Introduction
Mammalian spermatozoa require a period of incubation in the female reproductive tract to acquire the capacity to fertilize (Chang, Reference Chang1951; Austin, Reference Austin1952; Yanagimachi, Reference Yanagimachi1994). The biochemical processes that occur in this incubation are collectively called capacitation. The capacitated spermatozoon can bind to the zona pellucida (ZP) of an egg, to undergo the acrosome reaction, in which the outer acrosomal membrane fuses with the overlying plasma membrane (Huang & Yanagimachi, Reference Huang and Yanagimachi1985) and to fertilize the egg. A recent study has shown that mouse sperm began the acrosome reaction (AR) before reaching the ZP, and that these sperm were able to penetrate the ZP and fertilize the egg (Jin et al., Reference Jin, Fujiwara, Kakiuchi, Okabe, Satouh, Baba, Chiba and Hirohashi2011). Thus, it is not absolutely clear if indeed the ZP is the physiological inducer of the acrosome reaction, however it is clear that ZP can do it. The signal transduction cascades that occur in sperm capacitation, involve several protein kinases (Bailey, Reference Bailey2010; Ickowicz et al., Reference Ickowicz, Finkelstein and Breitbart2012) and an increase in the cytosolic calcium concentration ([Ca2+]i) followed by an increase in intracellular pH (Florman et al., Reference Florman, Arnoult, Kazam, Li and O’Toole1998).
Mitogen-activated protein kinases (MAPKs) are serine–threonine kinases activated by diverse stimuli ranging from cytokines, growth factors, neurotransmitters, hormones, cellular stress, and cell adherence (Widmann et al., Reference Widmann, Gibson, Jarpe and Johnson1999). MAPKs are expressed in all eukaryotic cells and their activity is dependent on three components that are conserved from yeast to human: a MAPK kinase kinase (MKKK), MAPK kinase (MKK), and MAPK (Widmann et al., Reference Widmann, Gibson, Jarpe and Johnson1999). One member, extensively studied, of the MAPK family is the extracellular-regulated kinase (ERK) (Liebmann, Reference Liebmann2001). ERK1/2 is classically associated with receptor tyrosine kinases (RTKs) such as the epidermal growth factor receptor (EGFR) (Liebmann, Reference Liebmann2001; Shah et al., Reference Shah, Baukal, Shah and Catt2005). Once phosphorylated, and thereby activated (Rossomando, Reference Rossomando1989), ERK1/2 can activate several signalling cascade such as transcription factors, regulatory enzymes such as phospholipase A2 and their own upstream regulators (e.g. EGFR or the Ras exchange factor Sos) (Liebmann, Reference Liebmann2001). ERK1/2 also regulates many cellular responses including proliferation and differentiation (Liebmann, Reference Liebmann2001). Spermatogenic cells at different stages express ERK1/2 in similar amounts. In ejaculated human sperm Shc, Grb2, Rasp21, Raf and ERK1/2 have been found (Luconi et al., Reference Luconi, Barni, Vannelli, Krausz, Marra, Benedetti, Evangelista, Francavilla, Properzi, Forti and Baldi1998a, de Lamirande & Gagnon, Reference Lax, Rubinstein and Breitbart2002) and it has also been shown that ERK1/2 is expressed mainly in the tail and in the head of human sperm (Luconi et al., Reference Luconi, Krausz, Barni, Vannelli, Forti and Baldi1998b, Almog et al., Reference Almog, Lazar, Reiss, Etkovitz, Milch, Rahamim, Dobkin-Bekman, Rotem, Kalina, Ramon, Raziel, Breitbart, Seger and Naor2008). ERK1/2 has been shown to be phosphorylated at early stages of spermatogenesis and in epididymal spermatozoa, and this phosphorylation was found to be increased with their progress in the epididymis (Lu et al., Reference Lu, Sun, Breitbart and Chen1999). Furthermore, ERK1/2 has been shown to contribute in mitotic proliferation of primitive spermatogonia and, in later stages, in acquiring motility (Lu et al., Reference Lu, Sun, Breitbart and Chen1999). The published information regarding the involvement of ERK1/2 in sperm motility and capacitation is controversial. One study has suggested that ERK1/2 has an inhibitory effect on human sperm motility (Weidinger et al., Reference Weidinger, Mayerhofer, Kunz, Albrecht, Sbornik, Wunn, Hollweck, Ring and Kohn2005), whereas other studies suggest that inhibition of ERK1/2 during sperm capacitation decreases tyrosine phosphorylation, motility and capacitation (de Lamirande & Gagnon, Reference de Lamirande and Gagnon2002; Almog et al., Reference Almog, Lazar, Reiss, Etkovitz, Milch, Rahamim, Dobkin-Bekman, Rotem, Kalina, Ramon, Raziel, Breitbart, Seger and Naor2008). Moreover, it has been shown that inhibition of ERK1/2 decreases the rate of the acrosome reaction induced by lysophosphatidylcholine (LPC), progesterone, human ZP, γ-amino butyric acid (GABA) or 12-O-tetradecanoyl phorbol-13-acetate (PMA) (Luconi et al., Reference Luconi, Barni, Vannelli, Krausz, Marra, Benedetti, Evangelista, Francavilla, Properzi, Forti and Baldi1998a, Reference Luconi, Krausz, Barni, Vannelli, Forti and Baldi1998b; du Plessis et al., Reference du Plessis, Page and Franken2001; de Lamirande & Gagnon, Reference de Lamirande and Gagnon2002; Chen et al., Reference Chen, Ni, Pan, Shi, Yuan, Chen, Mao, Yu and Roldan2005; Almog et al., Reference Almog, Lazar, Reiss, Etkovitz, Milch, Rahamim, Dobkin-Bekman, Rotem, Kalina, Ramon, Raziel, Breitbart, Seger and Naor2008). In this regard it has also been found that Ca2+ potentiating responses are mediated through activation of the ERK1/2 cascade, suggesting a role in the regulation of Ca2+ fluxes prior to the acrosome reaction (Moynihan et al., Reference Moynihan, Tolloczko, Michoud, Tamaoka, Ferraro and Martin2008).
EGFR and RTKs in general are activated by a large family of peptidic ligands that induce the formation of active auto(trans)phosphorylated receptor homo-/heterodimers (Schlessinger, Reference Schlessinger1988; Lemmon & Schlessinger, Reference Lemmon and Schlessinger1994; Jiang & Hunter, Reference Jiang and Hunter1999). The active dimers, upon recruitment of adaptor and signalling proteins, initiate multiple signalling events (Schlessinger, Reference Schlessinger2000). G-protein-coupled receptor (GPCR) signalling is mediated by RTK such as EGFR, in a process called transactivation (Prenzel et al., Reference Prenzel, Zwick, Daub, Leserer, Abraham, Wallasch and Ullrich1999; Jorissen et al., Reference Jorissen, Walker, Pouliot, Garrett, Ward and Burgess2003; Wetzker & Bohmer, Reference Wetzker and Bohmer2003; Shah & Catt, Reference Shah and Catt2004), which has also been found in bovine sperm (Etkovitz et al., Reference Etkovitz, Tirosh, Chazan, Jaldety, Daniel, Rubinstein and Breitbart2009). In the few last years, it became apparent that GPCRs may elicit mitogenic responses that stimulate MAPK cascades (Liebmann, Reference Liebmann2001). The activation of the EGFR generates a Ca2+ signal, broadly defined as the transient rise of the intracellular Ca2+ concentration (Tinhofer et al., Reference Tinhofer, Maly, Dietl, Hochholdinger, Mayr, Obermeier and Grunicke1996; Che & Carmines, Reference Che and Carmines2002; Kawanabe et al., Reference Kawanabe, Nozaki, Hashimoto and Masaki2003; Heo et al., Reference Heo, Lee and Han2006). We have previously shown that bovine sperm contains EGFR that can mediate the occurrence of the acrosome reaction and actin polymerization during sperm capacitation (Lax et al., Reference Lax, Rubinstein and Breitbart1994; Brener et al., Reference Brener, Rubinstein, Cohen, Shternall, Rivlin and Breitbart2003; Etkovitz et al., Reference Etkovitz, Tirosh, Chazan, Jaldety, Daniel, Rubinstein and Breitbart2009). Moreover, EGFR-phosphorylation/activation was increased during incubation under capacitating conditions, and further stimulation with EGF at the end of the capacitation period increased intracellular calcium levels leading to the AR (Etkovitz et al., Reference Etkovitz, Tirosh, Chazan, Jaldety, Daniel, Rubinstein and Breitbart2009). Recently, we have found that EGFR mediates the AR induced by activating sperm acetylcholine receptor and that it binds the ZP, suggesting that the EGFR serves as a novel sperm receptor to the egg ZP (Jaldety et al., Reference Jaldety, Glick, Orr-Urtreger, Ickowicz, Gerber and Breitbart2012).
In this study, we investigated the role of ERK1/2 in sperm capacitation and the acrosome reaction upon binding to ZP. We show for the first time the involvement of ERK1/2 in calcium influx, which leads to the occurrence of the acrosome reaction.
Materials and methods
Materials
U0126, A23187, Fluo4/AM and protease inhibitor cocktail were purchased from Calbiochem (San Diego, CA, USA). Antibodies against phospho-ERK1/2 and ERK2 were purchased from Santa-Cruz (Santa-Cruz, CA, USA). Antibody against phospho-EGFR (Y845) and EGFR were purchased from AbCam. Goat anti-mouse IgG–HRP conjugated and goat anti-rabbit IgG–HRP conjugated were purchased from Bio-Rad (Richmond, CA, USA). Goat anti-mouse IgG–Alexa586 conjugated was purchased from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA, USA). All other chemicals were purchased from Sigma (Sigma-Aldrich Israel Ltd, Rehovot, Israel) unless otherwise stated.
Mouse sperm preparation and capacitation
Sexually mature male mice (C57) were sacrificed by CO2 asphyxiation. The pair of cauda epididymis and part of the vas-deferens were rapidly removed and minced in 0.5 ml HM medium (modified Krebs-Ringer bicarbonate medium; Visconti et al., Reference Visconti, Bailey, Moore, Pan, Olds-Clarke and Kopf1995a, Reference Visconti, Moore, Bailey, Leclerc, Connors, Pan, Olds-Clarke and Kopf1995b). The sperm were released from the epididymal lumen for 5 min at 37°C. The medium was carefully collected and the cells were washed by centrifugation (780 g, 5 min) in the same medium, and then left for swim-up for 5 min at 37°C. The motile fraction was carefully collected and the washed cells were counted and maintained at 37°C until use.
Capacitation of mouse epididymal sperm (1×107 cells/ml) was induced as described previously (Visconti et al., Reference Visconti, Bailey, Moore, Pan, Olds-Clarke and Kopf1995a). Briefly, sperm pellets were resuspended to a final concentration of 107 cells/ml in HMB-modified Krebs Ringer bicarbonate medium (HMB-HEPES buffered) containing: 119.4 mM NaCl, 4.8 mM KCl, 1.7 mM CaCl2, 1.2 mM MgSO4, 10 mM NaHCO3, 25 mM HEPES (pH 7.4), 25 mM sodium lactate, 5.56 mM glucose, 0.001% phenol red, 10 IU/ml penicillin, 3 mg/ml bovine serum albumin (BSA). The cells were incubated in this capacitation medium for 1.5 h at 37°C with 5% CO2.
Assessment of mouse sperm acrosome reaction
Acrosome reaction was induced by adding the inducer to capacitated cells for 30 min of incubation. An aliquot of spermatozoa was smeared on a glass slide and allowed to air dry. Spermatozoa were then permeabilized by methanol for 15 min at room temperature, washed three times at 5 min intervals with 25 mM Tris-buffered saline pH 7.6 (TBS) and air-dried. Fluorescein isothiocyanate (FITC)-conjugated peanut lectin agglutinin (PNA–FITC) was used onto air-dried spermatozoa smears to trace microscopically acrosome-reacted spermatozoa. Cells were incubated with PNA–FITC (12.5 μg/ml in TBS) for 0.5 h, washed with H2O, and mounted with FluoroGuard Antifade (Bio-Rad Laboratories, Richmond, CA, USA). For each experiment, at least 200 cells per slide on duplicate slides were evaluated (400 cells for one experiment). Cells with green staining over the acrosomal cap were considered to be acrosome intact; those with equatorial green staining or no staining were considered to be acrosome reacted. Induced acrosome-reacted rate was calculated after subtraction of the spontaneous acrosome reaction for each treatment.
Isolation of zonae pellucidae
Zonae pellucidae were prepared from ovarian homogenates as described earlier by Bleil & Wassarman (Reference Bleil and Wassarman1986). Briefly, large numbers of zonae pellucidae (5–20 × 103) were isolated by Percoll gradient centrifugation of ovarian homogenates. Ovaries dissected from 20–30 mice (21-day-old) were homogenized on ice in 4 ml of a buffer containing 25 mM triethanolamine, pH 8.5, 150 mM NaC1, 1 mM MgC12, 1 mM CaCl2, and 1 mg DNase and 1 mg hyaluronidase were added. The homogenate was brought to 1% Nonidet P-40 and 0.1 mM phenylmethylsulfonyl fluoride and subjected to about 10 more strokes of the pestle. The homogenate was then brought to 1% deoxycholate, mixed with 9 ml of homogenization buffer containing Percoll (72%) in a sealed-cap tube, and centrifuged at 25,000 rpm for 45 min at 4°C in a rotor. Under these conditions, the ZP appeared as a narrow, opaque band at a density of 1.02 g/ml.
In vitro fertilization (IVF) and detection of sperm–egg binding
Female (C57BL/6) mice (8–9-week-old) were super-ovulated with 5 IU of pregnant mare's serum gonadotropin (PMSG) followed at a 48-h interval by 5 IU of human chorionic gonadotropin (hCG), and sacrificed between 12 and 17 h after the hCG injection. Oocytes were liberated from the ampule into M16 medium. Mouse epididymal sperm (1 × 107 cells/ml) were prepared and capacitated as described. A sample of 105 sperm cells was added and was incubated in a 100-μl droplet (on average 15–25 eggs were present in each droplet for each experiment) at 37°C in 5% CO2 for 24 h. Then the eggs examined under the ×50 magnification of the dissecting microscope to determine the number of 1-cell and 2-cell eggs present.
Immunoblot analysis
Sperm lysates were prepared by the addition of lysis buffer to three times TBS washed sperm pellets. Lysis buffer contained 6% sodium dodecyl sulfate (SDS); 1 M Tris–HCl (pH 7.5); 1 mM sodium orthovanadate; 1 mM benzamidine; 50 mM sodium fluoride; 0.1 M sodium pyrophosphate; 1:100 protease inhibitor cocktail; 1 mM phenylmethylsulfonyl fluoride (PMSF), sperm were incubated for 15 min and then centrifuged at 10,000 g at 4°C. The supernatant was transferred into a clean eppendorf tube and sample buffer (5×) was added and samples boiled for 5 min. The extracts were separated on 10% SDS-polyacrylamide gels and then electrophoretically transferred to nitrocellulose membranes (200 mA, 2 h) using a buffer composed of 25 mM Tris (pH 8.2), 192 mM glycine, and 20% methanol. For western blotting, nitrocellulose membranes were blocked with 5% BSA in Tris-buffered saline, pH 7.6, containing 0.1% Tween 20 (TBS-T), for 30 min at room temperature. The membranes were incubated overnight at 4°C with the primary antibodies. Next, the membranes were washed three times with TBS-T and incubated for 1 h at room temperature with specific horseradish peroxidase (HRP)-linked secondary anti-rabbit or anti-mouse antibody (Bio-Rad Laboratories, Richmond, CA, USA) diluted 1:5000 in 1% BSA solution. The membranes were washed three times with TBS-T and visualized by enhanced chemiluminescence (Amersham, Little Chalfont, UK).
Immunocytochemistry
Anti-ERK2 antibody was used at a 1:50 dilution on permeabilized sperm smears, to determine intracellular localization of protein, as described previously (Etkovitz et al., Reference Etkovitz, Rubinstein, Daniel and Breitbart2007). Non-specific staining was determined by incubating the sperm in the presence of goat anti-rabbit IgG (H+L)–Alexa Fluor 568 alone diluted 1:200, and no staining was detected.
Microscopy
All images were captured on an Olympus AX70 microscope at a magnification of ×400. This microscope was equipped with an Olympus DP50 digital camera and with ‘Viewfinder Lite’ software (version 1 from Pixera Corporation (Los Gatos, California, USA). All fluorescence determinations were done under non-saturated conditions. Each experiment and staining was performed on the same day, and sperm were photographed within 24 h to reduce fading. All cell preparations from a single experiment were photographed during the same session and at the same exposure time.
Determination of mouse intracellular calcium
The intracellular concentration of free Ca2+ was assessed using the fluorescent calcium indicator, Fluo-4/AM. Washed cells (1 × 107/ml) were incubated in HMB-modified Krebs Ringer bicarbonate medium (HMB-HEPES buffered) for 30 min, then 1 μM Fluo-4/AM was added for a further 1 h. The loaded cells were then washed three times to remove extracellular Fluo-4/AM. The cells were used immediately for fluorescence measurements using a plate reader, with an excitation wavelength of 485 nm and emission of 535 nm. During fluorescence measurements, sperm suspensions were maintained at 37°C.
Bovine sperm preparation
Ejaculated bull spermatozoa were obtained using an artificial vagina, and the ‘swim-up’ technique was applied to obtain motile sperm. Bovine sperm was supplied by the ‘SION’ Artificial Insemination Center (Hafetz-Haim, Israel). Sperm cells were washed three times by centrifugation (780 g for 10 min at 25°C) in NKM (Na-K-MOPS buffer) buffer that contained 110 mM NaCl, 5 mM KCl, and 20 mM 3-N-morpholino propanesulfonic acid (MOPS) (pH 7.4) and the sperm were allowed to swim up after the last wash. The washed cells were counted and maintained at room temperature until use. Only sperm preparations that contained at least 80% motile sperm were used in the experiments, and the motility was not significantly reduced at the end of the incubations.
Bovine sperm capacitation
In vitro capacitation of bovine sperm was induced as described previously (Etkovitz et al., Reference Etkovitz, Rubinstein, Daniel and Breitbart2007). Briefly, sperm pellets were resuspended to a final concentration of 108 cells/ml in mTALP (modified Tyrode solution) medium containing: 100 mM NaCl, 3.1 mM KCl, 1.5 mM MgCl2, 0.92 mM KH2PO4, 25 mM NaHCO3, 20 mM HEPES (pH 7.4), 0.1 mM sodium pyruvate, 21.6 mM sodium lactate, 10 IU/ml penicillin, 1 mg/ml BSA, 20 μg/ml heparin, and 2 mM CaCl2. The cells were incubated in this capacitation medium for 4 h at 39°C with 5% CO2. The capacitation state of the sperm was confirmed after the 4 h incubation in mTALP by examining the ability of the sperm to undergo the acrosome reaction. In all experiment the control cells were treated with appropriate vehicle (Me2SO or water).
Determination of bovine intracellular calcium
The intracellular concentration of free Ca2+ was assessed using the fluorescent calcium indicator, Fura-2. Washed cells (1 × 108/ml) were incubated in mTALP for 3.5 h, then 1 μM Fluo-4/AM was added for a further 30 min. The loaded cells were then washed three times to remove extracellular Fura-2. The cells were used immediately for fluorescence measurements using a Shimadzu (Columbia, MD) RF-5000 spectrofluorophotometer, with an excitation wavelength of 340 nm and emission of 510 nm. During fluorescence measurements, sperm suspensions were maintained at 37°C with stirring.
Statistical analysis
Data are expressed as the mean ± standard deviation (SD) of at least three experiments. Statistical significance was assessed between groups using Student's t-test and differences of P < 0.05 were considered to be significant.
Results
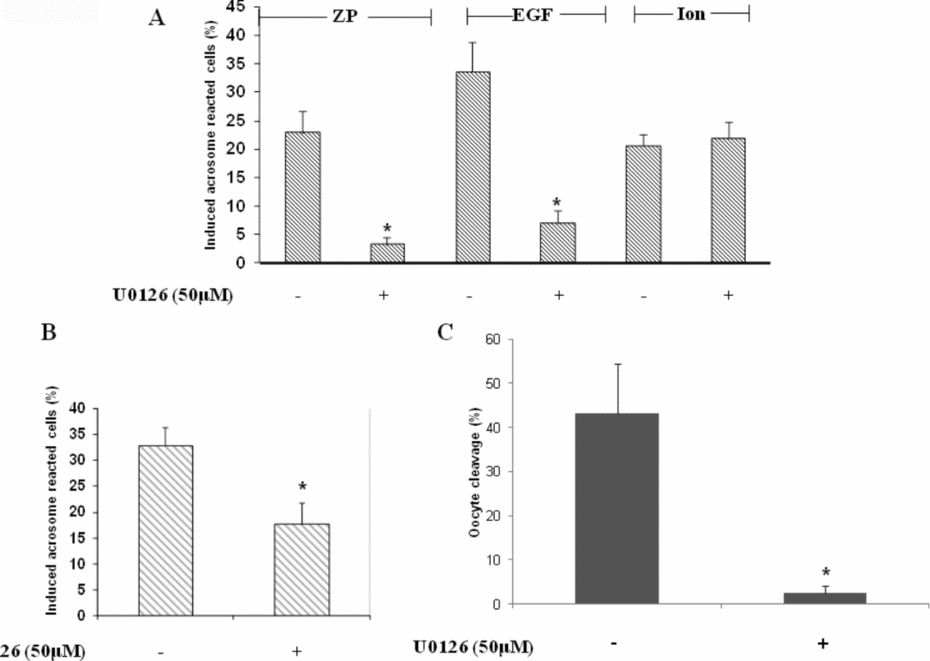
The presence of ERK2 in mouse sperm was examined using an immunocytochemistry assay, and the data revealed its localization in the acrosome region of the head as well as in the tail midpiece (Fig. 1A). Western blot analysis using anti-phopho-ERK1/2 revealed high phosphorylation after 10 min of incubation under capacitating conditions and this phosphorylation decreased with the progress of capacitation (Fig. 1B). The localization of ERK2 in the acrosomal region implies its possible involvement in the acrosome reaction. Evaluation of the acrosome reaction rate induced by ZP or EGF revealed 87% or 80% inhibition, respectively, by the ERK1/2 inhibitor U0126, however no inhibition was observed when the acrosome reaction was induced by the Ca2+ ionophore, A23187 (Fig. 2A). Furthermore, addition of U0126 at the end of the capacitation period revealed only 45% inhibition of the acrosome reaction induced by ZP (Fig. 2B). These data indicate that ERK1/2 mediates the acrosome reaction as well as the capacitation processes. Moreover, U0126 caused almost complete inhibition of the IVF rate (Fig. 2C), indicating the requirement of ERK1/2 in the fertilization process as well.

Figure 1 Presence and activation of ERK1/2 in mouse sperm. (A) 105 cells were smeared on a glass slide and were stained with anti-ERK2 antibody as described in Materials and methods. (B) Mouse sperm were incubated in capacitation medium and a sample for protein phosphorylation was taken in the indicated times. Sperm proteins were extracted with sodium dodecyl sulphate (SDS) lysis buffer and were separated on SDS-PAGE as described in Materials and methods. The nitrocellulose membrane was incubated with anti-phospho-ERK1/2 and with anti-ERK2 for protein amount. The data represent one experiment, typical of three repetitions performed.

Figure 2 Involvement of ERK1/2 in capacitation, acrosome reaction and fertilization. (A) Mouse sperm were incubated in capacitation medium for 1.5 h with or without U0126 (50 μM). At the end of capacitation period, zona pellucida (ZP) (~7.5 ZP/μl) or epidermal growth factor (EGF) (1 ng/ml) or Ca2+-ionophore (A23187, 10 μM) were added for an additional 30 min. (B) Mouse sperm were incubated in capacitation medium for 1.5 h, then U0126 (50 μM) was added for the last 15 min, then ZP (~7.5 ZP/μl) was added for an additional 30 min. Sperm samples were smeared on a slide for acrosome reaction determination as described in Materials and methods. Spontaneous acrosome reaction at zero time point ranged between 1–5% and after 90 min between 5–12% in the presence or absence of U0126. (C) Mouse sperm were incubated in capacitation medium for 1.5 h with or without U0126 (50 μM). Sperm (105) were added to metaphase II (MII) oocytes and the percentage of cleaved oocytes was determined after 24 h of incubation. The data represent the mean ± standard deviation (SD) of duplicates from at least three experiments. *Significant difference from the corresponding control, P < 0.05.
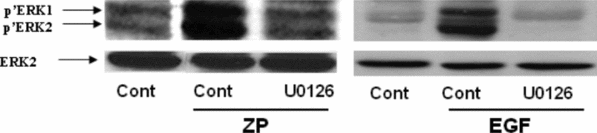
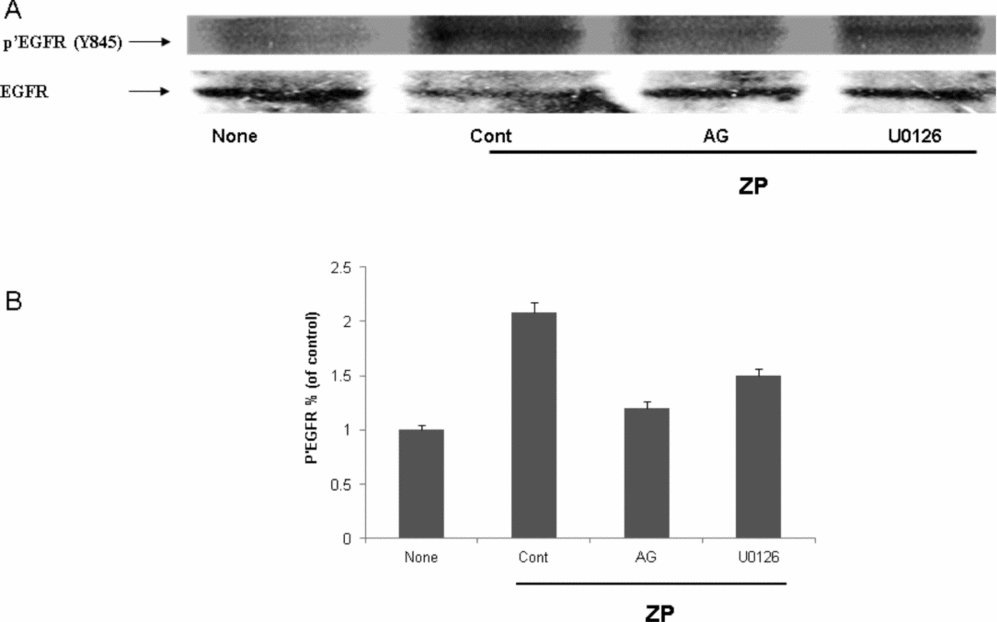
In order to further support the involvement of ERK1/2 activity in the acrosome reaction, its phosphorylation/activation was examined in response to ZP or EGF. For that analysis, ZP or EGF were added to capacitated sperm for 5 min and ERK1/2 phosphorylation was examined. It was shown that ZP or EGF increased the phosphorylation of ERK1/2 and U0126 inhibited this phosphorylation (Fig. 3). Furthermore, the phosphorylation/activation of EGFR on tyrosine 845 was enhanced in response to ZP and inhibited by AG1478, a specific inhibitor of the EGFR, but not significantly by U0126 (Fig. 4). These data indicate the participation of EGFR in the process of the acrosome reaction induced by ZP, and that ERK1/2 is located down-stream to the EGFR.

Figure 3 Activation of ERK1/2 in the acrosome reaction induced by zona pellucida (ZP) or epidermal growth factor (EGF). Mouse sperm were incubated in capacitation medium for 1.5 h with or without U0126 (50 μM), then EGF (1 ng/ml) or ZP (~7.5 ZP/μl) were added for an additional 5 min. Sperm proteins were extracted with sodium dodecyl sulphate (SDS) lysis buffer and The proteins were separated on SDS-PAGE as described in Materials and methods. The nitrocellulose membrane was incubated with anti-phospho-ERK1/2 (Y-204) and with anti-ERK2. The data represent one experiment, typical of three repetitions performed.

Figure 4 Activation of epidermal growth factor receptor (EGFR) in the acrosome reaction induced by zona pellucida (ZP). Mouse sperm were incubated in capacitation medium for 1.5 h with or without AG1478 (10 μM) or U0126 (50 μM). ZP (~7.5 ZP/μl) was added for an additional 2 min. Sperm proteins were extracted with sodium dodecyl sulphate (SDS) lysis buffer and the proteins were separated on SDS-PAGE as described in Materials and methods. The nitrocellulose membrane was incubated with anti-phospho-EGFR (Y-845) and with anti-EGFR. The data represent one experiment, typical of three repetitions performed (A). Densitometric analysis of the anti-phosphor-EGFR bands shown in (A). (B) The data represent the mean ± standard deviation (SD) from three different experiments. Significance from the corresponding control, P < 0.05.
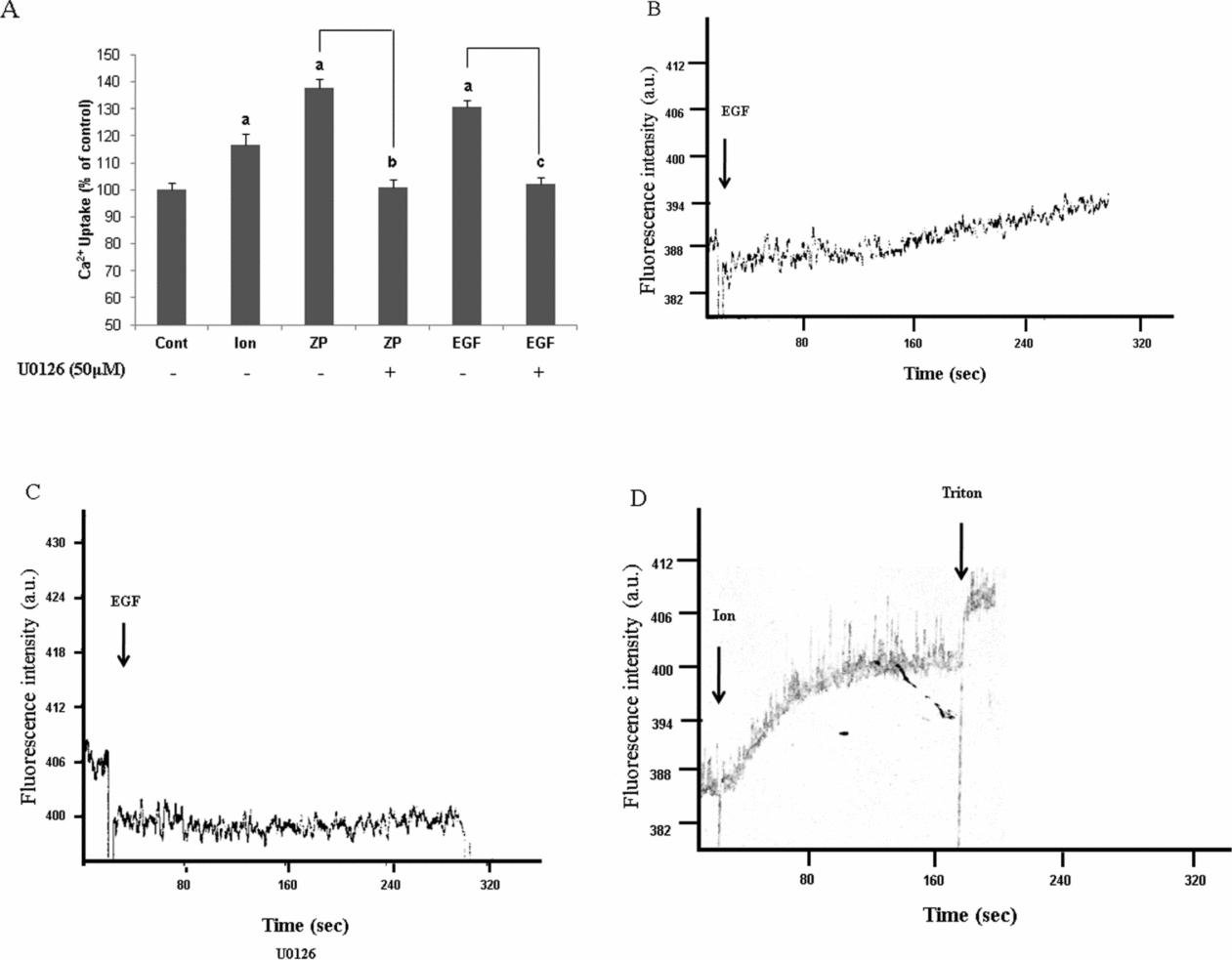
It is well accepted that calcium-ion influx is a critical event which enables the acrosome reaction to occur (Yanagimachi & Usui, Reference Yanagimachi and Usui1974; Spungin & Breitbart, Reference Spungin and Breitbart1996; Breitbart & Spungin, Reference Breitbart and Spungin1997). The fact that the acrosome reaction induced by the Ca2+-ionophore was not affected by U0126, while this inhibitor caused high inhibition in AR that was induced by ZP or EGF (Fig. 2A), suggests that ERK1/2 might mediate Ca2+ transport into the cell and that this mechanism is bypassed by the Ca2+ ionophore. Determination of Ca2+ influx using Fluo4/AM for the measurement of intracellular Ca2+ concentrations revealed that ZP or EGF manage to elevate intracellular Ca2+ levels by 30–40%, and that U0126 abolishes this increase completely (Fig. 5A). These data are further supported in bovine sperm in which we could measure intracellular calcium levels utilizing the spectro-fluorometer, which gave identical results, meaning a complete inhibition of Ca2+ influx in response to EGF in U0126 treated cells (Fig. 5B, C). The effect of ionomycin on Ca2+ influx is seen in Fig. 5D.

Figure 5 Involvement of ERK1/2 in Ca2+ influx during acrosome reaction. (A) Mouse sperm were incubated in capacitation medium for 30 min with or without U0126 (50 μM), then Fluo-4/AM was added for additional 1 h. The samples were washed three times in Ca2+-free medium and finally were resuspended in a medium that contained Ca2+, and then loaded on 96 wells plate. Ionomycin (10 μM) or zona pellucida (ZP) (~7.5 ZP\μl) or epidermal growth factor (EGF) (1 ng/ml) were added and the fluorescence was measured utilizing the ‘TECAN’ plate reader as described in Materials and methods. Control treatments were also conducted giving the values of 90% for Triton-X100 (0.1% v/v), when ethylene glycol tetraacetic acid (EGTA) (1 mM) was used there was no effect on intracellular calcium levels. The data represent the mean ± SD of duplicates from at least three experiments. a,b,cSignificant difference from the corresponding control, P < 0.05. (B, C, D) Bovine sperm were incubated in mTALP for 3 h with (C) or without (B) U0126 (50 μM) and loaded with Fluo-4/AM for additional 1 h. At the end of this period, cells were washed three times in Ca2+-free medium and finally were resuspended in a medium which contained Ca2+ and the fluorescence intensity was measured in response to EGF (1 ng/ml) or ionomycin (10 μM) (D). The data represent one experiment, typical of three repetitions performed with sperm from three different bulls.
Discussion
The mechanisms involved in sperm cells functions are still an enigma. The signalling processes that mediate sperm capacitation and the acrosome reaction are yet to be fully understood. ERK1/2 is a well defined kinase in many cell types, yet in sperm cells the ERK1/2 mechanism of action is not fully solved. To date, it is known that ERK1/2 has an indirect role in sperm protein tyrosine phosphorylation (de Lamirande & Gagnon, Reference de Lamirande and Gagnon2002; Awda & Buhr, Reference Awda and Buhr2010; Luna et al., Reference Luna, Colás, Pérez-Pé, Cebrián-Pérez and Muiño-Blanco2012), motility and hyperactivated motility (de Lamirande & Gagnon, Reference de Lamirande and Gagnon2002; Almog et al., Reference Almog, Lazar, Reiss, Etkovitz, Milch, Rahamim, Dobkin-Bekman, Rotem, Kalina, Ramon, Raziel, Breitbart, Seger and Naor2008) and in the acrosome reaction (Luconi et al., Reference Luconi, Barni, Vannelli, Krausz, Marra, Benedetti, Evangelista, Francavilla, Properzi, Forti and Baldi1998a, Reference Luconi, Krausz, Barni, Vannelli, Forti and Baldi1998b; du Plessis et al., Reference du Plessis, Page and Franken2001; de Lamirande & Gagnon, Reference de Lamirande and Gagnon2002; Chen et al., Reference Chen, Ni, Pan, Shi, Yuan, Chen, Mao, Yu and Roldan2005; Almog et al., Reference Almog, Lazar, Reiss, Etkovitz, Milch, Rahamim, Dobkin-Bekman, Rotem, Kalina, Ramon, Raziel, Breitbart, Seger and Naor2008). In this study it was demonstrated for the first time that mouse sperm ERK1/2 is activated upon ZP addition, and that ERK1/2 mediates the elevation of intracellular Ca2+ in the sperm cell prior to the occurrence of the acrosome reaction.
The localization of ERK1/2 in the mouse sperm head (Fig. 1A) implies that it has a role in capacitation and/or in the acrosome reaction processes. Almog et al. (Reference Almog, Lazar, Reiss, Etkovitz, Milch, Rahamim, Dobkin-Bekman, Rotem, Kalina, Ramon, Raziel, Breitbart, Seger and Naor2008) and Luconi et al., (Reference Luconi, Barni, Vannelli, Krausz, Marra, Benedetti, Evangelista, Francavilla, Properzi, Forti and Baldi1998a) also found that ERK1/2 is localized in the head as well as in the tail of human sperm. Characterization of the ERK1/2 activity in sperm cells during capacitation showed an intense increase in ERK1/2 phosphorylation/activation after 10 min of incubation in capacitation medium (Fig. 1B). Interestingly, ERK1/2 phosphorylation is elevated ‘spontaneously’ with no need for an external inducer, indicating that the capacitating ingredients were sufficient to cause this elevation. This ‘spontaneous’ increase in ERK1/2 phosphorylation during capacitation directed us to conclude that ERK1/2 has a key role in the process of capacitation.
In previous studies we have demonstrated a key role for the EGFR in mediating bovine and mouse sperm capacitation and the acrosome reaction (Etkovitz et al., Reference Etkovitz, Tirosh, Chazan, Jaldety, Daniel, Rubinstein and Breitbart2009; Jaldety et al., Reference Jaldety, Glick, Orr-Urtreger, Ickowicz, Gerber and Breitbart2012). It has also been shown that EGFR mediates capacitation in ram sperm (Luna et al., Reference Luna, Colás, Pérez-Pé, Cebrián-Pérez and Muiño-Blanco2012). The inhibition of ERK1/2 along the capacitation process attenuated the acrosome reaction rate in a higher manner (87% inhibition) (Fig. 2A) compared with inhibition rate (46%) of the acrosome reaction when ERK1/2 was blocked during the acrosome reaction only (Fig. 2B). These data suggest that ERK1/2 mediates the acrosome reaction as well as the capacitation process, a fact which is supported by the activation of ERK1/2 at the beginning of the capacitation (Fig. 1B). Inhibition of ERK1/2 also abolished the sperm's ability to in vitro fertilize eggs (Fig. 2C), indicating its pivotal role in promoting fertilization.
The specific inhibitory effect of U0126 on ERK1/2 activity is indicated by its inhibition of ERK1/2 phosphorylation induced by ZP or EGF (Fig. 3). Also, we recently demonstrated that EGFR mediates the acrosome reaction induced by ZP by serving as a sperm receptor for the ZP3 (Jaldety et al., Reference Jaldety, Glick, Orr-Urtreger, Ickowicz, Gerber and Breitbart2012). The ZP-induced EGFR-phosphorylation/activation is significantly inhibited by AG1478, a known EGFR specific inhibitor, but not by U0126 (Fig. 4). These data indicate that ERK1/2 is situated down-stream to EGFR in the cascade ZP–EGFR–ERK1/2, which leads to the occurrence of the acrosome reaction. As mentioned above, a role for the EGFR in the capacitation and the acrosome reaction processes has been demonstrated (Etkovitz et al., Reference Etkovitz, Tirosh, Chazan, Jaldety, Daniel, Rubinstein and Breitbart2009). It has been found that EGFR phosphorylation increases during capacitation, with no external inducer, and it is further phosphorylated with the stimulation of the acrosome reaction by EGF. These data are consistent with the results presented thus far.
The fact that the U0126 inhibition of the acrosome reaction induced by ZP or EGF by can be overcome when the acrosome reaction was induced by the Ca2+-ionophore, which bypasses the physiological Ca2+ transport mechanism (Fig. 2A), indicates that ERK1/2 mediate Ca2+ influx into the cells. Calcium assessment revealed that ZP or EGF stimulated Ca2+ influx into the sperm and that this stimulation is completely blocked by U0126 (Fig. 5). These data clearly indicate that Ca2+ influx into capacitated sperm is mediated by ERK1/2. This conclusion is supported elsewhere in which UO126 suppressed ZP-induced acrosome reaction by suppressing Ca2+ influx (Kirkman-Brown et al., Reference Kirkman-Brown, Punt, Barratt and Publicover2002).
In conclusion, this study demonstrates for the first time a role for ERK1/2 in the regulation of Ca2+ influx leading to the acrosome reaction. The following suggested mechanism is: sperm binding to ZP activates the EGFR, leading to ERK1/2 activation and Ca2+ influx and resulting in the occurrence of the acrosome reaction.
Financial support
‘IHEL’ Foundation to H.B.
Conflicts of interest
None.