Introduction
Many marine eukaryotes that release their gametes to the ocean (external fertilizers) use male gamete chemotaxis towards the female gamete to promote their successful fertilization (Miller, Reference Miller1985). As a common feature of the chemotaxis, the swimming male gametes (sperm) exhibit a change of their moving direction called chemotactic turn when they move away from the source of chemoattractant (Miller, Reference Miller1985; Maier & Müller, Reference Maier and Müller1986; Kinoshita et al., Reference Kinoshita, Nagasato and Motomura2017a). Calcium ion (Ca2+) in seawater is an essential factor for all male gamete (sperm) chemotaxis ever reported before (Maler & Calenberg, Reference Maler and Calenberg1994; Yoshida & Yoshida, Reference Yoshida and Yoshida2011).
Molecular identification of chemoattractants (previously called as sex pheromones) has advanced in fungi (Allomyces) (Nutting et al., Reference Nutting, Rapoport and Machlis1968) and brown algae (Müller et al., Reference Müller, Jaenicke, Donike and Akintobi1971). However, their signalling mechanisms mainly remain unknown. In sea urchin, the first chemoattractant (oligopeptide) was identified much later than those of fungi and brown algae (Suzuki et al., Reference Suzuki, Nomura, Ohtake and Isaka1981), but now in this animal major signalling mechanisms have been elucidated (Darszon et al., Reference Darszon, Nishigaki, Beltran and Trevino2011; Seifert et al., Reference Seifert, Flick, Bönigk, Alvarez, Trötschel, Poetsch, Müller, Goodwin, Pelzer, Kashikar, Kremmer, Jikeli, Timmermann, Kuhl, Fridman, Windler, Kaupp and Strünker2015; Espinal-Enríquez et al., Reference Espinal-Enríquez, Priego-Espinosa, Darszon, Espinal-Enríquez, Priego-Espinosa, Darszon, Beltrán and Martínez-Mekler2017). Initially, the oligopeptide activates a membrane-bound guanylyl cyclase that causes an increase in cGMP. Then, cGMP activates a K+ channel, called the tetraKCNG (or CNGK) channel (Galindo et al., Reference Galindo, de la Vega-Beltrán, Labarca, Vacquier and Darszon2007; Bonigk et al., Reference Bonigk, Loogen, Seifert, Kashikar, Klemm, Krause, Hagen, Kremmer, Strunker and Kaupp2009). Hyperpolarization of the membrane potential across the plasma membrane (Em) by K+ efflux upregulates several proteins, including the sperm-specific Na+/H+ exchanger (sNHE) (Wang et al., Reference Wang, King, Quill, Doolittle and Garbers2003; Nomura & Vacquier, Reference Nomura and Vacquier2006), soluble adenylyl cyclase (sAC) (Beltrán et al., Reference Beltrán, Zapata and Darszon1996; Nomura et al., Reference Nomura, Beltrán, Darszon and Vacquier2005) and hyperpolarization-activated cyclic nucleotide-gated (HCN) channels (Gauss et al., Reference Gauss, Seifert and Kaupp1998). Subsequently, a combination of intracellular pH increase (Nishigaki et al., Reference Nishigaki, Zamudio, Possani and Darszon2001; González-Cota et al., Reference González-Cota, Silva, Carneiro and Darszon2015) and Em repolarization and/or depolarization (Strünker et al., Reference Strünker, Weyand, Bönigk, Van, Loogen, Brown, Kashikar, Hagen, Krause and Kaupp2006) activates a sperm-specific Ca2+ channel named CatSper (Seifert et al., Reference Seifert, Flick, Bönigk, Alvarez, Trötschel, Poetsch, Müller, Goodwin, Pelzer, Kashikar, Kremmer, Jikeli, Timmermann, Kuhl, Fridman, Windler, Kaupp and Strünker2015). Finally, a transient increase in the intracellular Ca2+ concentration ([Ca2+]i) induces an asymmetric flagellar waveform, which in turn produces a change in swimming direction (Böhmer et al., Reference Böhmer, Van, Weyand, Hagen, Beyermann, Matsumoto, Hoshi, Hildebrand and Kaupp2005; Wood et al., Reference Wood, Nisihigaki, Furuta, Baba and Darszon2005). In sea urchin sperm chemotaxis, Em hyperpolarization mediated by the tetraKCNG channel plays a primordial role (Harumi et al., Reference Harumi, Hoshino and Suzuki1992; Nishigaki et al., Reference Nishigaki, Wood, Tatsu, Nishigaki, Wood, Tatsu, Yumoto, Furuta, Elias, Shiba, Baba and Darszon2004; Strünker et al., Reference Strünker, Weyand, Bönigk, Van, Loogen, Brown, Kashikar, Hagen, Krause and Kaupp2006). In fact, an increase in the K+ concentration of seawater from 10 mM to 50 mM (high K+ seawater) inhibits Em hyperpolarization and blocks almost all cell responses to the chemoattractant except for an enhanced elevation of cGMP (Harumi et al., Reference Harumi, Hoshino and Suzuki1992). As a consequence, sea urchin sperm chemotaxis is completely inhibited in high K+ seawater (Wood et al., Reference Wood, Nisihigaki, Furuta, Baba and Darszon2005).
Followed by the sea urchin (echinoderms), knowledge of the mechanism of sperm chemotaxis of ascidian, Ciona intestinalis, has been advanced in the last 2 decades. The chemoattractant of this marine invertebrate is a sulfated sterol called SAAF (sperm-activating and -attracting factor) (Yoshida et al., Reference Yoshida, Murata, Inaba and Morisawa2002). Although the molecular identity of the SAAF receptor is still under investigation, this factor induces fluctuations in sperm [Ca2+]i during chemotaxis (Shiba et al., Reference Shiba, Baba, Inoue and Yoshida2008) as observed in sea urchin sperm chemotaxis (Böhmer et al., Reference Böhmer, Van, Weyand, Hagen, Beyermann, Matsumoto, Hoshi, Hildebrand and Kaupp2005). By contrast with sea urchin spermatozoa, those of C. intestinalis remain quiescent upon spawning, and they initiate flagellar beating upon exposure to SAAF (Yoshida et al., Reference Yoshida, Inaba, Ishida and Morisawa1994). Therefore, as its name literally implies, this sulfated sterol functions as the motility initiation factor in addition to the chemoattractant. It was demonstrated that SAAF induces Em hyperpolarization by K+ efflux and high K+ seawater suppresses the initiation of sperm motility induced by SAAF (Izumi et al., Reference Izumi, Marian, Inaba, Oka and Morisawa1999). Therefore, a K+ channel in the ascidian spermatozoa plays a fundamental role in the SAAF signalling cascade.
In 2010, the complete genome DNA sequences of the brown alga Ectocarpus siliculosus were published (Cock et al., Reference Cock, Sterck, Rouzé, Scornet, Allen, Amoutzias, Anthouard, Artiguenave, Aury and Badger2010). Then, subsequent transcriptome analysis of the male and female gametes revealed that the male gametes expressed homologues of the tetraKCNG channel, sNHE, sAC and HCN channel, which are key proteins involved in sea urchin sperm chemotaxis (Lipinska et al., Reference Lipinska, D’Hondt, Van Damme and De Clerck2013). This report suggests that there might be a common signalling mechanism in male gamete chemotaxis between sea urchin and brown algae even though they belong to distant phylogenetic groups, Unikonta and Bikonta, respectively.
In this study, we focussed on the tetraKCNG channel to speculate the signalling mechanism of male gamete chemotaxis, as this channel is widely distributed among marine invertebrates (Fechner et al., Reference Fechner, Alvarez, Bönigk, Müller, Berger, Pascal, Trötschel, Poetsch, Stölting, Siegfried, Kremmer, Seifert and Kaupp2015) and plays a fundamental role in sea urchin sperm chemotaxis. Furthermore, we can easily and clearly demonstrate the involvement of a K+ channel in chemotaxis in high K+ seawater. Namely, this condition has been used to prevent K+ efflux through any K+ channels including tetraKCNG and inhibit all ion fluxes required for sperm chemotaxis in sea urchin. Initially, we confirmed the commonality underlying the sperm chemotaxis between sea urchin and ascidian as expected. Then, we explored the male gamete of the brown alga Mutimo cylindricus and found that the initial signalling cascade of the male gamete chemotaxis of this brown alga is distinct from that of the marine invertebrates. Followed by this unanticipated result, we performed a primary structural analysis of tetraKCNG-like channels in the brown alga and revealed that it has a distinct domain composition from that of tetraKCNG.
Materials and methods
Materials
SAAF was synthesized as described previously (Oishi et al., Reference Oishi, Tsuchikawa, Murata, Oishi, Tsuchikawa, Murata, Yoshida and Morisawa2003, Reference Oishi, Tsuchikawa, Murata, Yoshida and Morisawa2004). The ascidians Ciona intestinalis (type A; also called C. robusta) were obtained from the National BioResource Project for Ciona (http://marinebio.nbrp.jp/) and cultivated in Aburatsubo Bay (Kanagawa Prefecture), Japan. Then, they were maintained in an aquarium under constant light until use to prevent spontaneous spawning. Semen was obtained from the sperm duct by dissection and was stored on ice. Brown algal male and female gametes were obtained from the gametophytes of cultured strain M. cylindricus (Kinoshita et al., Reference Kinoshita, Nagasato, Tanaka and Motomura2016a). The gametophytes were cultured in autoclaved seawater containing half-strength Provasoli’s enriched seawater (PES) (Provasoli, Reference Provasoli1968) and incubated at 15°C under a 14:10 light:dark regime, with 20 to 40 µmol photons m–2 s–1 using white light.
Artificial seawater (ASW) for experiments with ascidian sperm contained (in mM): 462 NaCl, 9 KCl, 10 CaCl2, 48 MgCl2, and 10 HEPES (pH 8.2 by NaOH). Modified ASW (mASW) contained: 460 NaCl, 10 KCl, 9 CaCl2, 36 MgCl2, 17.5 MgSO4, 0.1 EDTA, and 10 HEPES (pH 8.2 by NaOH). High K+ (40 mM and 80 mM) was prepared by substituting Na+ by K+ in the ASW described above.
Centrifugation of the male gametes of the brown alga induces retraction of their flagella into their cell bodies. It is difficult, therefore, to replace all culture medium (autoclaved seawater that contains approximately 10 mM K+) that suspends the gametes. So, 100 mM K+ artificial seawater (100KASW) was prepared (in mM): 375 NaCl, 10 CaCl2, 100 KCl, 25 MgSO4, 25 MgCl2 and 10 Tris (pH 7.8 by HCl). Then, 100KASW was added to the gamete suspension to set up high K+ seawater in the ratios 3:7 (about 37 mM K+), 5:5 (about 55 mM K+), and 7:3 (about 73 mM K+).
Analysis of C. intestinalis sperm motility
Sperm motility was initiated by addition of 1 mM of theophylline (Yoshida et al., Reference Yoshida, Inaba, Ishida and Morisawa1994; Shiba et al., Reference Shiba, Baba, Inoue and Yoshida2008). Sperm chemotactic movements around SAAF-filled glass capillaries in ASW or ASW with altered K+ concentrations were recorded using a phase-contrast microscope with a Power LED stroboscopic illumination system and analyzed using Bohboh software (Bohboh Soft, Tokyo, Japan) as described previously (Shiba et al., Reference Shiba, Baba, Inoue and Yoshida2008). The linear equation chemotaxis index (LECI) was determined as described previously (Yoshida et al., Reference Yoshida, Murata, Inaba and Morisawa2002).
Assay of M. cylindricus male gametes chemotaxis
A droplet (10 µl) of the female gamete suspension on a coverslip was kept in dark for 5 min to induce settlement of female gametes on the coverslip and releasing chemoattractant. Quickly after removing the liquid of the droplet, 50 µl of the male gametes diluted in autoclaved seawater (or high K+ seawater) were added. The medium was covered with another coverslip using double-faced adhesive tapes. Immediately after the sample was set, images of swimming male gametes were recorded using a phase-contrast microscope (IX71, Olympus, Tokyo, Japan) and a ×10 objective (UPlan FLN, Olympus, Tokyo, Japan) connected to a high-speed CCD camera (HAS220, Ditect, Tokyo, Japan) at 50 frames per second (fps) with 2.5 ms exposure time. To prevent the effect of phototaxis induced by blue light (Kawai et al., Reference Kawai, Müller, Fölster and Häder1990), observations were conducted with a red LED (620–630 nm) in the dark room in normal seawater (10 mM K+) and high K+ conditions (approximately 10, 37, 55, 73 mM K+). The path curvatures of 30 gametes randomly selected in each condition were analyzed by Bohboh software (Shiba et al., Reference Shiba, Baba, Inoue and Yoshida2008). The path curvatures were determined using the trajectories of the initial 0.5 s. Similarly, straight-line paths of 20 gametes randomly selected were obtained by Bohboh software (the distance between the initial point and the position at 2 s). To obtain LECI values, trajectories of 15 gametes randomly selected (25 fps) were traced manually for 5 s using ImageJ software with a cell-tracking plug-in (Meijering et al., Reference Meijering, Dzyubachyk and Smal2012). To quantify the accumulation of the male gametes, the numbers of the male gametes inside of a circle with 50 µm radius were counted at two positions, one with a focus on the female gamete (a) and the other at randomly selected position with 400 µm-distance from the female gamete (b). Thereafter, the rate of a/b was calculated as an index of the male gamete accumulation.
Primary structure analysis of diKCNG channels of the brown alga
Amino acid sequences of two tetraKCNG-like channels expressed in the male gametes of Ectocarpus siliculosus have been previously reported as Esi0015_0103 and Esi0000_0275 (GenBank accession nos.: CBN76577 and CBN79924, respectively). However, version 2 of the genome information provides corrected amino acid sequences: Ec-04_001880 and Ec-27_005000 for Esi0015_0103 and Esi0000_0275, respectively (http://bioinformatics.psb.ugent.be/orcae/overview/EctsiV2). Therefore, amino acid sequences Ec-04_001880 and Ec-27_005000 were analyzed using SUPERFAMILY (Gough et al., Reference Gough, Karplus, Hughey and Chothia2001) to obtain their general structure. Then, Ec-04_001880 and Ec-27_005000 were used for alignment with the tetraKCNG channel of sea urchin Strongylocentrotus purpuratus (NP_001075433) and the SthK channel of Spirochaeta thermophila (WP_013313430). As Ec-04_001880, named diKCNG, and tetraKCNG have two and four repeating units of six transmembrane segments plus cyclic nucleotide-binding domain (6TMs-CNBD), the alignment was performed using Clustal Omega software (http://www.ebi.ac.uk/Tools/msa/clustalo/) using each 6TMs-CNBD unit as a separated protein sequence (I and II for diKCNG and I to IV for tetraKCNG). As Ec-27_005000 has some insertions and deletions in the pore, the C-linker and CNBD regions, this protein should not have the same channel activity as diKCNG and we named this protein ‘diKCNG-like channel’. The alignment obtained by Clustal Omega was manually modified according to the secondary structures analyzed by SPIDER2 (Heffernan et al., Reference Heffernan, Dehzangi, Lyons, Paliwal, Sharma, Wang, Sattar, Zhou and Yang2015a, Reference Heffernan, Paliwal, Lyons, Dehzangi, Sharma, Wang, Sattar, Yang and Zhoub).
Results
High [K+]ext inhibits ascidian sperm chemotaxis towards SAAF
Most vertebrates such as mammals do not possess a tetraKCNG channel, probably by loss of the gene in the process of evolution. Indeed, we found degenerate DNA fragments of putative tetraKCNG on human chromosome 5 with synteny to spotted gar chromosome LG6 (online Supplementary Fig. S1), this finding supports our hypothesis. Conversely, tetraKCNG is widely distributed in marine invertebrates including sea urchin and ascidian (Fechner et al., Reference Fechner, Alvarez, Bönigk, Müller, Berger, Pascal, Trötschel, Poetsch, Stölting, Siegfried, Kremmer, Seifert and Kaupp2015). Interestingly, marine invertebrates that have demonstrated their sperm chemotaxis (Miller, Reference Miller1985) conserve tetraKCNG channels (Fechner et al., Reference Fechner, Alvarez, Bönigk, Müller, Berger, Pascal, Trötschel, Poetsch, Stölting, Siegfried, Kremmer, Seifert and Kaupp2015); this suggests a possible involvement of this channel in sperm chemotaxis in marine invertebrates. Therefore, we first explored the effect of manipulation of K+ concentration of seawater ([K+]ext) on ascidian sperm chemotaxis. Once ascidian sperm started swimming due to theophylline, a phosphodiesterase inhibitor, the spermatozoa maintained their motility independent of the K+ concentration of the ASW. However, their swimming trajectories were prominently affected by altering [K+]ext (Fig. 1a and b). Namely, in 40 mM of [K+]ext, the index of the efficiency of sperm chemotaxis LECI was significantly decreased and, moreover, this index was almost zero in 80 mM [K+]ext, indicating the complete inhibition of sperm chemotaxis. Ca2+ imaging of spermatozoa revealed that the increase in Ca2+ concentration ([Ca2+]i), accompanied by the chemotactic turn observed in normal ASW, was highly reduced in 40 mM [K+]ext (online Supplementary Fig. S2).
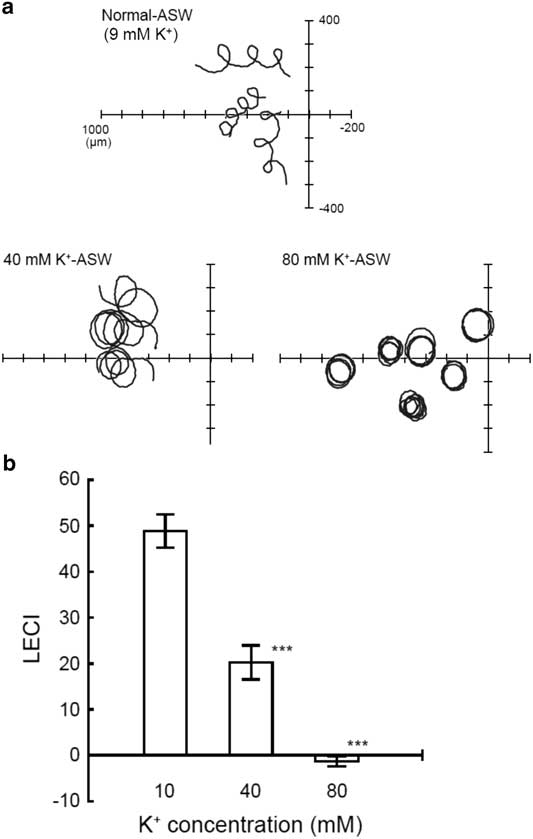
Figure 1 Effects of alteration of [K+]ext on ascidian sperm chemotaxis. (A) Representative sperm path trajectories are shown. The origin of the coordinates represents the tip of the glass capillary filled with 1 µM SAAF. (B) Comparison of linear equation chemotaxis indices (LECI) in various extracellular K+ concentration ([K+]ext). Values are means ± standard error (SE). n = 29 (10 mM, control), 21 (40 mM), 29 (80 mM). ***Significant at P<0.001 (Student’s t-test) as compared with the control.
High [K+]ext does not affect M. cylindricus male gamete chemotaxis towards the female gamete
In 2013, Lipinska et al. reported that the male gamete of brown alga, Ectocarpus siliculosus, expresses three tetraKCNG-like channels (Lipinska et al., Reference Lipinska, D’Hondt, Van Damme and De Clerck2013). We postulated that these channels might be involved in male gamete chemotaxis towards the chemoattractant derived from the female gamete, as the case of sea urchin spermatozoa. To examine our hypothesis, we chose M. cylindricus to perform experiments, as this species is easy to maintain as its gametophyte produces gametes constantly in a laboratory. As there is no synthetic chemoattractant available for M. cylindricus, we performed the chemotaxis assay using a settled female gamete on a coverslip as a source of chemoattractant (Fig. 2). Chemotaxis of M. cylindricus male gametes can be observed in normal ASW (10 mM [K+]ext) as small circles of thigmotactic gamete trajectories near the settled female gametes, as previously reported (Kinoshita et al., Reference Kinoshita, Nagasato, Tanaka and Motomura2016a; Fig. 2 upper right panel). Unexpectedly, in high K+ seawater (37, 55 and 72 mM [K+]ext), the male gametes exhibited apparently similar chemotactic swimming trajectories as in the normal ASW. To quantify the efficiency of chemotaxis in those conditions, we analyzed the swimming trajectories of the male gamete. However, we failed to obtain a mean positive LECI value in normal seawater (online Supplementary Fig. S3). This is probably due to our experimental conditions, namely the initial responses of many male gametes were not captured as we used the settled female gamete as the source of chemoattractant. Therefore, we determined the rate of male gamete accumulation around the female gamete as defined in Materials and methods. As shown in Table 1, no statistically significant difference was observed in the rate of male gamete accumulation between normal ASW and high K+ ASW. In addition, we determined the path curvature and relative velocity of the male gametes, as high curvature and low velocity are considered as typical features of the male gamete chemotaxis towards the settled female gamete in brown algae (Geller & Müller, Reference Geller and Müller1981; Kinoshita et al., Reference Kinoshita, Nagasato, Tanaka and Motomura2016a,Reference Kinoshita, Shiba, Inaba, Fu, Nagasato and Motomurab, Reference Kinoshita, Nagasato and Motomura2017b). Then, we confirmed that high path curvature (Fig. 3) and low straight-line path (the relative velocity shown in online Supplementary Fig. S4) are preserved in high K+ ASW in the presence of the female gamete; this finding also supports the idea that high external K+ does not affect male gamete chemotaxis in the brown alga.
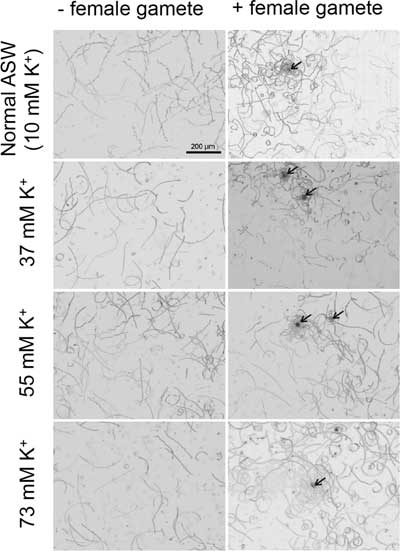
Figure 2 Chemotaxis of brown algal male gametes in high [K+]ext. The swimming trajectories of Mutimo cylindricus male gametes were obtained by image analysis over 2 s without female gametes (left) and with settled female gametes (right, indicated by arrows) in various [K+]ext: 10 mM (control), 37 mM, 55 mM and 73 mM.
Table 1 Number of male gametes of the brown alga close to the female gamete

a: Number of male gametes inside the 50-µm radius circle with a focus on the female gamete. b: Number of male gametes inside the 50-µm radius circle but 400 µm from the female gamete, randomly selected. a/b: the rate of male gamete accumulation (a divided by b). n = 8 for each condition. Mean values±standard deviation (SD) are shown.

Figure 3 Path curvature of the male gametes in high [K+]ext. Path curvatures of the male gamete under different conditions are shown by boxplots with their corresponding point distribution (n = 30). The leftmost (compressed box) represent results in the absence of the female gamete (− Female gamete). The others represent data in the presence of the female gamete with distinct external K+ concentrations (10, 37, 55 and 73 mM K+). Every distribution was not normal, therefore non-parametric statistics was used for this analysis. Multiple comparison tests after the Kruskal–Wallis test indicated that the curvature distributions in the absence of the female gamete were statistically different from those in the presence of the female gamete (*P-value<2.2×10–16). Conversely, no significant differences were observed among distinct K+ conditions in the presence of the female gamete.
Structure of tetraKCNG-like (diKCNG) channels of E. siliculosus
Although tetraKCNG-like channels were reported as highly expressed channels in the male gametes of E. siliculosus (Lipinska et al., Reference Lipinska, D’Hondt, Van Damme and De Clerck2013), their structures have not been analyzed in detail. Therefore, we performed a primary structure analysis of the two tetraKCNG-like channels, Ec-04_001880 and Ec-27_005000. Curiously, they do not have the same domain composition as that of the tetraKCNG channel. They rather possess a new architecture as a cyclic nucleotide-modulated channel (CNM channel) (Fig. 4 and online Supplementary Fig. S5). In general, CNM channels so far reported are composed of six transmembrane segments (TMs) followed by a cytoplasmic CNBD as a minimum unit of the channels (6TMs–CNBD). TetraKCNG channels are composed of 24 TMs, namely, four units of 6TMs–CNBD. Actually, tetraKCNG channel of C. intestinalis has four units of 6TMs–CNBD in the new database, although the previously reported channel (XP_002123955) contained only three units of 6TMs-CNBD (Fechner et al., Reference Fechner, Alvarez, Bönigk, Müller, Berger, Pascal, Trötschel, Poetsch, Stölting, Siegfried, Kremmer, Seifert and Kaupp2015). Conversely, at least, one of the two channels of the brown alga (Ec-04_001880) has two units of 6TMs–CNBD. Therefore, this protein can be classified into a new channel family and it could be called a diKCNG channel in comparison with tetraKCNG. Although the diKCNG channel conserves the general structure of the CNM channels, including the C-linker between the pore domain and the CNBD (Brams et al., Reference Brams, Kusch, Spurny, Benndorf and Ulens2014), the other channel (Ec-27_005000) lacks some essential segments such as pore domain and C-terminal CNBD (Fig. 4b). Therefore, we named this channel ‘diKCNG-like channel’, which is not likely to form a functional channel.

Figure 4 Structure of tetraKCNF-like (diKCNG) channels. (A) General topologies of cyclic nucleotide-modulated (CNM) channels. Upper left model represents channels possessing a single repeat of 6TMs–CNBD such as a bacterial K+ channel (SthK), HCN and CNG channels. Upper right represents tetraKCNF-like (diKCNG) channels found in brown algae, which possess two repeats of 6TMs–CNBD. Lower model shows tetraKCNG channels found in mainly marine animals. (B) Alignments of two diKCNG of the brown algae EsDiKCNG (Ec-04_001880), EsDiKCNGL (Ec-27_005000) and sea urchin SpTetraKCNG and SthK. Roman numbers indicate the position of the repeating unit (6TMs–CNBD). In S4, bold letters indicate positively charged residues found in every three amino acids. In the selectivity filter region, bold letters (GYGD) indicate K+ selective motif. In the C-terminus (C-linker and CNBD), blue letters represent predicted α-helixes and red letters represent β-sheets. Green arrow indicates arginine residues (bold) found in PBC of many functional CNBDs. EsDiKCNGLI has an atypical insertion in PBC, which is represented by # in the end of the figure. EsDiKCNGLII lacks the K+ selective motif, S6 (see online Supplementary Fig. S5) and the C-terminus (C-linker and CNBD) and the asterisk indicates the end of the proteins.
Discussion
Importance of K+ channel for sperm chemotaxis in ascidian spermatozoa
In this study, we first confirmed that Em hyperpolarization mediated by K+ efflux through the plasma membrane (Izumi et al., Reference Izumi, Marian, Inaba, Oka and Morisawa1999) is an essential step for ascidian sperm chemotaxis. Considering that the tetraKCNG channel plays a primordial role in sea urchin sperm chemotaxis (Harumi et al., Reference Harumi, Hoshino and Suzuki1992; Nishigaki et al., Reference Nishigaki, Wood, Tatsu, Nishigaki, Wood, Tatsu, Yumoto, Furuta, Elias, Shiba, Baba and Darszon2004; Strünker et al., Reference Strünker, Weyand, Bönigk, Van, Loogen, Brown, Kashikar, Hagen, Krause and Kaupp2006) and the orthologue is highly expressed in the testis of C. intestinalis (KH.C7.121.v1.A.ND1-1, Ghost Database: Ciona intestinalis genomic and cDNA resources, http://ghost.zool.kyoto-u.ac.jp), the tetraKCNG channel is a strong candidate of the molecular identity for this K+ channel. Recently, it was reported that zebrafish possesses a tetraKCNG that is regulated by cytoplasmic pH, not by cyclic nucleotides, even though the protein codes the highly conserved CNBD (Fechner et al., Reference Fechner, Alvarez, Bönigk, Müller, Berger, Pascal, Trötschel, Poetsch, Stölting, Siegfried, Kremmer, Seifert and Kaupp2015). This unique property of zebrafish tetraKCNG suggests that a channel from this family can be regulated by distinct factors in addition to cyclic nucleotides. Recently, a plasma membrane Ca2+-ATPase was identified as a possible SAAF receptor (Yoshida et al., Reference Yoshida, Shiba, Sakamoto, Ikenaga, Matsunaga, Inaba and Yoshida2018). However, it remains unknown how SAAF activates a K+ channel, probably a tetraKCNG channel. Further experiments are required to reveal the mechanism of activation of this ascidian K+ channel in the process of sperm chemotaxis.
K+ channels are not essential in M. cylindricus gamete chemotaxis
To study a possible involvement of tetraKCNG-like (diKCNG) channels in brown algae chemotaxis, we used M. cylindricus to perform experiments due to its facility for gamete preparation (Kinoshita et al., Reference Kinoshita, Nagasato, Tanaka and Motomura2016a) and because the behaviour of M. cylindricus male gametes (Kinoshita et al., Reference Kinoshita, Nagasato, Tanaka and Motomura2016a) is quite similar to that of E. siliculosus (Kinoshita et al., Reference Kinoshita, Shiba, Inaba, Fu, Nagasato and Motomura2016b). Contrary to our expectations, high [K+]ext did not inhibit male gamete chemotaxis to the female gamete in M. cylindricus (Figs 2, and 3, online Supplementary Figs S3 and S4 and Table 1). This result apparently indicates that no K+ channels, including diKCNG, are essential for male gamete chemotaxis of the brown alga, suggesting that the mechanism of male gamete chemotaxis between marine invertebrates and brown algae is different.
DiKCNG channels of E. siliculosus
Our structural analysis revealed that tetraKCNG-like channels expressed in E. siliculosus male gametes (Lipinska et al., Reference Lipinska, D’Hondt, Van Damme and De Clerck2013) can be classified into a novel channel family due to their domain compositions (Fig. 4). However, one channel (Ec-27_005000, named diKCNG-like channel) does not possess the complete functional domains, suggesting that it lacks functional channel activity. By contrast, the other channel (Ec-04_001880, named diKCNG channel) conserves all functional domains including the C-linker. In the diKCNG channel, the predicted S4 conserves six positively charged residues and none of the phosphate binding cassettes (PBC) of the putative CNBDs possessed arginine, which is a key residue for the specific molecular interaction between most CNBDs and cyclic nucleotides (Bonigk et al., Reference Bonigk, Loogen, Seifert, Kashikar, Klemm, Krause, Hagen, Kremmer, Strunker and Kaupp2009; Brelidze et al., Reference Brelidze, Carlson and Zagotta2009; Romero et al., Reference Romero, Santana-Calvo, Sánchez-Guevara and Nishigaki2017). These features suggested that diKCNG channels could be regulated by membrane potential rather than cyclic nucleotides as in KCNH channels (Brelidze et al., Reference Brelidze, Carlson, Sankaran and Zagotta2012; Haitin et al., Reference Haitin, Carlson and Zagotta2013). Nevertheless, there are some atypical CNBDs that lack arginine in their PBC, but are functional (Froese et al., Reference Froese, Breher, Waldeyer, Schindler, Nikolaev, Rinné, Wischmeyer, Schlueter, Becher, Simrick, Vauti, Kuhtz, Meister, Kreissl, Torlopp, Liebig, Laakmann, Müller, Neumann, Stieber, Ludwig, Maier, Decher, Arnold, Kirchhof, Fabritz and Brand2012; Jiang et al., Reference Jiang, Xu, Emery, Gerfen, Eiden and Eiden2017). Therefore, characterization of these channels through heterologous expression is required to understand the biophysical properties of these new channels. Alternatively, it is worth trying a direct patch-clamp recording to the male gamete of brown algae. As they have more abundant cytoplasm than most animal spermatozoa and their cell bodies are not covered with extracellular matrix, it could be easier to perform a whole cell patch-clamp recording with the brown alga male gametes than that with animal spermatozoa previously reported (Kirichok et al., Reference Kirichok, Navarro and Clapham2006; Lishko et al., Reference Lishko, Botchkina, Fedorenko and Kirichok2010; Orta et al., Reference Orta, Ferreira, José, Treviño, Beltrán and Darszon2012). It is worth mentioning that an orthologue of the diKCNG channel was detected by proteomics analysis of the brown algal flagellar protein of Colpomenia bullosa (Fu et al., Reference Fu, Nagasato, Oka, Fu, Nagasato, Oka, Cock and Motomura2014), suggesting a role for this channel in flagellar beating regulation such as phototaxis. Further experiments are required to understand the physiological function of the diKCNG channel.
Acknowledgements
We are grateful to all the staff members of the Education and Research Center of Marine Bio-Resources, Tohoku University, National BioResource Project (NBRP), and International Coastal Research Center and Misaki Marine Biological Station (MMBS), University of Tokyo for supplying Ciona intestinalis. We also thank Dr Toyoki Iwao, Fisheries Science Center of Toba for collecting M. cylindricus. TN appreciates Dr Takeaki Hanyuda for his help, and to the Kobe University International Exchange Division for the financial support of residential expenses during TN’s sabbatical stays in Kobe University.
Conflicts of interest
None.
Financial support
This work was supported by DGAPA-UNAM (TN, PAPIIT IN206116, IN205719 and PASPA); JSPS (NKT, Grant-in-Aid Research Fellow; MY, KAKENHI JP15H04398; HK, KAKENHI 16H04832).
Ethical standards
Not applicable.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0967199419000224 (DOI of that particular article).







