Introduction
Artificial insemination (AI) is currently widely used in cattle-breeding, and the use of frozen–thawed bull semen is a convenient option for this procedure. However, cryopreservation techniques currently in use still result in a loss of 40–50% viable sperm during the freezing–thawing process (Hu et al., Reference Hu, Zhao, Tian, Zan and Li2011). Several parameters can be used to evaluate semen quality after freezing–thawing, such as sperm motility, sperm survival time and activity of sperm antioxidant enzymes. These parameters could not only forecast the degree of sperm fertility, but also could reflect freezing resistance in diverse individual sperm samples (Casas et al., Reference Casas, Sancho, Briz, Pinart, Bussalleu, Yeste and Bonet2009). The range of sperm proteins includes alkaline proteins, acid proteins and membrane proteins. These proteins participate in energy production in mitochondria (Ruiz-Pesini et al., Reference Ruiz-Pesini, Diez, Lapena, Perez-Martos, Montoya, Alvarez, Arenas and Lopez-Perez1998), the release of nitric oxide (Lewis et al., Reference Lewis, Donnelly, Sterling, Kennedy, Thompson and Chakravarthy1996), protein phosphorylation (Vijayaraghavan et al., Reference Vijayaraghavan, Stephens, Trautman, Smith, Khatra, Silva and Greengard1996), and are also associated with sperm motility after freezing. Recently, several studies have revealed that the semen freezing process, even storage in liquid nitrogen, could cause some damage to the function of sperm proteins (Desrosiers et al., Reference Desrosiers, Legare, Leclerc and Sullivan2006), and to some large molecules such the main proteins in sperm (Cao et al., Reference Cao, Wang, Xiang and Li2003; Martin et al., Reference Martin, Cagnon, Sabido, Sion, Grizard, Durand and Levy2007). Comparative proteomic analysis of high-fertility and low-fertility bull sperm has shown that several proteins are related to sperm fertility (Gaviraghi et al., Reference Gaviraghi, Deriu, Soggiu, Galli, Bonacina, Bonizzi and Roncada2010), therefore proteomic analysis of sperm could be a valuable tool to identify expression variation of proteins related to freezing resistance and sperm fertility.
Mammalian cells can respond to environmental stress by synthesis of some conserved proteins, such as the family known as heat-shock proteins (HSPs). Among the heat-shock proteins, HSP90 is associated with activation of steroid hormone receptors (Pratt et al., Reference Pratt, Galigniana, Harrell and DeFranco2004). HSP90 has been found localized in the sperm tail in all species examined and plays a role in mediating sperm fertility. Genetic analysis of viable HSP90 alleles revealed that HSP90 plays a critical role in Drosophila spermatogenesis (Yue et al., Reference Yue, Karr, Nathan, Swift, Srinivasan and Lindquist1999) and decreased expression of HSP90 might be associated with low sperm motility during cooling of boar sperm (Huang et al., Reference Huang, Kuo, Lee, Tsou, Lee, Chang, Wu and Yang1999). To further study the novel function of HSP90 on the sperm motility, an HSP90-specific inhibitor geldanamycin (GA) was used and results showed that HSP90 might play a crucial role in regulating boar sperm motility (Huang et al., Reference Huang, Kuo, Tsou, Lee, King, Huang, Yang and Lee2000). In human spermatozoa, the expression level of HSP90 was decreased dramatically after cryopreservation (Cao et al., Reference Cao, Wang, Xiang and Li2003). During mouse sperm capacitation, HSP90 undergoes tyrosine phosphorylation (Ecroyd et al., Reference Ecroyd, Jones and Aitken2003). In the field of the bull sperm cryopreservation, only a few studies have been performed to investigate the expression of different protein (such as HSP90) in sperm of different quality.
Therefore, the present study was performed to investigate protein expression in bull sperm of different quality after freezing–thawing by analysis of the protein profile through SDS-polyacrylamide gel electrophoresis (SDS-PAGE). In addition, western blot was used to verify the relationship between HSP90 and frozen–thawed bull sperm quality.
Materials and methods
Semen collection and freezing processing
Semen was collected by artificial vagina from eight sexually mature Holstein bulls (4–5 years old) that belonged to the Domestic Animal Improvement Station (Jingyang, Shanxi, China). Semen was incubated in a water bath at 35 °C until the sperm concentration and initial percentage of motile sperm was estimated. Sperm concentration was evaluated by optical density using a calibration spectrophotometer (Shanghai Spectrophotometer Co., Ltd., Shanghai, China). The percentage of motile sperm was estimated at 37 °C by light microscope at ×200 magnification.
After evaluation of sperm quality, the fresh semen was diluted with commercial refrigeration extender and filled into polyvinyl chloride (PVC) straws (0.25 ml) (Biovet, Lyon, France). Then the straws were cooled from 37 °C to 4 °C for 1.5 h and kept at 4 °C for 2.5 h. Subsequently, straws were cooled from +4 °C to −120 °C at the speed of approximately 15 °C/min. Finally, these straws were transferred to a liquid nitrogen tank (−196 °C).
Semen thawing
Semen frozen in the 0.25 ml PVC straws was thawed at 37 °C for 40 s. For assessment of sperm parameters and protein expression, all samples were thawed simultaneously.
Analysis of the sperm quality
The percentage of linear motile sperm was observed visually. For each bull, four straws of semen were thawed and these samples were transferred into pre-warmed tubes immediately after thawing. After incubation at 37 °C for 5 min, 10-μl aliquots were transferred onto glass slides and covered with a glass cover slider. Sperm motility was assessed by evaluating the percentage of sperm that showed flagellum movement. The percentage of motile sperm was estimated at 37 °C by light microscope at ×400 magnification. At least 300 sperms were counted per slide.
Plasma membrane integrity was evaluated by the hypotonic swelling test (HOST) (Osinowo et al., Reference Osinowo, Bale, Oyedipe and Eduvie1982). Fifty microliters of semen were diluted in 1 ml hypo-osmotic solution that contained 7.35 g sodium citrate and 13.51 g fructose in 1000 ml distilled water. After incubation for 60 min at 37 °C, sperm swelling was assessed by dropping 15 μl of well mixed sample on a warm slide (37 °C) and observation under a light microscopy at ×400 magnification. Viable sperm had coiled tails after HOST. At least 300 sperms per slide were observed. Spermatozoa were classified as positive or negative based on the presence or absence of the coiled tail.
Acrosomal status was evaluated using fluorescein isothiocyanate-conjugated peanut agglutinin (FITC-PNA) according to the delineation by Aboagla & Terada (Reference Aboagla and Terada2003). Thirty microliters of the sperm sample were smeared on the microscope slide. Smears were fixed with methanol at 20–22 °C for 10 min and then air-dried. About 30 μl FITC-PNA solution (100 μg/ml, Sigma) in 1× phosphate-buffered saline (PBS, pH 7.4) were spread over each slide. Subsequently, the slides were incubated in a dark and moist chamber for 30 min at 37 °C. After incubation, the slides were rinsed with 1× PBS, air-dried and mounted with 10 μl of antifade solution to preserve fluorescence. The slide smear was covered by a coverslip and sealed with colorless nail polish. The smears were observed and photographed by microscope (LEIKA DM-IRB linked up to a Nikon digital camera DXM). The whole acrosome was visualized with strong green fluorescence under a fluorescence microscope and was scored as acrosome-intact sperm. The percentage of fluorescent acrosome-intact sperm was counted in at least 300 sperm cells per slide.
Experimental design
Semen from eight Holstein bulls was frozen using the freezing process above. After freezing–thawing, sperm quality was assessed. For sperm motility, semen samples were divided into two groups: HFR group in which the sperm motility was >40% after freezing–thawing and LFR group in which the sperm motility was <40% after freezing–thawing. Each group contained four different individual sperm samples. Eight ejaculates, belonging to the HFR or LFR group, were selected to perform the SDS-PAGE and western blot assay. The experiments were repeated three times.
Preparation of the sperm proteins
After thawing, semen samples were incubated at 37 °C for 1 min, and then were centrifuged (1000 g, 4 °C, 5 min) and washed three times in 1 ml of pro-cooled 1× PBS. The pellet was re-suspended with lysis buffer and incubated at 4 °C for 30 min to extract sperm proteins. After centrifugation (12,000 g, 15 min, 4 °C), the supernatant was boiled in the SDS-sample buffer for 5 min. Then the protein concentration was measured on a micro-titer plate using a bicinchoninic acid (BCA) kit (Pierce, Rockford, IL, USA) according to the manufacturer's instructions. Finally, the sperm proteins were stored at −70 °C until use.
SDS-PAGE
SDS-PAGE was performed to determine the molecular weight and relative content of various sperm proteins. Equal amounts of total proteins from each semen sample were separated by electrophoresis on a 10% SDS-PAGE gel. Pre-stained protein marker (Sigma) was used as a molecular weight standard.
Western blot
After the sperm proteins were separated on a 10% SDS-PAGE gel, the proteins were transferred to a nitrocellulose (NC) membrane (Bio-Rad, Hercules, CA, USA) using a semi-dry blotting apparatus (Model SD transfer cell, Bio-Rad) at 18 V per mini-gel for 1 h in the transfer buffer. The membrane was subsequently blocked for 30 min in blocking buffer (Tris-buffered saline (TBS) with 3% (w/v) milk and 0.1% (v/v) Tween 20) and then incubated at 4 °C overnight with the goat monoclonal anti-HSP90 antibody (Santa Cruz Biotechnology, Inc. California, USA), which was diluted to 1:1000 (v:v) in the blocking buffer. Then the membrane was washed three times with 0.1% TBST and incubated with the rabbit conjugate anti-goat IgG–horseradish peroxidase (HRP) (1:5000 diluted in the blocking buffer) for 1 h at room temperature. Finally, the membrane was washed three times with 0.1% TBST, and developed for 5 min with the chemiluminescent HRP substrate (Immobile Western Detection System. Millipore Corporation, Billerica, MA, USA).
Image acquisition and data analysis
Gel images and the protein patterns were scanned and quantified using a gel documentation system (Gel Doc XR; Bio-Rad) and Quantity One Version 4.6.5 software package (Bio-Rad). The protein levels were expressed by ‘band volume’, which was defined as the total signal intensity inside the boundary of a band measured in pixel intensity units (density) × mm2, the lowest intensity in a pixel is zero (white). To avoid background signals, band boundaries were defined on amplified digital images of the protein patterns. β-Actin was used as an internal standard to normalize the volume of protein bands.
Data analysis was performed using the SPSS software program (version 13.0). All values were expressed as mean ± standard deviation (SD). Analysis of variance (ANOVA) with Tukey's test was used for comparison of mean values at a significance level of P < 0.05.
Results
Post-thaw viability
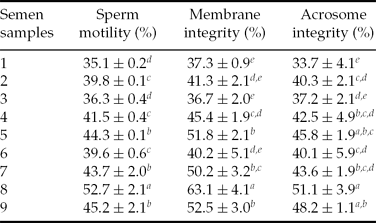
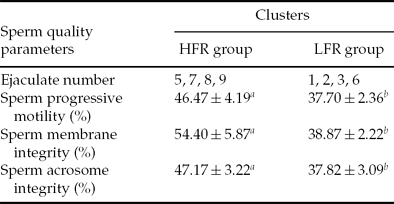
Frozen–thawed bull sperm motility, membrane integrity and acrosome integrity are shown in Table 1. The results showed that sperm quality from different bulls varied markedly. Eight ejaculates were classified into two clusters (HFR and LFR group, Table 2) based on the maximal dissimilarities of the sperm progressive motility after freezing–thawing. Ejaculate samples nos. 1, 2, 3 and 6 were assigned to the LFR group in which the sperm progressive motility was lower than 40% after freezing–thawing. Ejaculate semen samples nos. 5, 7, 8 and 9 were assigned to the HFR group in which sperm progressive motility exceeded 40% after freezing–thawing. Sperm quality parameters in the HFR group were significantly higher than those in the LFR group (P < 0.05). These eight ejaculates semen samples were used to perform western blot.
Table 1. Motility, membrane integrity and acrosome integrity of bull sperm after freezing–thawing

Note: Different superscript letters (a–e) in the same column represents significance difference at P < 0.05.
Table 2. Classification of ejaculate semen samples

Note: Values in the same row marked with different letters means significantly difference at P < 0.05. HFR: high freezing resistance; LFR: low freezing resistance.
Sperm proteins expressed in the HFR and LFR groups
Thirteen prominent protein bands were observed in which molecular weights ranged from 2.8–147.4 kDa. The band volumes of the 90 kDa protein in the HFR group were significantly higher than those in the LFR group (P < 0.05).
HSP90 expression level in the HFR and LFR groups
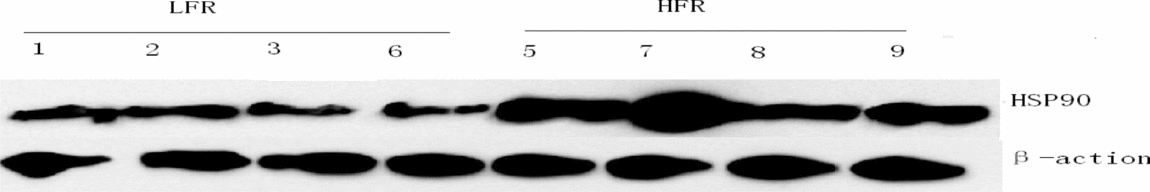
The most representative protein patterns are shown in Figs. 1 and 2. The average expression level of HSP90 in the LFR group was significantly lower than that in the HFR group (P < 0.05). Also, the relative expression level of HSP90 among different ejaculates in the LFR group were similar, however, those in the HFR group were markedly different. HS90 proteins were especially highly expressed in nos. 5 and 7 ejaculates compared with those of nos. 8 and 9. In general, the more sensitive the bull sperm to cold shock, the lower the expression of HSP90 after freezing–thawing. HSP90 was expressed more highly in sperm with higher motility and vice versa.

Figure 1. Expression pattern of 90 kDa heat-shock proteins (HSP90) in high freezing resistance (HFR) and low freezing resistance (LFR) sperm detected using western blot.

Figure 2. Expression of 90 kDa heat-shock proteins (HSP90). Note: The Gray value indicates expression of sperm HSP90 in clusters from different individual bulls. The unit is integral optical density (IOD). Different superscript letters (a, b) indicates significance difference at P < 0.05.
HSP90 expression was moderately related to the sperm quality parameters
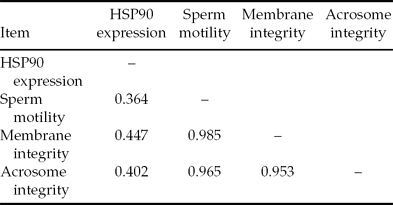
Correlation coefficients among different tests (HSP90 expression, sperm motility, acrosome integrity and membrane integrity) are summarized in Table 3. The correlation between HSP90 expression and sperm quality parameters was of moderate strength (ranging from 0.364–0.447).
Table 3. Correlation between HSP90 expression and different parameters for evaluating quality of bull sperm after freezing–thawing

Discussion
In this study, in which bull sperm was frozen and thawed, a change in quality parameters for bull sperm, including progressive motility, membrane integrity and acrosome integrity, was found. The degree of variation caused by cold shock was different in sperm in different ejaculates, including those that had undergone the same cryopreservation conditions. These results were consistent with results from previous studies (Roca et al., Reference Roca, Hernandez, Carvajal, Vazquez and Martinez2006; Gutierrez-Perez et al., Reference Gutierrez-Perez, de Lourdes Juarez-Mosqueda, Uribe Carvajal and Trujillo Ortega2009).
Previously, 125 putative biomarkers of fertility have been identified when high- and low-fertility bull sperm were evaluated by differential detergent fractionation multidimensional protein identification technology (DDF-Mud PIT) (Peddinti et al., Reference Peddinti, Nanduri, Kaya, Feugang, Burgess and Memili2008). Sperm from high-fertility bulls had a higher expression of proteins that were involved in energy metabolism, cell communication, spermatogenesis, and cell motility compared with sperm from low-fertility bulls. HSPs have been indicated as important key factors for the fertilizing ability of sperm in addition to having a protective function against stress (Casas et al., Reference Casas, Sancho, Ballester, Briz, Pinart, Bussalleu, Yeste, Fabrega, Rodriguez-Gil and Bonet2010). In a previous study, it was shown that HSP90 levels were markedly different between ejaculates that had either a good or poor freezing ability (Casas et al., Reference Casas, Sancho, Ballester, Briz, Pinart, Bussalleu, Yeste, Fabrega, Rodriguez-Gil and Bonet2010). In this study it was found that HSP90 from sperm from the LFR group displayed weaker reactivity compared with that from the HFR group. Therefore, it was possible to predict the potential freezing resistance ability of ejaculates based on the expression level of HSP90 by western blot, as sperm with a higher level of HSP90 had more resistance to freezing and vice versa.
Although HSP90 has been regarded as a cytosolic protein, its exact function still remains unclear. HSP90 plays an essential role in stress tolerance (Borkovich et al., Reference Borkovich, Farrelly, Finkelstein, Taulien and Lindquist1989), protein folding (Freeman & Morimoto, Reference Freeman and Morimoto1996), signal transduction (Pratt, Reference Pratt1998), and so on. Many proteins, including tyrosine kinases, serine–threonine kinases and other enzymes, may associate with HSP90 to affect the sperm motility (Pratt, Reference Pratt1998). HSP90 is a ubiquitous molecular chaperone that provides resistance against cell oxidative stress, apoptosis, and mediates cell protection and protein folding during thermal stress (Fukuda et al., Reference Fukuda, Osawa, Oda, Tanaka, Toyokuni and Uchida1996; Powers et al., Reference Powers, Clarke and Workman2008). It has been shown that the expression of HSP90 in HeLa cells was decreased significantly after cryopreservation (Wang et al., Reference Wang, Shu, He, Cui, Wang and Gao2005). Also, HSP90 could activate nitric oxide synthase (NOS), which is beneficial to sperm motility within the physiological range (Garcia-Cardena et al., Reference Garcia-Cardena, Fan, Shah, Sorrentino, Cirino, Papapetropoulos and Sessa1998). Nitric oxide (NO) is a reactive nitrogen species synthesized by NOS that may act as an antioxidant or free radical in inter-cellular and intra-cellular signalling (Dixit & Parvizi, Reference Dixit and Parvizi2001). It is well known that the freezing–thawing process could induce physical and chemical stress in the sperm plasma membrane that is associated with oxidative stress and the formation of free radical oxygen species (ROS), and resulting in impairment of sperm (Alvarez & Storey, Reference Alvarez and Storey1992). It has been found that HSP90 can protect cells from ROS (Fukuda et al., Reference Fukuda, Osawa, Oda, Tanaka, Toyokuni and Uchida1996). However, the protective roles of HSP90 against oxidative stress during semen freezing remain to be determined. In view of these studies, we could hypothesize that HSP90 might participate in the regulation of the sperm motility through modulation of the activity of its binding protein kinases. The freezing–thawing process results in cell oxidation and apoptosis in sperm, and HSP90 can protect sperm from this change through direct resistance to ROS or activation of NOS to produce NO that will eliminate ROS. Therefore, HSP90 could protect sperm from oxidative damage and improve sperm quality after freezing–thawing. In addition, HSP90 might contribute to changes during repair that occur in protamine–DNA complexes and in structural damage to chromatin during boar sperm cryopreservation (Flores et al., Reference Flores, Cifuentes, Fernandez-Novell, Medrano, Bonet, Briz, Pinart, Pena, Rigau and Rodriguez-Gil2008).
HSP90 had been shown to possess an inherent ATPase that is essential for the activation of authentic client proteins in vivo (Pearl & Prodromou, Reference Pearl and Prodromou2000). It has been claimed that the ATP level in sperm cell membranes diminishes after cold shock and is not restored subsequently (Watson, Reference Watson, Morris and Clarke1981). The diminishing levels of ATP might result in reduction in sperm motility. It has been demonstrated that HSP90 is involved in ATP metabolism (Prodromou et al., Reference Prodromou, Roe, Obrien, Ladbury, Piper and Pearl1997). During freezing–thawing, HSP90 initiates ATP depletion, which leads to lack of sperm dynamics such that the sperm stays in a resting state to reduce consumption of energy and reduce damage. Sperm motility functions could be affected either by the decrease in ATP, which is concomitant with lower mitochondrial potential (Pena et al., Reference Pena, Rodriguez Martinez, Tapia, Orteag Ferrusola, Gonzalez Fernandez and Macias Garcia2009), or by structural damage in the contractile apparatus of the flagellum (Guthrie et al., Reference Guthrie, Welch and Long2008). Our study showed that there were positive correlations between HSP90 expression and sperm motility, membrane integrity, and acrosome integrity; the correlation coefficient between HSP90 expression and sperm motility was 0.364. Moreover, HSP90 was expressed at a higher level in sperm with higher motility. A possible mechanism is that a surfeit of HSP90 inhibits ATP degradation and provides sufficient energy to ensure that spermatozoa have higher motility after freezing–thawing. These results illustrate that a higher HSP90 expression accurately predicts the increased freezing resistance of the sperm. Nevertheless, more research needs to be performed to investigate the mechanism of the protective role of HSP90 and to better understand the exact relationship between HSP90 expression and the sperm quality.
Conclusion
In this study, we found that expression of HSP90 in HFR bull sperm after freezing–thawing was dramatically higher than that in LFR bull sperm and, in addition, higher expression of HSP90 was related to lower sensitivity of HFR sperm to cold shock. Therefore, HSP90 could be considered as a cold shock molecular marker for frozen bull semen. This study indicated the need to search for cold shock markers to improve freezing ability prediction tests for frozen semen ejaculates, an approach that would have a high impact on bull semen cryopreservation methods.
Acknowledgements
This research was supported by the 12th Five-year Plan Rural Areas State Science and Technology Support Projects (2011BAD19B04-3), National Agriculture Science and Technology Achievements Transfer Capital Support Project (2010GB2G000466), Program for Yangtze Rive Scholar and Innovative Research Team of the Ministry of Education (IRT0940), the ‘13115’ Sci- Tech Innovation Project of Shaanxi Province (2010ZDGC-01), Scientific and Technological Project of Shaanxi Province (2011K01-04 and 2010K01-05), Agricultural Sci-Tech Innovation Project of Shaanxi Province (2011NXC01-11), Science and Technology Innovation Engineering Project of Shaanxi Province (2011KTCL02-11), the Basic Scientific Research Expense of Major Project of Northwest A&F University (PY200901) and the Basic Scientific Research Expense of Sci-Tech Innovation Major Project of Northwest A&F University (QN2011061).