinoshita et al., Reference Kinoshita, Sakamoto, Kashio, Shimizu and Yamasoba2013). Van Remmen et al. (Reference Van Remmen, Ikeno, Hamilton, Pahlavani, Wolf, Thorpe, Alderson, Baynes, Epstein, Huang, Nelson, Strong and Richardson2003) using heterozygous Mn-SOD knock-out mice demonstrated a key role of Mn against oxidative stress. The mechanism by which Mn prevents apoptosis is unclear, but several reports have established a correlation between Mn-SOD activity and a higher resistance to cell injury and apoptosis (Epperly et al., Reference Epperly, Sikora, DeFilippi, Gretton, Zhan, Kufe and Greenberger2002; Holley et al., Reference Holley, Bakthavatchalu, Velez-Roman and St Clair2011; Keller et al., Reference Keller, Kindy, Holtsberg, St Clair, Yen, Germeyer, Steiner, Bruce-Keller, Hutchins and Mattson1998). Reactive oxygen species have been implicated as mediators of apoptosis (Hampton & Orrenius, Reference Hampton and Orrenius1997). The SOD protects cell from damage caused by free radicals, and catalyze the dismutation of superoxide to hydrogen peroxide (Chihuailaf et al., Reference Chihuailaf, Contreras and Wittwer2002). Superoxide dismutase has three isoforms, depending on the metal it contains: SOD-Cu and SOD-Zn, which are found mostly in cytosol, while isoform SOD-Mn is located in the mitochondrial matrix (Chihuailaf et al., Reference Chihuailaf, Contreras and Wittwer2002). Mitochondria are important sites for the initiation and progression of apoptosis, upon mitochondrial dysfunction many molecules are released to initiate and propagate apoptosis (Mohr et al., Reference Mohr, Buneker, Gough and Zwacka2007; Holley et al., Reference Holley, Bakthavatchalu, Velez-Roman and St Clair2011). Manganese appears to be able to counteracts oxidative stress and modulate apoptosis depending on the cell type and concentration used (Schrantz et al., Reference Schrantz, Blanchard, Mitenne, Auffredou, Vazquez and Leca1999). It has been reported that Mn deficiency induces apoptosis in chick chondrocytes by a remarkably decreasing Bcl-2 antiapoptotic protein expression (Wang et al., Reference Wang, Wang, Wang, Liu, Liu and Wang2014). Our study demonstrated that COC matured with Mn supplementation reduced apoptosis rates in cumulus cells. It is well known that the degree of apoptosis in cumulus cells correlates with the developmental competence of bovine enclosed oocytes (Ikeda et al., Reference Ikeda, Imai and Yamada2003).
In our study, the degree of cumulus expansion did not vary in presence of Mn. After IVM, COC undergo dramatic changes. Cumulus cells synthesize and secrete large amounts of a hyaluronic acid (HA)-enriched extracellular matrix (Chen et al., Reference Chen, Mao and Larsen1992). The matrix is then deposited into extracellular spaces leading to the process of expansion (Eppig, Reference Eppig1979). The HA is a linear polysaccharide synthetized by glycosyltransferases that can be activated only in the presence of Mn (De Angelis, Reference De Angelis1999; Keen et al., Reference Keen, Ensunsa, Lönnerdal, Zidenberg-Cherr and Caballero2009). However, the addition of Mn to IVM medium at these concentrations did not vary the degree of cumulus expansion.
Within the follicle, granulose cells can be divided into two functional groups: the cumulus cells and the mural granulose cells around the antrum (Edson et al., Reference Edson, Nagaraja and Matzuk2009). Cumulus cells maintain a proximity relationship with the oocyte, providing nutrients, maturation-enabling factors, and an optimal microenvironment to ensure successful maturation and further developmental competence (Eppig, Reference Eppig1991; Pangas & Matzuk, Reference Pangas and Matzuk2005; Gilchrist et al., Reference Gilchrist, Lane and Thompson2008). Cumulus cells have gap junctions that allow the transfer of low molecular-weight molecules, ions and amino acids between oocyte and cumulus cell (Eppig, Reference Eppig1982; Larsen & Wert, Reference Larsen and Wert1988; Larsen, Reference Larsen, Sperelakis and Cole1989). In addition, gap junctions participate in oocyte meiotic regulation by allowing the passage of small regulatory molecules such as cAMP and purines (Dekel & Beers, Reference Dekel and Beers1980; Salustri & Siracusa, Reference Salustri and Siracusa1983; Eppig & Downs, Reference Eppig and Downs1984; Racowsky, Reference Racowsky1985; Racowsky & Satterlie, Reference Racowsky and Satterlie1985). Premature interruption of cumulus–oocyte gap junction communication affects the oocyte developmental capacity (Modina et al., Reference Modina, Luciano, Vassena, Baraldi-Scesi, Lauria and Gandolfi2001). This is likely to be due to the lack of transfer of specific molecular signals that coordinate oocyte final maturation (Gilchrist et al., Reference Gilchrist, Ritter and Armstrong2004; Lodde et al., Reference Lodde, Modina, Galbusera, Franciosi and Luciano2007).
In the present study, blastocyst rates increased when the oocytes were maturated in the presence of cumulus cells and was significantly higher in cumulus-intact oocytes (COC). These results are consistent with that observed by other researchers who found that removal of cumulus cells before IVM was detrimental to oocyte maturation in various species (such as, pigs, rats and cattle), and co-culture with COC or cumulus cells restored partially the developmental potential of cumulus-denuded oocytes (Vanderhyden and Armstrong, Reference Vanderhyden and Armstrong1989; Zhang et al., Reference Zhang, Jiang, Wozniak, Yang and Godke1995; de Matos et al., Reference de Matos, Furnus and Moses1997; Wongsrikeao et al., Reference Wongsrikeao, Kaneshige, Ooki, Taniguchi, Agung, Nii and Otoi2005). This can be explained by the fact that cellular communications between cumulus cells and the oocyte not only occurs through gap junction, but also via paracrine factors (Gilchrist et al., Reference Gilchrist, Ritter and Armstrong2004). Recent findings have demonstrated that diffusible factors secreted by cumulus cells play a key role in the acquisition of developmental competence of the bovine and mouse denuded oocytes (Luciano et al., Reference Luciano, Lodde, Beretta, Colleoni, Lauria and Modina2005; Ge et al., Reference Ge, Han, Lan, Zhou, Liu, Zhang, Sui and Tan2008). Conversely, in our study Mn only improved embryo quality when oocytes were matured with their intact cumulus mass. This result suggests that the gap junction might be involved in the improvement of embryo quality achieved by adding Mn to IVM medium. Manganese homeostasis is crucial for all mammalian cells. It has been established that Mn can also be transported by different mechanisms including the divalent metal transporter (DMT1) (Gunshin et al., Reference Gunshin, Mackenzie, Berger, Gunshin, Romero, Boron, Nussbeger, Gollan and Hediger1997; Garrick et al., Reference Garrick, Dolan, Horbinski, Ghio, Higgins, Porubcin, Moore, Hainsworth, Umbreit, Conrad, Feng, Lis, Roth, Singleton and Garrick2003; Kim et al., Reference Kim, Buckett and Wessling-Resnick2013), ZIP-8 and ZIP-14 (He et al., Reference He, Girijashanker, Dalton, Reed, Li, Soleimani and Nerbert2006; Himeno et al., Reference Himeno, Yanagiya and Fujishiro2009) and transferrin receptor (TfR) (Aschner & Gannon, Reference Aschner and Gannon1994; Davidsson et al., Reference Davidsson, Lonnerdal, Sandstrom, Kunz and Keen1989). Although the relative contribution of each of these transporters remains unknown, it is likely that optimal tissue Mn concentrations might be maintained by the involvement of all these transporters (Au et al., Reference Au, Benedetto and Aschner2008).
In conclusion, Mn increased the blastocyst rates regardless of the presence of cumulus cells during IVM, highlighting the importance of this mineral in oocyte cytoplasmic maturation. In addition, Mn improved embryo quality when oocytes were matured with intact cumulus–oocyte cell complex (COC).
Acknowledgments
We are grateful to Centro de Inseminación Artificial La Elisa S.A. (CIALE) for make available bovine frozen semen; and the staff of Frigorífico Gorina S.A. for providing the bovine ovaries.
Financial support
This work was supported by grants from Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET/PIP0990); Ministerio de Ciencia, Tecnología e Innovación Productiva de la Nación Argentina.
Conflicts of interest
There are no conflicts of interest.







