Introduction
For the evaluation of DNA integrity and other qualitative criteria of sperm, such as viability, intact acrosome or mitochondrial activity, flow cytometry has been be used (Graham et al., Reference Graham, Kunze and Hammerstedt1990; Nagy et al., Reference Nagy, Hallap, Johannisson and Rodriguez-Martinez2004; Gillan et al., Reference Gillan, Evans and Maxwell2005; Evenson and Wixon, Reference Evenson and Wixon2006). The assessment of chromatin integrity of bovine sperm by the Sperm Chromatin Structure Assay (SCSA) has been described by Evenson et al. (Reference Evenson, Larson and Jost2002). So far this method it has been used to assess sperm chromatin integrity mostly in fresh or frozen-thawed semen of men, bulls and boars (Rybar et al., 2004; Smit et al., Reference Smit, Dohle, Hop, Wildhagen, Weber and Romijn2007).
In cattle, high yields of in vitro embryos can be achieved if enough motile spermatozoa, with an intact acrosome and DNA, are separated from bull semen. Several methods are used to recover a population of motile spermatozoa from frozen-thawed bull semen, the fast and efficient technique of centrifugation on a Percoll gradient being most frequently used. In comparison with other separation methods, this method provides a relatively high yield of motile spermatozoa usable for in vitro fertilization (Parrish et al., Reference Parrish, Krogenaes and Susko-Parrish1995). Although this population has a high proportion of viable spermatozoa with intact acrosomes (Alomar et al., Reference Alomar, Mahieu, Verhaeghe, Defoin and Donnay2006), their DNA may be damaged, because the sperm of some bulls can be highly sensitive to this treatment and may require the use of more gentle separation techniques, such as the swim-up technique or centrifugation on a Sephadex column. It has been reported that centrifugation itself may increase the levels of oxygen free radicals that bring about the risk of damage to sperm DNA (Aitken and Clarkson, Reference Aitken and Clarkson1988; Zalata et al., Reference Zalata, Hafez and Comhaire1995).
A successful outcome of fertilization also requires that, at an appropriate time, a high proportion of these spermatozoa were able to undergo the acrosomal reaction. In order to stimulate the acrosomal reaction, fertilization media are supplemented with various capacitacion agents; the most common is heparin whose effect on sperm is related to its concentration, glucose and calcium levels and pH of the capacitacion medium (Van Soom and de Kruif, Reference Van Soom and de Kruif1996; Pereira et al., 2000; Mendes et al., Reference Mendes, Burns, De La Torre-Sanchez and 2003). Nevertheless, even under standard conditions of capacitacion, the proportion of acrosome-reacted spermatozoa can be highly variable depending on the bull's breed and its individual characteristics, as shown by Sumantri et al. (Reference Sumantri, Ooe, Saha and Boediono1996). Similarly, to separation, also capacitacion of sperm may produce specific damage to DNA (Silva and Gadella, Reference Silva and Gadella2006).
In bulls it has been suggested that reduced in vitro fertility can be caused by changes in DNA integrity of sperm and that these changes can occur during either the separation or the capacitacion procedure (Boe-Hansen et al., Reference Boe-Hansen, Averz, Christensen, Lehn-Jensen and Greve2003). Similar results have been reported by Ahmadi and Soon-Chye (Reference Ahmadi and Soon-Chye1999) in mice. In human sperm, it was observed that physical damage to DNA was related to a lower in vitro fertility and a subsequent poor embryo development (Filatov et al., Reference Filatov, Semenova, Vorobeva, Leonteva and Drobchenko1999; Larson-Cook et al., Reference Larson-Cook, Brannian, Hansen, Kasperson, Aamold and Evenson2003; Payne et al., Reference Payne, Raburn, Couchman, Price, Jamison and Walmer2005). Virant-Klun (Reference Virant-Klun, Tomazevic and Meden-Vrtovec2002) described that the human oocytes fertilized with sperm containing a higher proportion of damaged chromatin resulted in a significantly higher percentage of embryos that were fragmented, retarded or arrested in their development. A correlation between the quality of chromatin and the patho-morphological assessment of the sperm in human (Spano et al., 2000; Saleh et al., Reference Saleh, Agarwal, Nelson, Nada, El-Tonsy, Alvarez, Thomas and Sharma2002) as well as in bulls has been reported (Sailer et al., Reference Sailer, Jost and Evenson1996). Fatehi et al. (Reference Fatehi, Bevers, Schoevers, Roelen, Colenbrander and Gadella2006) report that although bovine spermatozoa with damaged chromatin may be capable of fertilization, their embryos show lesser development that correlates with the degree of sperm chromatin damage.
In bulls, the efficiency of in vitro fertilization is highly variable amongst individual sires. Sometimes their in vitro fertility does not correspond to their field fertility. In order to eliminate that variability in the fertilization efficiency is not related to damage caused by separation and capacitacion procedures we evaluated sperm chromatin integrity at various time intervals before and after separation and during capacitacion.
Materials and Methods
Bulls
A group of 17 AI bulls, Czech pied breed, with good non-return rates (52.3 to 67.3%) but variable results of embryo production (3.7 to 37.2%), were used in our experiments. These bulls were selected on the basis of a preliminary experiments in which embryos were produced by the standard protocol (Machatkova et al., Reference Machatkova, Hanzalova, Horakova, Reckova and Hulinska2006).
Motile sperm
Separation, capacitacion and incubation
From each bull, semen from five insemination doses was used. The semen was thawed in a water bath 37 °C warm for 60 s and pooled. Then, the semen was then layered onto a Percoll gradient and centrifuged for 30 min at 700 g. The pellet containing motile sperm was washed twice in SP–TALP medium at 200 g for 10 min. Subsequently, sperm were resuspended in IVF–TALP medium to a final concentration of 25 × 106 sperm/ml. Sperm capacitacion took place in the presence of 10 μg/ml heparin (H+) and sperm incubation was carried out in medium without heparin (H−) for 6 h at room temperature each.
Sample collection and preparation
For each bull, samples of semen before separation, motile sperm after separation and sperm at 3 h and 6 h of capacitacion and at the same intervals of incubation were collected. They were diluted with TNE buffer (0.15 M NaCl, 0.01 M Tris–HCl, 1 mM disodium EDTA, pH 7.4) to give a final concentration of 2 × 106 sperm/ml and then were stored at −80 °C until examination.
Sperm chromatin structure assay (SCSA)
Sample examination
The method described by Evensonem et al. (2002) was used as follows: Samples were thawed in a water bath at 37 °C and then maintained on ice at ±4 °C. A 100 μl sperm suspension was mixed with 200 μl of acidified detergent solution (0.08 M HCl, 0.1% Triton X 100, pH 1.2) and after 30 s spermatozoa were stained by adding 600 μl of acridine orange staining solution (0.037 M citric acid, 0.126 M Na2HPO4, 0.0011 M disodium EDTA, 0.15 M NaCl, pH 6.0). Samples were examined at 3 min after adding the acidified detergent in a flow cytometer (Dickinson) in duplicate; 10 000 spermatozoa were evaluated in each sample.
Sample evaluation
The flow cytometric assay allows us to evaluate damage to sperm chromatin structure on the basis of an increased susceptibility of DNA to acid-induced denaturation and express it by means of the DNA fragmentation index (DFI). Spermatozoa with intact chromatin structure show no DNA fragmentation and, therefore, have a non-detectable fragmentation index (non-DFI-sperm), while spermatozoa with damaged chromatin integrity have a detectable fragmentation index (det-DFI-sperm), as calculated by the SCSA-soft software. For the purpose of this study, the proportion of non-DFI-sperm was used. This measurement was determined in all samples and the kinetics of chromatin integrity changes was defined for each bull. Similarities in chromatin change kinetics allowed us to categorize the bulls into three groups.
Statistical analysis
The data analysed by Wilcoxon's test using Statistica software were expressed as mean percentage ± S.M.E. and ± S.D. values and presented as box-and-whisker diagrams.
Results
Bull categorization by chromatin change kinetics
The sperm response to separation and capacitacion evaluated by changes in their chromatin integrity was expressed as a non-DFI-sperm proportion. On the basis of this criterion, the bulls fell into three groups, namely, DNA-unstable, DNA-stable and DNA-most stable bulls (DNA-us, n = 3; DNA-s, n = 5; and DNA-ms, n = 9, respectively).
DNA-us bulls
In this bull group, separation resulted in a significant increase of the mean non-DFI-sperm proportion (p ≤ 0.01), as compared with its value before separation (minimal non-DFI-sperm value, 79.4% versus 92.5%, the increase was 13.1%). The subsequent capacitacion produced a significant decrease in the mean non-DFI-sperm proportion in H+ sperm (p ≤ 0.01; Fig. 1).

Figure 1 Mean percentage of non-DFI-sperm in DNA-us bulls during separation and capacitacion. PS, before separation; P0, after separation; P3, after 3-h capacitacion or incubation; P6, after 6-h capacitacion or incubation.
In spermatozoa of DNA-us bulls, after separation variance in non-DFI-sperm proportions markedly decreased (Fig. 2). During capacitacion of H+ sperm variance in non-DFI-sperm proportion values remained practically the same (Fig. 3). The variance in non-DFI-sperm proportion values in H− sperm increased with incubation time (Fig. 4).

Figure 2 Variance of non-DFI-sperm percentage in DNA-us bulls before and after separation. PS, before separation; P0, after separation.

Figure 3 Variance of non-DFI-sperm percentage in DNA-us bulls during capacitacion. P0, after separation; P3, after 3-h capacitacion or incubation; P6, after 6-h capacitacion or incubation.

Figure 4 Variance of non-DFI-sperm percentage in DNA-us bulls during incubation. P0, after separation; P3, after 3-h capacitacion or incubation; P6, after 6-h capacitacion or incubation.
DNA-s bulls
In this bull group, separation significantly increased the mean non-DFI-sperm proportion (p ≤ 0.01) in comparison with that before separation, but the increase was only about half of the value achieved in the DNA-us bulls (minimal non-DFI-sperm values, 80.9% and 92.6%, respectively; increase, 11.7%). During capacitacion as well as incubation, the mean non-DFI-sperm proportion remained almost unchanged. (Fig. 5).

Figure 5 Mean percentage of non-DFI-sperm in DNA-s bulls during separation and capacitacion. PS, before separation; P0, after separation; P3, after 3-h capacitacion or incubation; P6, after 6-h capacitacion or incubation.
In spermatozoa of DNA-s bulls, after separation variance in non-DFI-sperm proportion values decreased (Fig. 6). During capacitacion of H+ sperm, variance in the non-DFI-sperm proportions decreased slightly at 3 h and remained unchanged at 6 h (Fig. 7). The incubation of H− sperm had a little effect on variance in the non-DFI-sperm proportion values (Fig. 8).

Figure 6 Variance of non-DFI-sperm percentage in DNA-s bulls before and after separation. PS, before separation; P0, after separation.

Figure 7 Variance of non-DFI-sperm percentage in DNA-s bulls during capacitacion. P0, after separation; P3, after 3-h capacitacion or incubation; P6, after 6-h capacitacion or incubation.

Figure 8 Variance of non-DFI-sperm percentage in DNA-s bulls during incubation. P0, after separation; P3, after 3-h capacitacion or incubation; P6, after 6-h capacitacion or incubation.
DNA-ms bulls
Separation had no effect on the mean non-DFI-sperm proportion in this bull group. (minimal non-DFI-sperm values, 81.4% and 86.6%, respectively; increase 5.2%). The mean non-DFI-sperm proportion remained unchanged either during capacitacion or during incubation (Fig. 9).

Figure 9 Mean percentage of non-DFI-sperm in DNA-ms bulls during separation and capacitacion. PS, before separation; P0, after separation; P3, after 3-h capacitacion or incubation; P6, after 6-h capacitacion or incubation.
In spermatozoa of DNA-ms bulls, separation had a little effect on variance in non-DFI-sperm proportion (Fig. 10). The capacitacion of H+ sperm affected variance in non-DFI-sperm proportions to a certain extent (Fig. 11) as well as the incubation of H− sperm (Fig. 12).

Figure 10 Variance of non-DFI-sperm percentage in DNA-ms bulls before and after separation. PS, before separation; P0, after separation.
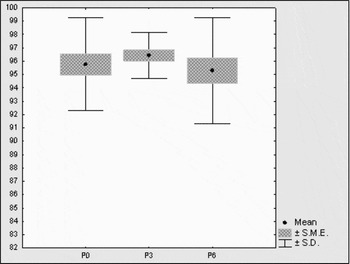
Figure 11 Variance of non-DFI-sperm percentage in DNA-ms bulls during capacitacion. P0, after separation; P3, after 3-h capacitacion or incubation; P6, after 6-h capacitacion or incubation.

Figure 12 Variance of non-DFI-sperm percentage in DNA-ms bulls during incubation. P0, after separation; P3, after 3-h capacitacion or incubation; P6, after 6-h capacitacion or incubation.
Discussion
No explanation for the finding that bulls with high field fertility can greatly vary in their in vitro fertility has so far been provided in the literature yet. To ascertain that the procedure before in vitro oocyte fertilization is not involved, we examined the spermatozoa chromatin integrity of 17 AI bulls and focused on reaction of their spermatozoa to separation and capacitacion treatment. On the basis of changes in the non-DFI-sperm proportion during separation and capacitacion, the sires were categorized as DNA-unstable, DNA-stable and DNA-most stable bulls.
The effect of different separation methods on chromatin integrity of motile spermatozoa has already been studied. Halap et al. (2005) provided evidence that the swim-up method significantly increased the proportion of bovine spermatozoa with intact chromatin structure in comparison with spermatozoa in thawed semen. Chohan et al. (Reference Chohan, Griffin, Lafromboise, De Jonge and Carrell2004) showed in human sperm that, with the use of gradient centrifugation, 90% of frozen-thawed semen showed no damage to chromatin structure.
Also in our study, the separation of motile spermatozoa on a Percoll gradient resulted in a significant increase in proportions of spermatozoa with intact chromatin. The effect of separation on the proportion of sperm with intact chromatin, however, varied with the bulls examined. The spermatozoa of DNA-unstable bulls were affected most, spermatozoa of DNA-stable bulls were affected less and those of DNA-most stable bulls were not influenced at all. Regardless of the bull category, the process of separation reduced variance in non-DFI-sperm proportion values and of these most markedly in the spermatozoa of DNA-unstable bulls.
The effect of incubation on semen chromatin integrity has also been reported. Damage to DNA increasing with the incubation period was demonstrated by Boe-Hansen et al. (Reference Boe-Hansen, Ersbøll, Greve and Christensen2005) in boar spermatozoa incubated for 72 h. The harmful effect of exogenous H2O2 on sperm chromatin in incubated bull spermatozoa was found by Krzyzosiak et al. (Reference Krzyzosiak, Evenson, Pitt, Jost, Molan and Vishwanath2000). For bull spermatozoa, however, no data on changes in chromatin integrity in relation to separation and capacitacion procedures are available. In our study, incubation for 6 h brought about a decrease in the non-DFI-sperm proportion only in the spermatozoa of DNA-unstable bulls, in which heparin treatment intensified this change even more with capacitacion time.
The role of chromatin integrity in the fertilizing ability of spermatozoa has, in the first place, been studied in human medicine, as a potential cause of male infertility. The results suggests that there is a correlation between damage of chromatin structure integrity and reduced human male fertility (Larson et al., Reference Larson, De Jonge, Barnes, Jost and Evenson2000; Zini et al., Reference Zini, Bielecki, Phang and Zenzes2001; Virro et al., Reference Virro, Larson-Cook and Evenson2004; Lewis and Aitken, Reference Lewis and Aitken2005; Evenson and Wixon, Reference Evenson and Wixon2006). Chohan et al. (Reference Chohan, Griffin, Lafromboise, De Jonge and Carrell2004) showed that, in healthy donor semen, damage of sperm DNA was lower than in semen of infertile patients.
The authors who have studied relationships between chromatin integrity of bull sperm and its fertilizing ability have arrived to inconsistent conclusions. Madrid-Bury et al. (Reference Madrid-Bury, Perez-Gutierrez, Perez-Garnelo, Moreira, Sanjuanbenito, Gutierrez-Adan and Martinez2005) confirmed a high correlation between chromatin integrity in frozen bovine semen and the non-return rate. Conversely, Hallap et al. (Reference Hallap, Nagy, Haard, Jaakma, Johannisson and Rodriguez-Martinez2005) did not find any correlation between sperm chromatin integrity and bull fertility. In addition, attempts have been made to find relationships between sperm chromatin integrity, morphology of spermatozoa and sperm fertilizing ability; however, they differ in their results. Sailer et al. (Reference Sailer, Jost and Evenson1996) hold that abnormal morphology of spermatozoa is related to abnormal chromatin structure and fertility. On the other hand, Katska-Ksiazkiewicz et al. (Reference Katska-Ksiazkiewicz, Bochenek and Rynska2005) have found that bovine spermatozoa with normal morphology but damaged chromatin can penetrate oocytes. They also suggest that the morphology of bull spermatozoa is related to their fertilizing ability and that the chromatin structure shows high inter-individual variability. This finding is in agreement with the results of our study, which confirmed that, during the standard treatment of spermatozoa for in vitro oocyte fertilization, the response of spermatozoa in terms of chromatin integrity was different in each group of bulls. No relationship, however, between sperm chromatin quality of the bulls selected for this study and their ability to produce in vitro embryos (evaluated in preliminary experiments) was found.
It can be concluded that chromatin integrity of motile spermatozoa was influenced by separation and capacitacion procedures. Separation significantly increased the proportion of sperm with intact chromatin in the DNA-unstable and the DNA-stable bulls and but had no effect in the DNA-most stable bulls. Heparin treatment during capacitacion had a negative effect on chromatin integrity only in the DNA-unstable bulls but not on the DNA-stable or the DNA-most stable bulls. The different in vitro fertility of bulls was not associated with damage to sperm chromatin during separation and capacitacion, because enough sperm with intact chromatin was available for oocyte fertilization.
Acknowledgements
This study was supported by Grants MZE 0002716201 and 1B44018 of Ministry of Agriculture of Czech Republic.














