Introduction
The morphology and quality of mammalian blastocysts is related to the in vitro culture (IVC) conditions. Therefore, the number of cells in blastocysts produced by in vitro fertilization (IVF) or somatic cell nuclear transfer (SCNT) may be directly affected by the IVC medium in which they are cultured. Various types of IVC media have been developed in which to culture porcine embryos, such as modified Whitten's medium (Beckmann & Day, Reference Beckmann and Day1993), North Carolina State University 23 and 37 (NCSU-23 and 37) media (Petters & Wells, Reference Petters and Wells1993), Iowa State University medium (Youngs et al., Reference Youngs, Ford, McGinnis and Anderson1993), Beltsville embryo culture medium 3 (Im et al., Reference Im, Lai, Liu, Hao, Wax, Bonk and Prather2004), and porcine zygote medium 3 (Mao et al., Reference Mao, Tessanne, Whitworth, Spate, Walters, Samuel, Murphy, Tracy, Zhao and Prather2012). In these media, the mean number of cells per porcine blastocyst ranges from 14 to 92 (Beckmann & Day, Reference Beckmann and Day1993; Petters & Wells, Reference Petters and Wells1993; Youngs et al., Reference Youngs, Ford, McGinnis and Anderson1993; Im et al., Reference Im, Lai, Liu, Hao, Wax, Bonk and Prather2004; Mao et al., Reference Mao, Tessanne, Whitworth, Spate, Walters, Samuel, Murphy, Tracy, Zhao and Prather2012; Yamanaka et al., Reference Yamanaka, Sugimura, Wakai, Kawahara and Sato2009;). Moreover, various media have been reported in which to culture bovine embryos, including Charles Rosenkrans (CR) medium supplemented with amino acids (CR1-aa) (Fakruzzaman et al., Reference Fakruzzaman, Bang, Lee, Kim, Ha, Ghanem, Han, Cho, White and Kong2013; Ha et al., Reference Ha, Lee, Jeon, Park, Lee, Jin, Sessions, Wang, White and Kong2014a,Reference Ha, Park, Jin, Lee, Ko, Lee, White and Kongb), CR2 (Wang et al., Reference Wang, Panter, Holyoak, Molyneux, Liu, Evans and Bunch1999), synthetic oviduct fluid (SOF) (Sagirkaya et al., Reference Sagirkaya, Misirlioglu, Kaya, First, Parrish and Memili2007), modified SOF (Lim et al., Reference Lim, Lee, Kang and Hwang2003), tissue culture medium 199 (Balasubramanian & Rho, Reference Balasubramanian and Rho2007), potassium simplex optimized medium (KSOM) (Arias et al., Reference Arias, Ross and Felmer2013), G1.2/G2.2 sequential media (Lane et al., Reference Lane, Gardner, Hasler and Hasler2003), bovine embryo culture medium (Lim et al., Reference Lim, Okitsu, Okuda and Niwa1994) and Menezo's B2 medium (Farin et al., Reference Farin, Hasler, Martus and Stokes1997). In these media, the mean number of cells per bovine blastocyst ranges from 82 to 207 (Lim et al., Reference Lim, Okitsu, Okuda and Niwa1994, Reference Lim, Lee, Kang and Hwang2003; Farin et al., Reference Farin, Hasler, Martus and Stokes1997; Wang et al., Reference Wang, Panter, Holyoak, Molyneux, Liu, Evans and Bunch1999; Lane et al., Reference Lane, Gardner, Hasler and Hasler2003; Balasubramanian & Rho, Reference Balasubramanian and Rho2007; Sagirkaya et al., Reference Sagirkaya, Misirlioglu, Kaya, First, Parrish and Memili2007; Arias et al., Reference Arias, Ross and Felmer2013; Fakruzzaman et al., Reference Fakruzzaman, Bang, Lee, Kim, Ha, Ghanem, Han, Cho, White and Kong2013; Ha et al., Reference Ha, Lee, Jeon, Park, Lee, Jin, Sessions, Wang, White and Kong2014a,Reference Ha, Park, Jin, Lee, Ko, Lee, White and Kongb). In most studies on porcine and bovine embryos, culture in IVC media increased the percentage of embryos that developed to the blastocyst stage, but did not significantly increase the number of cells per blastocyst. To produce high-quality blastocysts that contain a higher number of cells, IVC media need to be improved.
Blastocysts of an improved quality and containing more cells are important for several reasons. First, blastocysts that contain a high number of cells can assist single blastocyst analyses. Several blastocysts are required to obtain sufficient DNA for analyses, whereas only single blastocysts would be required if they contained an increased number of cells. Second, blastocyst transfer is a commonly used method in bovine transplantation trials. Hence, blastocysts with an improved morphology and a higher number of cells may improve the pregnancy rate following such transplantation. Embryonic stem (ES) cells are derived from the inner cell mass (ICM) of blastocysts and can be established from mice and humans (Evans & Kaufman, Reference Evans and Kaufman1981; Martin Reference Martin1981; Thomson et al., Reference Thomson, Kalishman, Golos, Durning, Harris, Becker and Hearn1995; Thomson et al., Reference Thomson, Itskovitz-Eldor, Shapiro, Waknitz, Swiergiel, Marshall and Jones1998). Attempts to establish ES cell lines from domestic animals have been unsuccessful, due in part to the inability to develop suitable culture conditions. Therefore, development of new IVC systems to produce blastocysts with a larger ICM may increase the rate with which ES cell lines are successfully established.
In this study, we cultured porcine and bovine early blastocysts in human induced pluripotent stem cell (hiPS) medium for 2 days and then investigated the morphological quality of the blastocysts, as well as the total number of cells per blastocyst. Culture in hiPS medium increased the total number of cells per blastocyst by two-fold.
Materials and methods
All chemicals and reagents used in this study were purchased from Sigma Chemical Company (St. Louis, MO, USA). The various components of the hiPS medium were purchased from Invitrogen Inc. Cryopreserved semen was procured from Animal Husbandry Development, Inc., Yanbian City, Jilin Province, China.
Preparation and procurement of media
NCSU-37 and CR1-aa media were used to culture porcine and bovine embryos, respectively. hiPS medium was used to culture both porcine and bovine blastocysts. Detailed information about these media is provided in Table 1.
Table 1 Compositions of media used to culture porcine and bovine embryos

bFGF, basic fibroblast growth factor; BME EAA, Basal Medium Eagle containing essential amino acids; BSA, bovine serum albumin; FBS, fetal bovine serum; MEM NEAA, Minimum Essential Medium containing non-essential amino acids; P/S, penicillin–streptomycin; SR, serum replacement.
a All the components of human induced pluripotent stem cell (hiPS) medium were obtained from Invitrogen Inc.
b hiPS medium was prepared without bFGF and then supplemented with fresh bFGF immediately before use.
Production of porcine SCNT embryos
Ovaries were collected at a local abattoir and transported to the laboratory at 30–35°C. Cumulus–oocyte complexes (COCs) were aspirated from antral follicles (3–6 mm) using an 18-gauge needle and a regulated vacuum pump (KMAR-5100; Cook, Eight Mile Plains, Australia) and collected into a 50 ml centrifuge tube. COCs were pooled and washed three times with HEPES-buffered Tyrode medium (TALP-HEPES). Only COCs with a uniform ooplasm were transferred to 500 μl of maturation medium (NCSU-37 medium). The detailed information was previously published (Kang et al., Reference Kang, Li, Lu, Wang, Liang, Liu, Jin, Hong, Yan and Yin2013).
To prepare donor cells, a porcine fetus was obtained from a hybrid pig on day 30 of pregnancy. The tissue was cut into small pieces then washed three times and cultured at 38°C in an atmosphere of 5% (v/v) CO2 in air in Dulbecco's modified Eagle medium (DMEM). Detailed procedures have been published previously (Jin et al., Reference Jin, Li, Hong, Jin, Zhu, Guo, Gao, Yan, Kang and Yin2014).
The nuclear transfer protocol was adapted from a previous study (Yin et al., Reference Yin, Tani, Yonemura, Kawakami, Miyamoto, Hasegawa, Kato and Tsunoda2002). Mature eggs with the first polar body were enucleated. A single donor cell was inserted into the perivitelline space of each egg and electrically fused in 0.28 mol/l mannitol supplemented with 0.1 mmol/l MgSO4 and 0.01% polyvinyl alcohol (v:v). Fused eggs were cultured for 1 h in medium containing 0.4 μg/ml demecolcine before electroactivation and then cultured for 4h in medium supplemented with 2 mmol/l 6-dimethylaminopurine. The reconstructed oocytes were activated by two direct pulses of 100 V/mm for 20 μs in 0.28 mol/l manitol supplemented with 0.1 mmol/l MgSO4 and 0.05 mmol/l CaCl2. Activated eggs were cultured in NCSU-37 medium for 5 days at 38.5°C under a humidified atmosphere of 5% CO2 in air. Day 5 porcine SCNT blastocysts were cultured in 500 μl of hiPS medium or NCSU-37 medium for an additional 2 days. Moreover, the number of porcine blastocysts cultured in NCSU-37 medium or hiPS medium was 16 or 16, respectively.
In vitro production of bovine embryos and embryo transfer(ET)
Ovaries were collected from a local abattoir. Embryos were produced from oocytes in vitro as described previously (Jeong et al., Reference Jeong, Cho, Lee, Deb, Lee, Kwon and Kong2009). In brief, COCs were recovered from follicles 2–8 mm in diameter using an 18-guage needle connected to a 10 ml syringe, as previously described (Jo et al., Reference Jo, Bang, Kim, Choi, Jin, Kim, Jung, Suh, Ghanem, Wang and Kong2014), washed with in vitro maturation (IVM) medium (Ha et al., Reference Ha, Park, Jin, Lee, Ko, Lee, White and Kong2014b), and transferred to a well of a 4-well dish containing 700 μl of IVM medium for 23–24 h.
COCs were fertilized with sperm obtained from frozen semen, which was thawed by warming at 38°C for 1 min and washed in Dulbecco's phosphate-buffered saline by centrifugation at 750 g for 5 min at room temperature. The sperm pellet was dissolved with 500 μl heparin (20 μg/ml) in fertilization medium (Ha et al., Reference Ha, Lee, Jeon, Park, Lee, Jin, Sessions, Wang, White and Kong2014a) and incubated at 38.5°C in a humidified atmosphere of 5% CO2 in air for 15 min. Sperm, which were presumed to be capacitated, were diluted in IVF medium (concentration, 1 × 106 sperm/ml). Matured oocytes were transferred to IVF medium containing sperm for 18–20 h.
After IVF (day 0), COCs were removed by repeated pipetting and denuded zygotes were placed into a well of a 4-well plate containing 700 μl of CR1-aa medium (Rosenkranz et al., Reference Rosenkranz, Dickie, Auer and Holzmann1993), supplemented with 44 μg/ml sodium pyruvate, 14.6 μg/ml glutamine, 10 μl/ml penicillin–streptomycin, 3 mg/ml bovine serum albumin (BSA), and 310 μg/ml glutathione for 3 days (IVC-I). The presumed zygotes were cultured until day 7 of embryonic development (day 1 was the day on which IVC was performed) in the same medium, except that BSA was replaced with 10% (v:v) FBS from day 3 (IVC-II) at 38.5°C under a humidified atmosphere of 5% CO2 in air. Day 7 bovine blastocysts were transferred to 500 μl of hiPS medium or IVC-II medium for another 2 days. Moreover, the number of bovine blastocysts cultured in IVC-II medium or hiPS medium was 16 or 19, respectively.
For ET, representative day 8 blastocysts were removed from IVC-I (4 days), IVC-II (3 days) and hiPS medium (1 day). Two individual embryos to be transferred were loaded into a 1/4 insemination straw, fitted onto an ET gun, and transported to the farm where ET was performed with 1 h. Single embryos were transferred non-surgically to the uterine horn ipsilateral to the corpus luteum. Day 8 blastocysts were transferred to three recipient females. The recipients were checked for pregnancy at 35–40 days after embryo transfer.
Differential staining and evaluation of morphology
The quality of day 7 porcine blastocysts was assessed by differential staining of the ICM and trophectoderm (TE) cells. The number of cells in day 9 bovine blastocyst was evaluated by Hoechst 33342 staining. The staining procedures have been previously published (Thouas et al., Reference Thouas, Korfiatis, French, Jones and Trounson2001). Briefly, The TE of porcine SCNT blastocysts were stained when blastocysts were treated with a permeabilizing solution containing the ionic detergent Triton X-100 and the fluorochrome propidium iodide. Then, porcine blastocysts were incubated in a second solution containing 100% ethanol (for fixation) and the secondary fluorochrome bisbenzimide. Fixed and stained whole blastocysts were mounted and assessed for cell number using UV illumination of an epifluorescence microscope (IX71 Olympus, Tokyo, Japan).
The method used to evaluate the morphology of blastocysts is shown in Fig. 1. The mean diameter of the portion of the procine blastocyst that was extruded from the zona pellucida (D1) and the mean diameter of the portion of the bovine blastocyst (D2) were calculated as described previously (Laowtammathron et al., Reference Laowtammathron, Lorthongpanich, Ketudat-Cairns, Hochi and Parnpai2005).

Figure 1 Methods used to evaluate the morphology of porcine somatic cell nuclear transfer (SCNT) blastocysts and bovine in vitro fertilization (IVF) blastocysts. (A) D1: diameter of the portion of the porcine blastocyst extruded from the zona pellucida. (B) D2: diameter of the portion of the bovine blastocyst. Method used to calculate D1 of a porcine SCNT blastocyst below. Method used to calculate D2 of a bovine IVF blastocyst below. The mean value of D1 was calculated using a previously published equation (Laowtammathron et al., Reference Laowtammathron, Lorthongpanich, Ketudat-Cairns, Hochi and Parnpai2005).
Experimental design
Two experiments were conducted, and each experiment was performed more than four times. In the first experiment, porcine blastocysts were cultured in NCSU-37 medium for 5 days, and then in NCSU-37 medium or hiPS medium for an additional 2 days. Thereafter, the morphological quality of the blastocysts (day 7) was examined, as described previously (Laowtammathron et al., Reference Laowtammathron, Lorthongpanich, Ketudat-Cairns, Hochi and Parnpai2005) (Fig. 1 A). In addition, the number of cells per blastocyst was assessed by differential staining.
In the second experiment, bovine blastocysts were cultured in IVC-I medium for 3 days, then in IVC-II medium for 4 days, and then in IVC-II medium or hiPS medium for an additional 2 days. Thereafter, the diameter of the portion of the blastocyst (day 9) was calculated as shown in Fig. 1 B. The number of cells was evaluated by Hoechst 33342 staining.
Day 8 bovine blastocysts cultured in hiPS medium for 1 day were transferred to synchronized recipient heifers.
Statistical analysis
All data were obtained from more than three replicates. Diameters and nuclei numbers were analyzed by T-test (independent samples) using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). P-values <0.01 were regarded as statistically significant.
Results
Assessment of pig SCNT blastocysts morphological quality
Hatching of porcine blastocysts was clearly enhanced when they were cultured in NCSU-37 medium for 5 days and then in hiPS medium for another 2 days. The diameter of the portion of the blastocyst that was extruded from the zona pellucida was significantly lower in blastocysts that were continually cultured in NCSU-37 medium that in those that were switched to hiPS medium (221.47 ± 38.94 μm versus 481.87 ± 40.61 μm, P < 0.01). Therefore, the morphological quality of porcine SCNT blastocysts was significantly improved by hiPS medium. Blastocysts cultured in hiPS medium (Fig. 2 B) were much larger than those cultured in NCSU-37 medium (Fig. 2 A). In particular, the hatched portion of the blastocyst was much larger in the former blastocysts than in the latter blastocysts.
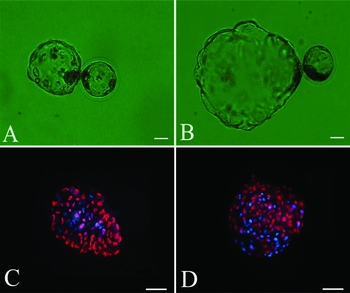
Figure 2 Differential staining of day 7 porcine blastocysts. Bright field images (A, B) and differential staining (C, D) of porcine somatic cell nuclear transfer (SCNT) blastocysts cultured in North Carolina State University (NCSU)-37 medium (A, C) or human induced pluripotent stem cell (hiPS) medium (B, D). Inner cell mass (ICM) cells and trophectoderm (TE) cells appear blue and red, respectively. (A, B) Original magnification, ×200. Scale bar = 80 μm. (C, D) Original magnification, ×200. Scale bar = 100 μm.
Cell numbers of porcine SCNT blastocysts
Table 2 shows the effects of culture in the two types of media on the quality of porcine SCNT blastocysts. The number of day 7 blastocysts evaluated was the same in the two conditions (i.e., 16). However, the total number of cells per blastocyst differed between the two conditions (44.33 ± 5.28 and 143.33 ± 16.05, P < 0.01). The total number of cells per blastocyst was more than two-fold higher in blastocysts cultured in hiPS medium than in those cultured in NCSU-37 medium (Fig. 2 C, D). The highest number of cells in a blastocyst was 164 (Fig. 2 D).
Table 2 Effects of culture in the two types of medium on the quality of porcine somatic cell nuclear transfer (SCNT) blastocysts

North Carolina State University (NCSU)-37: porcine SCNT embryos were cultured in NCSU-37 medium for 7 days, with a change of medium on day 5.
Human induced pluripotent stem cell (hiPS): porcine SCNT embryos were cultured in NCSU-37 medium for 5 days and then in hiPS medium for an additional 2 days (7 days in total).
D1, diameter of the portion of the blastocyst extruded from the zona pellucida.
a,b,c,d Different superscripts in a column indicate significant differences (P < 0.01).
Evaluation of bovine IVF blastocysts morphological quality
Culture of bovine blastocysts in IVC-I medium for 3 days, then in IVC-II medium for 4 days, and then in hiPS medium for another 2 days improved blastocyst hatching. The number of day 9 blastocysts assessed was 11 for blastocysts that were cultured in IVC-II medium and 19 for blastocysts that were cultured in hiPS medium. The diameter of the portion of the blastocyst that was markedly lower in blastocysts cultured in IVC-II medium than in those cultured in hiPS medium(150.30 ± 29.49 μm versus 195.58 ± 41.59 μm, P < 0.01). Therefore, culture in hiPS medium significantly improved the morphological quality of bovine blastocysts. Blastocysts cultured in hiPS medium (Fig. 3 B) were much larger than those cultured in IVC-II medium (Fig. 3 A). The hatched portion of the blastocyst was much larger in the former blastocysts than in the latter blastocysts.

Figure 3 Staining of day 9 bovine IVF blastocysts. Bright field images (A, B) and Hoechst 33342 staining (C, D) of bovine blastocysts cultured in in vitro culture (IVC)-II medium (A, C) or human induced pluripotent stem cell (hiPS) medium (B, D). (A, B) Original magnification, ×50. Scale bar = 150 μm. (C, D) Original magnification, ×200. Scale bar = 50 μm.
Cell numbers of bovine blastocysts
Table 3 shows the beneficial effects of hiPS medium on the quality of bovine blastocysts. The total number of cells per bovine blastocyst was significantly lower in blastocysts cultured in IVC-II medium than in those cultured in hiPS medium (172.12 ± 45.08 and 604.83 ± 242.64, P < 0.01). The total number of cells per blastocyst was more than two-fold higher in blastocysts cultured in hiPS medium than in those cultured in IVC-II medium (Fig. 3 C, D). The highest number of cells in a blastocyst was 992 (Fig. 3 D).
Table 3 Comparison of the quality of bovine in vitro fertilization (IVF) blastocysts cultured in the two types of media

CR1: bovine IVF embryos were cultured in in vitro culture (IVC)-I medium for 3days and then in IVC-II medium for 6 days; the IVC-II medium was changed once on day 7.
Human induced pluripotent stem cell (hiPS): bovine IVF embryos were cultured in IVC-I medium for 3 days, in IVC-II medium for 4 days, and then in hiPS medium for an additional 2 days (9 days in total).
D2, diameter of the portion of the blastocyst within the zona pellucida.
a,b,c,d Different superscripts in a column indicate significant differences (P < 0.01). SD, standard deviation.
In summary, culture in hiPS medium generated porcine and bovine blastocysts with an increased number of cells and an improved morphology.
Bovine embryo transfer
Bovine embryos were cultured in IVC-I (4 days), IVC-II (3 days) and hiPS medium (1 day). Then day 8 bovine blastocysts were transferred to three recipient heifers (two embryos/recipient). One of three recipients was in pregnancy.
Discussion
The present study demonstrated that in vitro-produced porcine and bovine early blastocysts was significantly improved by culture in hiPS medium for 2 days. Similar observations were occasionally made during a trial of human–pig chimera production in our experiment. In this trial, hiPS were injected into pig early blastocysts (day 5), and then the blastocysts were cultured in hiPS medium. After 2 days, the blastocysts were rather larger than control blastocysts. Subsequently, we investigated the in vitro development of porcine and bovine embryos cultured in NCSU-37, CR1, and hiPS medium.
NCSU-37 and CR-1aa have been reported in which to culture porcine and bovine embryos, respectively. hiPS medium is a comprehensive medium for hiPS cells with minimal spontaneous differentiation, high levels of pluripotency markers, differentiation potential and possession of a normal karyotype. DMEM/F-12, a 1:1 mixture of DMEM and Ham's F-12 Nutrient Mixture, serves as a basal medium consisting of typical amino acids, glucose, salts and vitamins. It has broad applicability in the serum-free culture of not only normal mouse and chicken cells, but also transformed cells, whose growth characteristics are likely altered in some way. The growth-promoting factors and hormones in DMEM/F-12 may have important effects on porcine and bovine blastocysts. Addition of essential amino acids (EAAs) and non-essential amino acids (NEAAs) to culture medium promotes the development of murine (Gardner & Lane, Reference Gardner and Lane1993), bovine (Takahashi & First, Reference Takahashi and First1992; Liu & Foote, Reference Liu and Foote1995), and porcine (Van Thuan et al., Reference Van Thuan, Harayama and Miyake2002; Suzuki & Yoshioka, Reference Suzuki and Yoshioka2006). NEAAs could provide favorable conditions for early cleavage and the expansion of porcine parthenogenetic diploid blastocysts, whereas EAAs could increase the total number of cells and the number of ICM cells (Van Thuan et al., Reference Van Thuan, Harayama and Miyake2002). However, EAAs and NEAAs were added even in the IVC-II medium, the cell numbers in bovine blastocysts cultured in IVC-II medium were not improved. Hence, the abnormal cell number in bovine blastocysts from hiPS medium might be due to EAAs and NEAAs interact with other ingredients. Besides DMEM/F-12 and NEAAs, hiPS medium also comprises other components, namely, KnockOut™ Serum Replacement, GlutaMAX™-1, β-mercaptoethanol, and basic fibroblast growth factor (bFGF). KnockOut™ Serum Replacement is a solution of BSA containing lipids, vitamins, trace elements, and low concentration of transferring and insulin. GlutaMAX™-1 contains a stabilized form of a dipeptide form of l-glutamine, namely, l-alanyl–l-glutamine which prevents degradation and ammonia build-up even during long-term cultures. β-Mercaptoethanol, a hybrid of ethylene glycol, is used to reduce disulfide bonds and can act as a biological antioxidant by scavenging hydroxyl radicals. bFGF is a bioactive protein intended for use in cell culture applications. It is involved in a number of biological processes, including embryonic development and differentiation, and the proliferation of cells of mesodermal and endodermal origins. The critical component of hiPS medium that plays a critical role in the development of porcine and bovine blastocyst is unknown; however, we are currently undertaking experiments to identify this factor. We have cultured porcine reconstructed embryos in hiPS medium for 7 days, during which time the medium was changed once, on day 5. In this condition, the blastocyst formation rate and blastocyst quality were not improved (unpublished data); thus hiPS medium may be unsuitable for the early development of porcine SCNT embryos. So far, only hiPS medium broke through the limit of number of cells per porcine and bovine blastocyst. Human iPS medium is suitable for the culture of early porcine and bovine blastocysts, but additional mechanistic studies and the expression levels of developmentally important genes need to be researched. Moreover, the effects of hiPS medium on embryos of other species also need to be investigated in the future.
Based on results of this study, we concluded that hiPS medium improves the morphological quality of porcine and bovine blastocysts, as well as the number of cells per blastocyst (more than two-fold higher than in other media).
Acknowledgements
This work was supported by the State Key Development Program for Basic Research of China (grant no. 2012CB526710).








