Introduction
At least 25 discoveries of physiological mechanisms have been made using sea urchin embryos that have been shown to be of importance in higher organisms including humans (Davidson & Cameron, Reference Davidson and Cameron2002; Davidson, Reference Davidson2006). As a result, the U.S. National Institutes of Health designated the sea urchin embryo as a model system that provides insights into mechanisms important in human health and disease (Davidson & Cameron, Reference Davidson and Cameron2002; Davidson, Reference Davidson2006). The sea urchin egg and embryo extracellular matrix have long been the subjects of study due, in part, to the spectacular structural and biochemical changes that occur in fertilization and early development. With the advent and maturation of molecular technique and the NIH designation of the sea urchin system as a medically relevant model, we have reinvestigated the possible function(s) of the hyaline layer and its constituent proteins/glycoproteins in embryogenesis.
Hyalin, a large glycoprotein of the sea urchin hyaline layer, and its hyalin repeat domain, a sequence of about 84 amino acids that is associated with the immunoglobulin-like fold, is found in organisms as diverse as bacteria, sea urchins, worms, flies, mice and humans (Wessel et al., Reference Wessel, Berg, Adelson, Cannon and McClay1998; Callebaut et al., 2000). In addition, p62 and p56, two proteins found in hamster egg cortical granules and in the zygote cortical granule envelope share a common immunological epitope with IL2, a polyclonal rabbit antibody prepared against 11.6 S hyalin (Justice & Carroll, Reference Justice and Carroll1989) isolated from Strongylocentrotus purpuratus embryos (Gray et al., Reference Gray, Justice, Nagel and Carroll1986).
The protein hyalin (actually a complex of three separate, related molecules, see below) is contained within the cortical granules of the unfertilized sea urchin egg (Hylander & Summers, Reference Hylander and Summers1982; Vater & Jackson, Reference Vater and Jackson1989), and forms the hyaline layer extracellular matrix in the fertilized egg within 20 min postinsemination (see the reviews by Schuel, Reference Schuel1978; Shapiro et al., 1981). The protein has been characterized and operationally defined by its solubility in divalent cation free media and its Ca2+ and Mg2+ precipitability. Extensive work from the laboratories of Carroll & Nagel (Gray et al., Reference Gray, Justice, Nagel and Carroll1986; Justice et al., Reference Justice, Gottschling, Carroll and Nagel1988, Reference Justice, Nagel, Gottschling, Damis and Carroll1992) has shown, in S. purpuratus, that hyalin consists of three large asymmetric molecules of 11.6S, 9.6S, and 6.4S. Polyclonal rabbit antibody work has shown that the three molecules exist in two immunological families (Justice, Reference Justice1989; Justice & Carroll, Reference Justice and Carroll1989). Although hyalin has been suggested to function in adhesive interactions, some direct evidence for this function has been obtained in sea urchin development, (Herbst, Reference Herbst1900; Fink & McClay Reference Fink and McClay1985; Adelson & Humphreys, Reference Adelson and Humphreys1988; Wessel et al., Reference Wessel, Berg, Adelson, Cannon and McClay1998) and in hamster cleavage (Hoodbhoy et al., Reference Hoodbhoy, Carroll and Talbot2000).
Microassays have been used to investigate the function of hyalin and other putative adhesive proteins in sea urchin embryos, often using disaggregated single cells (Fink & McClay, Reference Fink and McClay1985; McClay, Reference McClay and Schroeder1986; Wessel et al., Reference Wessel, Berg, Adelson, Cannon and McClay1998). Recently, we reported the use of a microplate assay that allowed for the quantification of developmental effects of exogenously added hyalin and other molecules in whole, living sea urchin embryos (Alvarez et al., Reference Alvarez, Nnoli, Carroll, Hutchins-Carroll, Razinia and Oppenheimer2007; Razinia et al., Reference Razinia, Carroll and Oppenheimer2007; Sajadi et al., Reference Sajadi, Rojas and Oppenheimer2007) and showed in part I of this series that hyalin isolated from S. purpuratus embryos blocked archenteron elongation/attachment to the blastocoel roof in S. purpuratus embryos (Razinia et al., Reference Razinia, Carroll and Oppenheimer2007). In part II of this series, we showed that S. purpuratus hyalin blocked this adhesive interaction in Lytechinus pictus embryos. Here we take these studies one important step further to learn if hyalin isolated from L. pictus has the same biological activity as S. purpuratus hyalin. We isolated hyalin, for the first time, from L. pictus embryos and tested its effects on early L. pictus development using the microplate assay. We found inhibition of archenteron elongation and attachment to the blastocoel roof and therefore provide evidence for a specific adhesive function of hyalin in addition to its role as an extracellular matrix molecular set.
Materials and Methods
Solutions
Artificial seawater (ASW; 423 mM NaCl, 9 mM KCl, 9.3 mM CaCl2, 22.9 mM MgCl2, 25.5 mM MgSO4, 2.1 mM NaHCO3, pH 8.0) was prepared by following the Marine Biological Laboratory (Woods Hole, MA) formula. Low-calcium seawater (LCSW) was prepared by reducing the calcium concentration to 1.5 mM (Bidwell & Spotte, Reference Bidwell and Spotte1985). All chemicals and reagents were prepared using materials obtained from Sigma Chemical Co, St Louis, MO, USA.
Preparation of hyalin
L. pictus sea urchins were obtained from Marinus Scientific, Garden Grove, CA. Spawning was induced by injecting 0.55 M KCl into the coelomic cavity. Eggs were collected by inverting the female and letting it shed its eggs over a beaker filled with artificial seawater at 11 °C. Sea urchin sperm was collected ‘dry’ in a petri dish and kept on ice until use. Eggs were filtered through 180 μm Nitex mesh (Tetco Inc.) and washed three times with large amounts of seawater. Subsequent dejellying of the eggs was achieved by bringing a suspension of 0.5% eggs in ASW immediately to pH 5.5–5.7 with 1 N HCl. The eggs were allowed to settle without being disturbed for 2 min before returning the suspension to pH 8.0 with 2 M Tris-base. The dejellied eggs were then washed three times with 500 ml of artificial seawater and their vitelline envelopes were disrupted with 0.01 M dithiothreitol (DTT), 0.1 M Tris-base in ASW, pH 9.2 for 3 min. The eggs were washed several times with 0.01 M Tris-ASW, pH 8.0. Four volumes of eggs were inseminated with 1 volume of dilute sperm (1 ml sperm/25 ml 0.01 M Tris-ASW, pH 8.0). During the 45–90 s post-insemination, the suspension was diluted in 8 volumes of ASW and the hyaline layers were allowed to form for 30–45 min while the eggs settled in the incubator at 15 °C. Hyalin protein was isolated and purified by the method described by Gray et al., Reference Gray, Justice, Nagel and Carroll1986 and Razinia et al., Reference Razinia, Carroll and Oppenheimer2007 with the following variations. After the fertilized eggs settled, the supernatant was aspirated leaving adherent cells, and the hyaline layers were dissociated from the egg surfaces by the addition of 50 ml of 0.475 M NaCl–0.025 M KCl. Embryos were stirred in this solution for 15 min until the hyaline layers appeared substantially reduced. After the embryos settled, the supernatant that contained the hyalin was collected. Hyalin protein was centrifuged at 20 000 g for 15 min at 4 °C to eliminate residual sperm and insoluble contaminants. The supernatant solution containing hyalin protein was collected and frozen at −20 °C.
Protein concentration
Protein concentration was determined at 260 nm and 280 nm (Warburg and Christian, Reference Warburg and Christian1941).
Gel electrophoresis
In order to assess hyalin purity, electrophoresis was done with 4% (w/v) polyacrylamide gradient gels on vertical submarine slabs. Proteins were visualized by silver staining using the method of Merrill et al. (Reference Merrill, Goldman and Van Keuren1984), or using Coomassie Brilliant Blue G250. Prior to silver staining, gels were fixed with 40% methanol, and 10% acetic acid for 30 min.
Embryo extraction/culture
L. pictus gametes were obtained as previously detailed. Eggs were extensively washed three times at 15 min intervals with 500 ml of ASW, pH 8.0. The diluted sperm (1.2 ml concentrated sperm/5 ml ASW, pH 8.0) was combined with 6 ml of eggs suspended in 500 ml of ASW, pH 8.0. Early embryos were washed two times at 15 min intervals with 500 ml of ASW, pH 8.0 to eliminate excess sperm. The embryos were then transferred to a pyrex dish and were maintained in an incubator at 15 °C for a period of 24 h.
Microassay experiments
Using wide-mouthed pipette tips, 25 μl of 24-hour-old L. pictus embryos were transferred to 40 wells of a 96-well polystyrene flat-bottom microplate. There were about 10–15 live embryos per well. As the embryos were swimming, a precise sample quantity (number of embryos/well) could not be added to the wells. The embryos in each well were incubated at 15 °C for 24 h with 25 μl of various hyalin concentrations (0.00 mg/ml, 0.018 mg/ml, 0.036 mg/ml, 0.075 mg/ml, 0.100 mg/ml and 0.150 mg/ml) diluted with low calcium seawater. The same process was repeated 10 times for each concentration. Medium containing ASW, 0.475 M NaCl–0.025 M KCl, and LCSW were used as controls for the hyalin protein experimental samples. At 48 h, the embryos were fixed with 2.5 μl of 2.5% formaldehyde solution. Embryos in each well were observed using a Zeiss Axiolab (Oberkochen) photomicroscope and the archenteron morphologies were scored (complete, unattached, exogastrula or no invagination). For each treatment, the total sample size was obtained by combining the number of embryos in each well for the 10 replicates. The particular archenteron morphologies for the 10 replicates were recorded as mean percentages ± standard error of the mean (SEM). An unpooled two-sample t-test was used to analyse the significance of the observed differences in numbers of complete archenterons in the combined controls versus the high hyalin-treated samples. The differences were highly significant (p < 0.001).
Results
Hyalin was isolated in L. pictus as described by Gray et al. (Reference Gray, Justice, Nagel and Carroll1986) for S. purpuratus and modified by Razinia et al. (Reference Razinia, Carroll and Oppenheimer2007). The purity of hyalin preparations (0.2 mg protein/ml) was assessed on 4% polyacrylamide gels (Fig. 1). A deliberately overloaded gel is shown in Fig. 1, which indicates the presence of one major band of 11.6S hyalin. In this overloaded condition the small amount of a few minor species that are typically present is shown. These minor species are normally observed in hyalin preparations and probably represent smaller hyalin species as observed and described by Gray et al. (Reference Gray, Justice, Nagel and Carroll1986) in S. purpuratus.

Figure 1 4% (w/v) polyacrylamide gradient gel electrophoresis of L. pictus hyalin preparations. The massive 11.6 S hyalin band is indicated with an arrow. The direction of migration, toward the positive pole, is from top to bottom. The other minor species are present in purified hyalin preparations and are believed to represent smaller hyalin subunits (Gray et al., 1985) or minor contaminants.
In order to determine the morphological effects of exogenous hyalin on gastrulation, 24 h L. pictus embryos were incubated for 24 h with a variety of hyalin concentrations (using the preparation described above) and control media. The total sample numbers of embryos for 10 replicates treated with the various hyalin concentrations of 0.00, 0.18, 0.036, 0.075, 0.10 and 0.15 mg/ml in LCSW were: 267, 274, 212, 212, 227, and 262 and the controls were 319, 294, 312 and 208. As shown in Fig. 2a, each of the control media embryo samples showed normal archenteron elongation and attachment to the blastocoel roof, while embryos in the high hyalin concentration tested showed a failure of the archenteron attachment to the blastocoel roof.

Figure 2 (a) The effects of various media on archenteron morphology. L. pictus hyalin is at a concentration of 0.2 mg/ml LCSW. Error bars are ± SEM. (b) A dose-dependent response curve for various archenteron morphologies. This graph demonstrates the percentage of 48 h embryos that developed the following morphologies: complete archenterons, unattached archenterons and no invaginations when incubated with increasing hyalin concentrations. The data were plotted as mean percentages ± SEM of 10 experiments.
Figure 2b represents a hyalin dose–response curve for the microassay trials. The mean percentages ± SEM for each morphology (complete archenteron, unattached archenteron and no invagination) were plotted. In the absence of hyalin, 95.1 ± 1.80% of embryos developed full archenteron attachment as seen in Fig. 2b. At lower hyalin concentrations, (0.018 and 0.036 mg/ml), there was a reduction in archenteron attachment to 83.6 ± 7.6% and 66.0 ± 4.8% of the embryos respectively. At higher hyalin concentrations (0.10, 0.15 mg/ml), the percentage of embryos with attached archenterons decreased even more notably to 22.9 ± 8.3% and 6.1 ± 2.3% respectively. The differences in archenteron morphology between no hyalin controls and highest hyalin concentration were statistically highly significant (p < 0.001). Exogastrulation and inhibition of invagination were almost never observed in any samples. Prior to fixation, embryos were determined to be viable as they were moving. Figure 3a–c provides photographs of embryos incubated in the experimental and control media showing attached and unattached archenterons. Figure 3a shows control embryos with normal development and complete archenteron attachment. Figure 3b shows an unattached archenteron when embryos were exposed to low hyalin concentration (0.018 mg/ml). Figure 3c shows the high incidence of unattached archenterons noted at the high hyalin concentration (0.150 mg/ml).
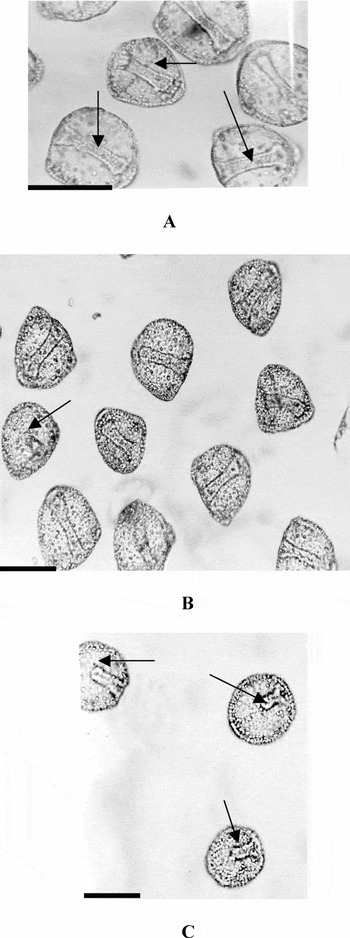
Figure 3 (a) 48 h L. pictus embryos incubated in low calcium seawater, pH 8.0, at 24 h in absence of L. pictus hyalin. Arrow show complete archenterons attached to the blastocoel roof (scale bar = 80 μm). (b) 48 h L. pictus sea urchin embryos treated with low L. pictus hyalin concentrations (0.018 mg/ml) incubated in low calcium seawater at 24 h demonstrating unattached and attached archenterons. Arrows show unattached archenterons (scale bar = 80 μm). (c) 48 h L. pictus embryos treated with high L. pictus hyalin concentration (0.150 mg/ml) incubated in low calcium seawater at 24 h. Note the unattached archenterons (scale bar = 80 μm).
Discussion
In the sea urchin S. purpuratus, in part I of this series, hyalin blocked invagination in gastrula stage S. purpuratus embryos in a dose-dependent manner from low concentrations (e.g. 0.05 mg/ml) to high hyalin concentrations (0.15 mg/ml and higher), where most embryos exhibited totally blocked invagination at the highest concentrations tested (Razinia et al., Reference Razinia, Carroll and Oppenheimer2007). At low hyalin concentrations (e.g. 0.05 mg/ml) most of the embryos invaginated and exhibited unattached archenterons (Razinia et al., Reference Razinia, Carroll and Oppenheimer2007). In the present study, high concentration of L. pictus hyalin (0.15 mg/ml) had no effect on archenteron invagination at the stages and times tested, while they inhibited archenteron elongation/attachment to the blastocoel roof in L. pictus embryos. The dose–response curve for L. pictus hyalin showed a nearly straight line of increasing hyalin activity in inhibiting archenteron elongation/attachment to the blastocoel roof, with increasing hyalin concentrations, with about 90% of the embryos exhibiting blocked archenteron attachment at 0.15 mg/ml low calcium seawater. The L. pictus results shown here indicate that hyalin blocks archenteron elongation/attachment in viable, swimming embryos, possibly by blocking hyalin/hyalin receptor or ligand interaction within the embryos, with no effect on invagination. It has been previously shown that large molecules can enter the sea urchin blastocoel in normal artificial seawater (Latham et al., Reference Latham, Martinez, Cazares, Hamburger, Tully and Oppenheimer1998) and with increasing speed in low calcium sea water (Latham et al., Reference Latham, Martinez, Cazares, Hamburger, Tully and Oppenheimer1998; Itza & Mozingo, Reference Itza and Mozingo2005). We suggest that exogenous hyalin may exert the effects described herein following entry into the blastocoel.
The microplate approach is one of the assay systems that we use in analysing the effects of molecules on sea urchin embryo cellular interactions. We also use a novel assay in which we dissect the archenteron and blastocoel roof from the embryo proper and study the interactions of these isolated components in a pristine environment away from possible confounding factors present in intact embryos (Coyle-Thompson & Oppenheimer, Reference Coyle-Thompson and Oppenheimer2005). The third assay system that we are beginning to use involves microinjection of molecules, such as hyalin into the sea urchin blastocoel. In collaboration with Dr Kathleen Foltz at the University of California, Santa Barbara, we have manually microinjected (total volume injected = 35 pl) of varying concentrations of L. pictus hyalin, from the same hyalin preparation as used here, into 24 h L. pictus embryo blastocoels using mercury-filled micropipettes (Jaffe & Terasaki, Reference Jaffe and Terasaki2004). The results of these initial experiments indicated that microinjected hyalin inhibited archenteron attachment in living embryos as compared with control injections that had no effect. Complete microinjection studies will be the subject of another paper. We are unaware of any other studies that approach functional analysis of molecules in the sea urchin model that utilize all three of these assay systems.
For many decades, studies have indicated that hyalin is important in maintaining proper adhesive interactions in developing sea urchin embryos (Herbst, Reference Herbst1900; Fink & McClay, Reference Fink and McClay1985; McClay, Reference McClay and Schroeder1986; Wessel et al., Reference Wessel, Berg, Adelson, Cannon and McClay1998). We are not aware of any studies that have focused on the role of hyalin in the model specific adhesive interaction described here. Sea urchin hyalin consists of units of the hyalin repeat domain that also have been identified in proteins in organisms as diverse as bacteria, flies, worms, mice and humans (Wessel et al., Reference Wessel, Berg, Adelson, Cannon and McClay1998; Callebaut et al., 2000) and non-repeated regions (Wessel et al., Reference Wessel, Berg, Adelson, Cannon and McClay1998). We are not aware of any studies that have focused on the specific function of the hyalin repeat domain, although the hyalin repeat is similar to the immunoglobulin-like fold that has been associated with the function of various cell adhesion molecules (Edelman, Reference Edelman1987; Whittaker et al., Reference Whittaker, Bergeron, Whittle, Brandhorst, Burke and Hynes2006). Further utilization of the three assay systems just described and the use of anti-hyalin antibody will help elucidate the function of hyalin, which we have termed a cell adhesion molecule that appears to be required for archenteron attachment to the blastocoel roof. In addition, isolation of hyalin binding receptors or ligands, so far under-investigated, should be feasible using hyalin derivatized beads and the three assay systems for assessing activity of isolated hyalin binding ligands or receptors.
The S. purpuratus sea urchin genome includes about 400 gene predictions of molecules that include immunoglobulin-like domains as well as many gene predictions of a variety of so-called cell adhesion molecules found across the animal phyla (Whittaker, et al., Reference Whittaker, Bergeron, Whittle, Brandhorst, Burke and Hynes2006). These gene predictions have been recently identified with the sequencing of the S. purpuratus genome (Whittaker et al., Reference Whittaker, Bergeron, Whittle, Brandhorst, Burke and Hynes2006). Little work, however, has been done in identifying specific functions for most of theses molecules during sea urchin development (Whittaker et al., Reference Whittaker, Bergeron, Whittle, Brandhorst, Burke and Hynes2006).
The use of the three assay systems could help reveal some of these specific functions. We have used two of the assays in identifying a putative role for glucose/mannose groups in the specific adhesive interaction studied here (Latham et al., 1999; Khurrum et al., Reference Khurrum, Hernandez, Eskalaei, Badali, Coyle-Thompson and Oppenheimer2004; Coyle-Thompson & Oppenheimer, Reference Coyle-Thompson and Oppenheimer2005; Sajadi et al., Reference Sajadi, Rojas and Oppenheimer2007) and are currently examining the relationship between these past findings with respect to sugar involvement, and the hyalin results reported here, in Razinia et al. (Reference Razinia, Carroll and Oppenheimer2007), and in Alvarez et al. (Reference Alvarez, Nnoli, Carroll, Hutchins-Carroll, Razinia and Oppenheimer2007).
Acknowledgements
This work was supported by grants from NIH NIGMS SCORE (S0648680), NIH NIGMS RISE, NIH NIGMS MARC, and the Joseph Drown Foundation.





