Introduction
Mammalian testes operate at the limit of hypoxia (Reyes et al., Reference Reyes, Farias, Henríquez-Olavarrieta, Madrid, Parraga, Zepeda and Moreno2012). Long-term exposure to high temperatures results in metabolic rate increase and higher oxygen demand without blood influx elevation, causing testicular hypoxia (Waites and Setchell, Reference Waites and Setchell1964) and consequently oxidative stress (OS). OS is triggered when there is an imbalance between reactive oxygen species (ROS) production and antioxidant activity (Halliwell and Gutteridge, Reference Halliwell and Gutteridge2007), including enzymes such as superoxide dismutase (SOD), glutathione reductase (GR), glutathione peroxidase (GPx) and catalase. OS causes alterations in the testicular environment and compromises spermatogenesis (Hamilton et al., Reference Hamilton, Castro, Delgado, Assis, Siqueira, Mendes, Goissis, Muiño-Blanco, Cebrián-Perez, Nichi, Visintin and Assumpção2016a, b), mainly inducing sperm DNA damage (Hamilton et al., Reference Hamilton, Siqueira, Castro, Mendes, Delgado, Assis, Mesquita, Maiorka, Nichi, Goissis, Visintin and Assumpção2018). Sperm DNA damage can compromise embryo development (Virro et al., Reference Virro, Larson-Cook and Evenson2004) leading to low pregnancy rates. Identifying which cells of the testicular germ line are responsible for maintaining oxidative balance by activating antioxidant enzymes in OS situations will allow us to develop strategies to minimize the deleterious effects of OS during spermatogenesis. Therefore, intensifying oxidative control pathways to avoid compromising spermatogenesis during heat stress (HS) conditions is an important control mechanism.
The aim of this study was to verify intensity of staining and to immunoidentify which germ cells in ram seminiferous tubules respond to heat stress-induced OS by increasing antioxidant enzymes SOD, GR and GPx.
Material and Methods
We used 12 Santa Inês adult (8-month-old) rams divided in the control (n = 6) and treated (n = 6) groups. Animals were submitted to uniform nutritional conditions, and the experiments were approved by the Bioethics Committee of the School of Veterinary Medicine and Animal Sciences, University of São Paulo (protocol no. 2445-2011). The experiment was conducted during the Brazilian winter (months of July/August). The mean environment temperature in this period was 17.01 ± 0.52°C and relative humidity was 78.08 ± 0.73%. All animals were examined clinically and reproductively before starting the experiment. None of the animals presented any type of pathology and reproductive evaluations, such as motility, sperm concentration, mass motility, sperm morphology, and sperm membrane integrity, were similar (P > 0.10) between them. In the treated group, animals underwent testicular HS using an insulating bag placed in the scrotum for 240 h consecutively. This condition elevated the scrotal temperature by 5°C. After removal of the bags, each animal was subjected to unilateral orchiectomy with resection of the left testicle. Testicles fragments of 1 cm3 were fixed in methanol-Carnoy and processed in paraffin. Silanized slides containing the fragments were submitted to immunohistochemistry against SOD, GR and GPx. Endogenous peroxidase was blocked with 5% hydrogen peroxide solution (v/v) in methanol (1:1) and antigen retrieval was performed using 10 mM sodium citrate buffer pH 6.0 at 96°C for 20 min. Non-specific sites were blocked with 5% solution (w/v) skimmed milk powder in PBS at room temperature for 30 min. After washing in PBS, the slides were incubated for 16–18 h at 4°C with the primary antibodies diluted 1:500 for anti-SOD (ab16831) and anti-GPx (ab22604), and diluted 1:200 for anti-GR (ab16801) (rabbit polyclonal antibodies; Abcam, Cambridge, UK). Intestine and mammary gland were used as the positive control and homologous non-immune serum as the negative control. Posteriorly, slides were incubated with complement (10 min) and then with horseradish peroxidase (HRP) conjugate (15 min) from the Reveal Biotin-Free Polyvalent HRP Kit (Spring Bioscience, Pleasanton, Canada). After washing in PBS, the diaminobenzidine reaction was performed for 5 min. Slides were counterstained with Harris haematoxylin. The evaluation was carried out using an optical light microscope coupled with a photographic camera (Olympus IX, Tokyo, Japan). First, slides were subjectively analyzed for antioxidant enzyme staining. We evaluated the cellular types inside the seminiferous tubule that presented SOD, GR and GPx staining. Therefore, we analyzed the intensity of cell staining, considering 0 (zero) as null; one cross (+) as moderate, and two crosses (++) as strong staining. Then, we used ImageJ software (Image Processing and Analysis in Java version 1.52 j, public domain, National Institutes of Health, USA) to quantitate the pixel intensity of seminiferous tubule staining. Seminiferous tubule staining was quantified by SOD, GR and GPx staining intensity and adjusted for white staining intensity (values expressed in mean ± standard error of the mean (SEM)). We examined, for both analyses, one slide per animal for each enzyme evaluated, in total 36 slides (18 slides per treatment). For each slide, five images from different fields were observed at ×1000 magnification, and quantified at ×400 magnification, computing 15 seminiferous tubules evaluated per experimental unit (90 seminiferous tubules evaluated per treatment). We used Statistical Analysis System software (SAS Institute, Cary, NC, USA) for statistical analysis. Data were tested for normality of residues and homogeneity of variances, and we used the GLM procedure for comparing the groups.
Results and Discussion
The objective of this study was to identify which germ cells from seminiferous tubules responded to heat stress-induced OS by increasing antioxidant enzymes SOD, GR and GPx. We did not observe SOD staining in spermatids, spermatocytes and spermatogonia in both control and treated groups (Table 1), however many stained desquamated cells (DC) were found in the lumen of the seminiferous tubule and moderate or strong SOD staining of Sertoli cells (SC) was seen in the control and treated groups respectively (Fig. 1a, b). In the control group, we observed high GR staining in SC, and absence of staining in spermatogonia, spermatids and spermatocytes (Table 1 and Fig. 1c). In the treated group, only SC presented GR staining (Table 1 and Fig. 1d). Control and treated groups presented DC in the tubular lumen. Also, we observed intense GPx staining of SC in both the control and treated groups. In the control group an absence of staining was present in spermatogonia, with moderate GPx staining in round spermatids and spermatocytes, whereas strong staining was seen in DC (Table 1 and Fig. 1e). However, in the treated group, there was moderate GPx staining of spermatogonia, and intense staining of spermatocytes, spermatids and DC on the lumen of the seminiferous tubules (Table 1 and Fig. 1f). Pixel intensity quantification using image analysis software revealed no differences in SOD staining between control and treated groups (94.06 ± 1.38; 94.44 ± 1.88, respectively, p = 0.87) (Fig. 2). Similarly, no differences were found in GR staining between control and treated groups (127.90 ± 1.85 and 126. 53 ± 2.33, respectively, p = 0.64) (Fig. 3). However, pixel intensity quantification revealed high GPx staining inside seminiferous tubules from the treated group compared with the control group (101.27 ± 2.14; 94.20 ± 2.02, respectively, p = 0.018) (Fig. 4). Long-term HS promotes OS in ram testis (Hamilton et al., Reference Hamilton, Mendes, Castro, Assis, Siqueira, Delgado, Goissis, Muiño-Blanco, Cebrián-Perez, Nichi, Visintin and Assumpção2016b). Histological studies have demonstrated that spermatocytes and early spermatids are the cells in the testes most susceptible to HS. SC and spermatogonia may be affected when the duration of heat exposure is increased (reviewed by Setchell, Reference Setchell2006). Some of these cells survived cell death or apoptosis and completed spermatogenesis; however these sperm had damaged DNA, as previously shown by our group (Hamilton et al., Reference Hamilton, Siqueira, Castro, Mendes, Delgado, Assis, Mesquita, Maiorka, Nichi, Goissis, Visintin and Assumpção2018) using the same experimental design. Antioxidant enzymes act by promoting neutralization of ROS induced by HS that could be present in cells involved in spermatogenesis (Halliwell and Gutteridge, Reference Halliwell and Gutteridge2007). In this study, we identified which germ cells from the seminiferous tubules responded to heat stress-induced OS by immunolocalization of SOD, GR and GPx. No differences were verified in immunolocalization of GR and SOD between control and treated groups after induction of HS in ram testis. We verified that, after induced stress, GPx was immunolocalized in more testicular germ cells types such as spermatogonia, spermatocytes, spermatids, SC and DC, while the control group mainly presented SC and DC staining. This pattern would characterize more efficient transcription or translation of GPx than other enzymes in an attempt to maintain testicular oxidative balance. High lipid peroxidation triggers the activation of GPx, the main antioxidant pathway of hydrogen peroxide (H2O2) catalysis (Bansal and Bilaspuri, Reference Bansal and Bilaspuri2011). Decomposition of H2O2 by GPx occurs by catalysis of reduced glutathione (GSH), resulting in H2O and oxidized glutathione (GSSG). GSSG can be reduced to GSH by GR, this mechanism will activate the catalytic system of glutathione peroxidases by the action of GPx in an OS environment (Halliwell and Gutteridge, Reference Halliwell and Gutteridge2007). Although H2O2 is not a ROS, it has the ability to penetrate biological membranes and induce ROS formation (Mathai and Sitaraman, Reference Mathai and Sitaraman1994). In a previous study, we observed an increase in GPx enzymatic activity in the seminal plasma and an increase in the percentage of sperm with intracellular marking of free radicals in rams submitted to testicular long-term HS (Hamilton et al., Reference Hamilton, Mendes, Castro, Assis, Siqueira, Delgado, Goissis, Muiño-Blanco, Cebrián-Perez, Nichi, Visintin and Assumpção2016b). This event led to an increase in GPx, that, in combination with the data presented here, will presumably act to neutralize H2O2 in spermatocytes, spermatogonia and SC in an attempt to maintain the oxidative balance and reduce damage during spermiogenesis, especially in sperm chromatin.
Table 1. Analysis of cell types stained by anti-SOD (ab16831, rabbit polyclonal antibodies; Abcam, Cambridge, UK), anti-GR (ab16801; Abcam) and anti-GPx (ab22604; Abcam) in ram seminiferous tubules after long-term heat stress, n = 90

Staining: 0 (zero) null, + (one cross) moderate, and ++ (two crosses) strong.
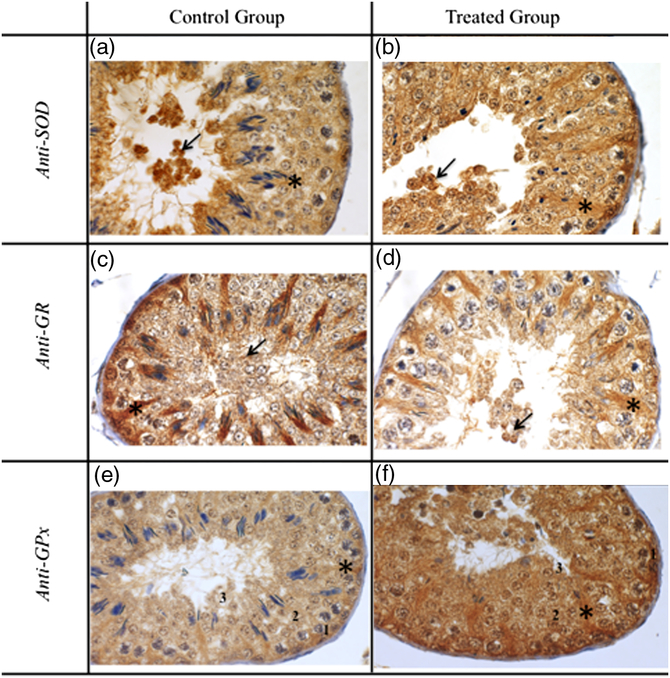
Figure 1. Immunolocalization of SOD, GR and GPx in ram testis after heat stress. (a, b) SOD labelling in control and treated groups, evidencing desquamated cells in the lumen (arrow) and moderate staining of Sertoli cells (asterisk). (c, d) GR labelling in control and treated groups evidencing desquamated cells in the lumen (arrow) and strong staining of Sertoli cells (asterisk). (e) GPx labelling in the control group evidencing strong staining of Sertoli cells (asterisk), null staining of spermatogonia (1) and moderate staining of spermatocyte (2) and round spermatid (3). (f) GPx labelling in the treated group evidencing desquamated cells in the lumen (arrow), moderate staining of spermatogonia (1) and strong staining of SC (*), spermatocyte (2) and round spermatid (3). Scale bar: ×1000 magnification.

Figure 2. Pixel intensity quantification of anti-SOD on ram seminiferous tubules after long-term heat stress in control and treated groups. Presented as staining intensity on ram seminiferous tubule (mean ± SEM), n = 90.

Figure 3. Pixel intensity quantification of anti-GR on ram seminiferous tubules after long-term heat stress in control and treated groups. Presented as staining intensity on ram seminiferous tubule (mean ± SEM), n = 90.

Figure 4. Pixel intensity quantification of anti-GPx on ram seminiferous tubules after long-term heat stress in control and treated groups. Presented as staining intensity on ram seminiferous tubule (mean ± SEM), n = 90. a,bDifferent superscript letters in each bar represent significant differences (P < 0.05).
Acknowledgements
We thank Bruno Cogliati for antibody assistance and Marcelo Goissis for English review (School of Veterinary and Animal Science, USP, Brazil).
Financial support
This work was supported by FAPESP (grant no. 2011/11231-8) and CNPq (grant no. 800585/2016-0).
Conflict of interest
Authors declare that they have no conflict of interest.
Ethics statements
Authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.







