Introduction
Exposure of cows to thermal stress at oestrus can reduce oocyte competence for subsequent development after fertilization (Putney et al., Reference Putney, Drost and Thatcher1988). This action of heat stress may involve direct effects of elevated body temperature on the function of the maturing oocyte. Exposure of oocytes during maturation to elevated culture temperatures (i.e. heat shock) can disrupt mitochondrial function (Rodriques et al., Reference Rodrigues, Ispada, Risolia, Rodrigues, Lima, Assumpção, Visintin and Paula-Lopes2016; Payton et al., Reference Payton, Rispoli, Nagle, Gondro, Saxton, Voy and Edwards2018) and reduce oocyte competence to complete nuclear maturation (Roth & Hansen, Reference Roth and Hansen2005; Nabenishi et al., Reference Nabenishi, Ohta, Nishimoto, Morita, Ashizawa and Tsuzuki2012; Cebrian-Serrano et al., Reference Cebrian-Serrano, Salvador, Raga, Dinnyes and Silvestre2013; Meiyu et al., Reference Meiyu, Liu and Roth2015), be fertilized and undergo cleavage (Roth & Hansen, Reference Roth and Hansen2004a, Reference Roth and Hansen2005; de Castro e Paula & Hansen, Reference de Castro e Paula and Hansen2007; Meiyu et al., Reference Meiyu, Liu and Roth2015). Moreover, the competence of the resultant embryos to develop to the blastocyst stage can be compromised (Roth et al., 2004a, b; Nabenishi et al., Reference Nabenishi, Ohta, Nishimoto, Morita, Ashizawa and Tsuzuki2012; Rodriques et al., Reference Rodrigues, Ispada, Risolia, Rodrigues, Lima, Assumpção, Visintin and Paula-Lopes2016).
Heat shock may disrupt oocyte function, at least in part, by increasing the generation of reactive oxygen species (ROS). Production of ROS is increased by heat shock (Nabenishi et al., Reference Nabenishi, Ohta, Nishimoto, Morita, Ashizawa and Tsuzuki2012; Ispada et al., Reference Ispada, Rodrigues, Risolia, Lima, Gonçalves, Rettori, Nichi, Feitosa and Paula-Lopes2018) and antioxidants such as retinol (Lawrence et al., Reference Lawrence, Payton, Godkin, Saxton, Schrick and Edwards2004), cysteine (Nabenishi et al., Reference Nabenishi, Ohta, Nishimoto, Morita, Ashizawa and Tsuzuki2012) and astaxanthin (Ispada et al., Reference Ispada, Rodrigues, Risolia, Lima, Gonçalves, Rettori, Nichi, Feitosa and Paula-Lopes2018) can reduce the negative consequences of heat shock on the oocyte. Moreover, the function of the maturing oocyte can be disrupted by other oxidative stresses, as shown for the nitric oxide donor sodium nitroprusside in the bovine (Soto et al., Reference Soto, Natzke and Hansen2003; Cheuquemán et al., Reference Cheuquemán, Loren, Arias, Risopatrón, Felmer, Álvarez, Mogas and Sánchez2015) and hydrogen peroxide in the mouse (Tamura et al., Reference Tamura, Takasaki, Miwa, Taniguchi, Maekawa, Asada, Taketani, Matsuoka, Yamagata, Shimamura, Morioka, Ishikawa, Reiter and Sugino2008) and pig (Yazaki et al., Reference Yazaki, Hiradate, Hoshino, Tanemura and Sato2013).
One molecule that may exert protective effects on the oocyte is the multifunctional hormone melatonin (N-acetyl-5-methoxytryptamine). Administration of melatonin to heat-stressed females improved the embryo competence for development in mice (Matsuzuka et al., Reference Matsuzuka, Sakamoto, Ozawa, Ushitani, Hirabayashi and Kanai2005) and fertility in lactating cows (Garcia-Ispierto et al., Reference Garcia-Ispierto, Abdelfatah and López-Gatius2013). Melatonin affects cellular function by interacting with membrane and nuclear receptors and by functioning as an antioxidant (Tan et al., Reference Tan, Reiter, Manchester, Yan, El-Sawi, Sainz, Mayo, Kohen, Allegra and Hardeland2002; Mayo et al., Reference Mayo, Sainz, González-Menéndez, Hevia and Cernuda-Cernuda2017). Melatonin improved oocyte maturation in the pig (Shi et al., Reference Shi, Tian, Zhou, Wang, Gao, Zhu, Zeng, Tian and Liu2009), sheep (Tian et al., Reference Tian, Wang, Zhang, He, Ji, Wang, Zhang, Lv, Abulizi, Wang, Lian and Liu2017) and bovine (El-Raey et al., Reference El-Raey, Geshi, Somfai, Kaneda, Hirako, Abdel-Ghaffar, Sosa, El-Roos and Nagai2011; Tian et al., Reference Tian, Wang, He, Zhang, Tan, Reiter, Xu, Ji and Liu2014; Marques et al., Reference Marques, da Silva Santos, Diesel, Leme, Martins, Dode, Alves, Costa, de Oliveira and Gambarini2018).
Experiments to test whether melatonin protects oocytes from heat shock have yielded inconclusive results, however. Effects of melatonin in the pig were evaluated in heat-shocked oocytes but not in oocytes cultured at normal temperature (Li et al., Reference Li, Zhang, He, Zhu, Xu, Ma, Tao and Liu2015, Reference Li, Wang, Zhang, Yi, He, Wang, Tian, Yang, Song, He and Liu2016). Protective effects of high concentrations of melatonin (10 mM) on bovine oocytes cultured at 41.5°C were difficult to interpret because 10 mM melatonin reduced oocyte competence in the absence of heat shock (Cebrian-Serrano et al., Reference Cebrian-Serrano, Salvador, Raga, Dinnyes and Silvestre2013).
In the current experiment, we evaluated the capacity of melatonin to protect oocytes using two models of oxidative stress. In addition to heat shock, cytoprotective properties of melatonin against the pro-oxidant menadione were examined. Menadione (2-methyl-l,4-naphtoquinone) is a vitamin K precursor that can react with a single electron to produce a free radical derivative that can cause superoxide anion formation (Comporti, Reference Comporti1989). Menadione can reduce developmental capacity of the preimplantation bovine embryo (Moss et al., Reference Moss, Pontes and Hansen2009) and act on bovine spermatozoa to reduce fertilizing ability and compromise developmental competence of the resultant embryos (Hendricks & Hansen, Reference Hendricks and Hansen2010).
Materials and methods
Oocyte collection and maturation
Ovaries were obtained from a local abattoir from cows of a variety of genotypes including Bos taurus and admixtures of B. taurus and B. indicus. Ovaries were transported within 10h to the laboratory at 23°C in a solution of 0.9% (w/v) NaCl. Cumulus−oocyte complexes (COCs) were harvested from follicles 2 to 8 mm in diameter by cutting the surface of the ovary with a scalpel and swirling the ovary in BoviPRO™ Oocyte Washing Medium (with BSA) (MOFA Global, Verona, WI, USA). Only COCs with at least three or four layers of compact cumulus and with an oocyte with a uniform cytoplasm were selected for maturation. Selected COC were washed and matured in 6-well plates in groups of 25–30 in 300μl BO-IVM oocyte maturation medium (IVF Bioscience, Falmouth, UK) that was prepared ± 1 µM melatonin (Santa Cruz Biotechnology, Dallas, TX, USA) and with either 5 µM menadione (Sigma-Aldrich, St. Louis, MO, USA) or an equivalent volume of vehicle. Melatonin was prepared as described by Ortega et al. (Reference Ortega, Rocha-Frigoni, Mingoti, Roth and Hansen2016) and menadione as described by Moss et al. (Reference Moss, Pontes and Hansen2009). Depending on the experiment, maturation was carried out for 3 or 22 h at 38.5°C or 41°C under an atmosphere of either 5% (v/v) CO2 in humidified air (38.5°C) or 7% (v/v) CO2 in humidified air (41°C). The higher CO2 for maturation at 41.0°C was used to maintain pH at 7.4 in the face of lower solubility of CO2 at the higher temperature. The control temperature of 38.5°C was chosen because it is similar to the body temperature of the cow in the absence of heat stress. Heat shock was performed at 41°C because cows subjected to heat stress often experience rectal temperatures of 41°C or higher (Dikmen & Hansen, Reference Dikmen and Hansen2009). Moreover, exposure of bovine oocytes during maturation to 41°C disrupted mitochondrial function (Rodriques et al., Reference Rodrigues, Ispada, Risolia, Rodrigues, Lima, Assumpção, Visintin and Paula-Lopes2016; Payton et al., Reference Payton, Rispoli, Nagle, Gondro, Saxton, Voy and Edwards2018), increased apoptosis (Roth & Hansen, Reference Roth and Hansen2004a; Rodriques et al., Reference Rodrigues, Ispada, Risolia, Rodrigues, Lima, Assumpção, Visintin and Paula-Lopes2016) and reduced oocyte competence to complete nuclear maturation (Roth & Hansen, Reference Roth and Hansen2005) and be fertilized, undergo cleavage and develop to the blastocyst stage (Roth & Hansen, Reference Roth and Hansen2004a, Reference Roth and Hansen2005; de Castro e Paula & Hansen, Reference de Castro e Paula and Hansen2007; Rodriques et al., Reference Rodrigues, Ispada, Risolia, Rodrigues, Lima, Assumpção, Visintin and Paula-Lopes2016).
Production of ROS
An experiment was conducted to determine whether heat shock and menadione would increase the production of ROS by oocytes at the beginning of exposure to stress and whether increased production would be blocked by melatonin. For each replicate, COCs (~100) were randomly assigned to one of six treatments in a 3 × 2 factorial arrangement with the main effects of stress (control, heat shock, or menadione) and melatonin (0 or 1 µM). Controls were cultured at 38.5°C, heat shock was 41°C and menadione involved culture at 38.5°C in BO-IVM containing 5 µM menadione. This concentration of menadione was chosen because treatment of bovine embryos at day 6 after insemination with 5 µM menadione increased ROS production and apoptosis, while almost completely blocking development to the blastocyst stage (Moss et al., Reference Moss, Pontes and Hansen2009). For all treatments, medium contained 5 µM CellROX® Green, a cell-permeant dye that exhibits bright green photostable fluorescence upon oxidation and subsequent binding to DNA.
After 3 h of maturation, COCs were denuded of cumulus cells by vortexing groups of 25 to 30 for 5 min in 200 µl hyaluronidase as described earlier (Ortega et al., Reference Ortega, Wohlgemuth, Tribulo, Siqueira, Cole and Hansen2017). Oocytes were then washed three times in 50 µl droplets of Dulbecco’s phosphate-buffered saline (DPBS) containing 1% (w/v) polyvinylpyrrolidone (PVP), fixed in 4% (w/v) paraformaldehyde in DPBS, washed three more times in DPBS-PVP and mounted in groups of 10 oocytes on microscope slides using Prolong Gold anti-fade reagent (Invitrogen, Carlsbad, CA, USA). Oocytes were examined individually for fluorescence within 10 h after labelling using fluorescence microscopy and a green emission filter with a Zeiss Axio Plan 2 epifluorescence microscope (Zeiss, Göttingen, Germany). Digital images of each oocyte were acquired using AxioVision software (v.4.8.2; Zeiss) and a high-resolution black and white Zeiss Axiocam MRM digital camera. Analysis of the images was performed using ImageJ sotware v.1.48 (National Institutes of Health, Bethesda, MD, USA). Net fluorescent intensity was calculated by obtaining the average pixel intensity of each oocyte (obtained after manually drawing a boundary around the oocyte) and subtracting the background intensity obtained from a region of the image not containing the oocyte. The experiment was replicated four times using 41−72 oocytes per treatment (n=326 total).
Competence of oocytes to cleave and develop after fertilization
An experiment was conducted to determine whether: (1) heat shock and menadione would alter competence of matured oocytes to cleave after fertilization and alter ability of the resultant embryos to develop to the blastocyst stage; and (2) if actions of heat shock and menadione would be blocked by melatonin. For each replicate (n = 12 replicates total), COCs (~200) were randomly assigned to one of six treatments in a 3×2 factorial arrangement with main effects of stress (control, heat shock, or menadione) and melatonin (0 or 1 µM). Control COCs were matured for 22 h at 38.5°C, heat-shocked COCs were matured for 14 h at 41.0°C and for 8 h at 38.5°C and menadione-treated COCs were matured for 22 h at 38.5°C in medium containing 5 µM menadione.
After maturation, COCs were washed three times in HEPES-TALP (Tyrode’s albumin−lactate−pyruvate) and placed in wells of 6-well plates containing 425 µl fertilization medium [in vitro fertilization-TALP; see Ortega et al. (Reference Ortega, Wohlgemuth, Tribulo, Siqueira, Cole and Hansen2017) for recipes for TALP medium] and 1 × 106 ml−1 spermatozoa. For each replicate, fertilization was performed with semen pooled from three individual bulls of various taurine breeds; the total number of bulls used in the experiment was 17. Sperm were purified from frozen−thawed straws of extended semen using an Isolate® gradient [Irvine Scientific, Santa Ana, CA USA; 50% (v/v) and 90% (v/v) Isolate]. In addition, 20 µl of penicillamine−hypotaurine−epinephrine solution (Ortega et al., Reference Ortega, Wohlgemuth, Tribulo, Siqueira, Cole and Hansen2017) was added to each fertilization well to improve sperm motility. Fertilization proceeded for 14 to 16 h at 38.5°C in an atmosphere of 5% (v/v) CO2 in humidified air.
Putative zygotes (i.e. oocytes exposed to sperm) were denuded from the surrounding cumulus cells at the end of fertilization (14 to 16 h) by vortexing groups of 25 to 30 zygotes for 5 min in 200 µl of HEPES-TALP containing 10 000 U/ml of hyaluronidase. Embryos were cultured in groups of 25–30 in 50 µl drops of culture medium (SOF-BE2; Ortega et al., Reference Ortega, Wohlgemuth, Tribulo, Siqueira, Cole and Hansen2017) that were covered with mineral oil. Embryos were cultured at 38.5°C in a humidified atmosphere of 5% (v/v) O2 and 5% (v/v) CO2 with the balance N2. The percentage of oocytes that cleaved was determined at day 3.5 of development (day 0=day of fertilization) and the per cent of cleaved embryos that became blastocysts was determined at day 7.5 of development.
The experiment was replicated 12 times with the total number of COCs ranging from 299 to 444 per treatment (total number=2491).
Statistical analysis
Data were analyzed using SAS v.9.4 (SAS Institute Inc., Cary, NC, USA). Data on ROS production were analyzed by analysis of variance using the MIXED procedure. The model included main effects of stress and melatonin and the interaction of stress and melatonin as fixed effects and replicate as a random effect. Effects of stress and melatonin on the proportion of oocytes cleaving and on the proportion of cleaved embryos becoming blastocysts was evaluated using the GLIMMIX procedure. Each embryo was considered an observation with binary response (0=not developed to blastocyst; 1=developed to blastocyst) and analysis was performed by logistic regression fitting binary data distribution. The statistical model included the fixed effects of stress, melatonin, stress×melatonin interaction and random effect of replicate.
For both analyses, two sets of orthogonal contrasts were used to make individual degree-of-freedom comparisons of resolve multilevel effects of stress and interactions of stress and melatonin. In the first set of contrasts, differences between types of stress (control, heat shock and menadione) were evaluated by two orthogonal contrasts: (1) the comparison of control v. heat shock+menadione; and (2) the comparison of heat shock v. menadione. In the second set of contrasts, differences in the effect of melatonin for each stress were evaluated by comparisons of: (1) control v. control+melatonin; (2) heat shock v. heat shock+melatonin; and (3) menadione v. menadione+melatonin.
Results
Production of ROS
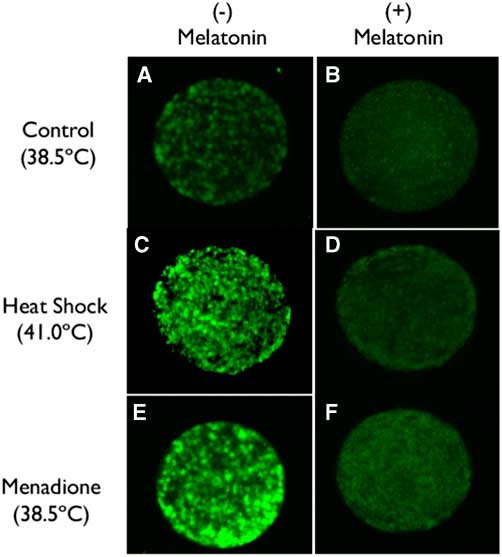
Representative images of labelling of oocytes using CellROX are shown in Fig. 1. Intensity of fluorescence was higher for oocytes exposed to heat shock (41°C) or 5 µM menadione than for oocytes matured at 38.5°C (compare Fig. 1C and Fig. 1E with Fig. 1A). Addition of 1 µM melatonin reduced fluorescence intensity under all culture conditions (compare Fig. 1A, C and E with Fig. 1B, D and F).
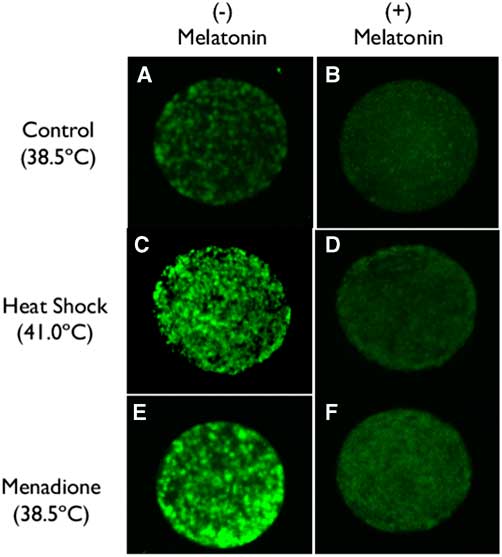
Figure 1 (A–F) Representative images of oocytes labelled with CellROX (Thermo Fisher Scientific, Waltham, MA, USA) to assess the production of ROS as affected by incubation temperature, menadione and melatonin.
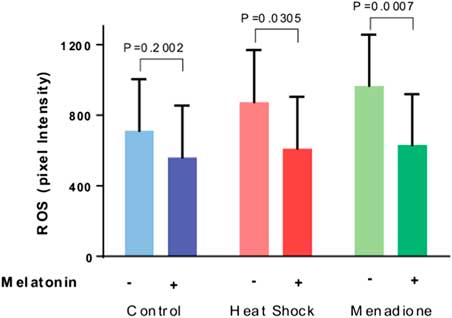
Results of quantification of ROS labelling are shown in Fig. 2. The intensity of ROS was greater (P=0.0577) for oocytes exposed to heat shock and menadione than for control oocytes. Overall, melatonin reduced ROS intensity (P=0.0002) and there was no interaction between stress and melatonin (P=0.4806). However, analysis of the effects of melatonin for each stress indicated that melatonin reduced ROS intensity for heat-shocked oocytes (P=0.0305) and oocytes exposed to menadione (P=0.0007) but not for control oocytes (P=0.2002).
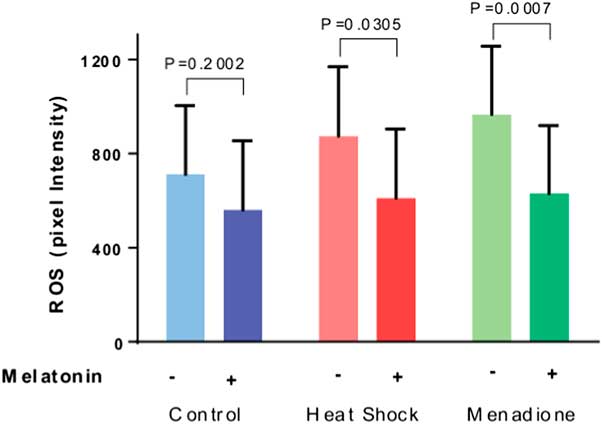
Figure 2 Effects of melatonin (1 µM) on the production of reactive oxygen species (ROS) by maturing oocytes exposed to control conditions (38.5°C), heat shock (41.0°C) and menadione (5 µM at 38.5°C). Data are least-squares means±standard error of the mean (SEM) of pixel intensity. Overall, the intensity of ROS was greater (P=0.0577) for oocytes exposed to heat shock and menadione than for control oocytes. Probability values for the effect of melatonin for each stress are indicated above the bars.
Competence of oocytes to cleave and develop after fertilization
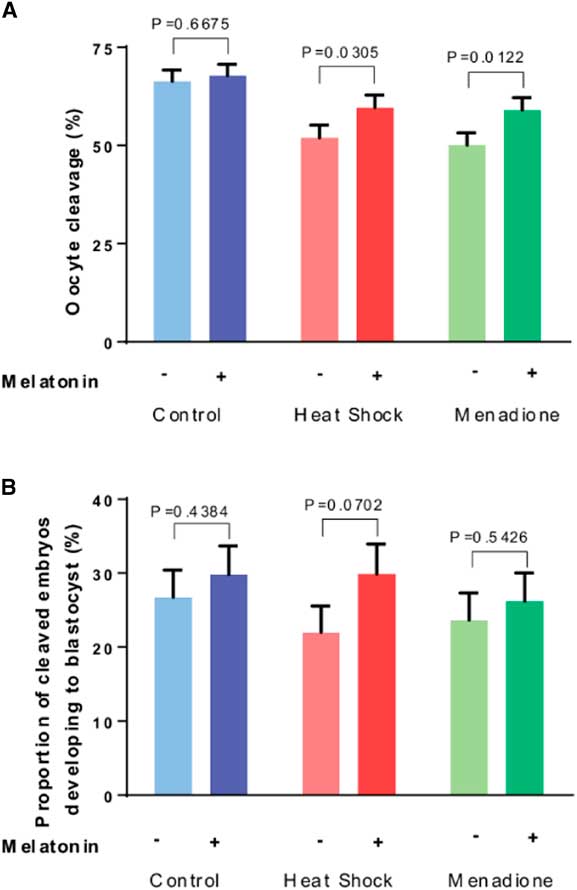
Results on cleavage of oocytes after fertilization are presented in Fig. 3A. The proportion of oocytes that cleaved after fertilization was lower (P<0.0001) for oocytes exposed to heat shock and menadione than for control oocytes. Overall, melatonin increased cleavage (P=0.0041). While the interaction between stress and melatonin was not significant (P=0.2944), analysis of the effects of melatonin for each stress indicated that melatonin increased cleavage for heat-shocked oocytes (P=0.0305) and oocytes exposed to menadione (P=0.0122) but not for control oocytes (P=0.6675).
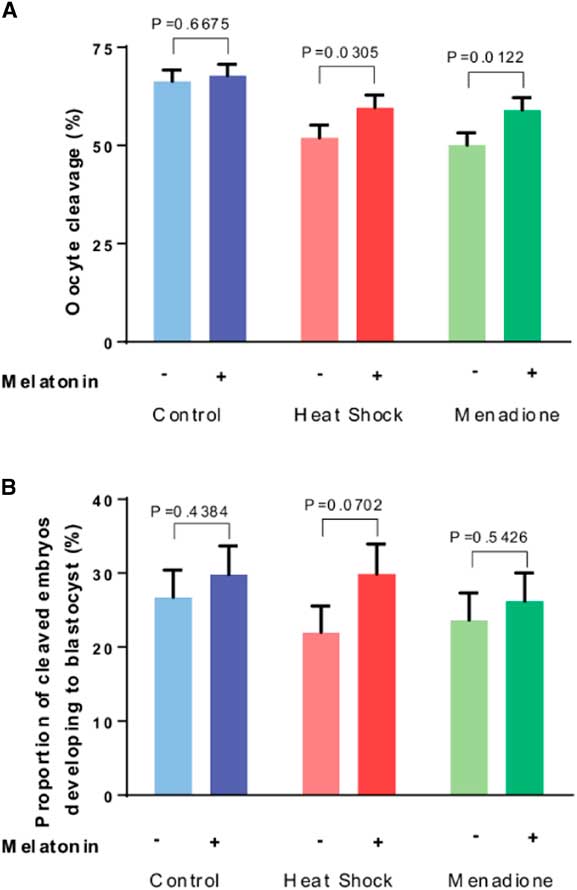
Figure 3 Effects of melatonin (1 µM) on developmental competence of oocytes exposed to control conditions (38.5°C), heat shock (41.0°C) and menadione (5 µM at 38.5°C) during in vitro maturation. Data are least-squares means±standard error of the mean (SEM) of the proportion of oocytes that cleaved after fertilization (A) and the proportion of cleaved embryos developing to the blastocyst stage (B). Overall, the proportion of oocytes that cleaved after fertilization was lower (P<0.0001) for oocytes exposed to heat shock and menadione than for control oocytes. The proportion of cleaved embryos that became blastocysts was not affected by stress (heat shock+menadione v. control, P=0.7871). Probability values for the effect of melatonin for each stress are indicated above the bars.
As shown in Fig. 3B, the proportion of cleaved embryos that became blastocysts was not affected by stress (heat shock+menadione v. control, P=0.7871) but was increased by melatonin (P=0.0634). Despite a lack of a stress × melatonin interaction (P=0.5991), analysis of effects of melatonin for each stress indicated that melatonin tended to increase development for embryos from heat-shocked oocytes (P=0.0702) but not for embryos from oocytes exposed to menadione (P=0.5426) or from control oocytes (P=0.4384).
Discussion
Present results indicate that melatonin can reduce ROS production by the bovine oocyte exposed to conditions that promote production of ROS and partially preserve developmental competence of the oocyte exposed to those stresses. These results confirm the importance of oxidative stress for damaging the oocyte and show how administration of an antioxidant can block that effect.
It has been repeatedly demonstrated, both in the present experiments and by others, that heat shock increases ROS production by the bovine oocyte (Nabenishi et al., Reference Nabenishi, Ohta, Nishimoto, Morita, Ashizawa and Tsuzuki2012; Ispada et al., Reference Ispada, Rodrigues, Risolia, Lima, Gonçalves, Rettori, Nichi, Feitosa and Paula-Lopes2018) and reduces the percentage of oocytes that cleave after coincubation with spermatozoa (Roth & Hansen, Reference Roth and Hansen2004a; de Castro e Paula & Hansen, Reference de Castro e Paula and Hansen2007). In some cases, the deleterious actions of heat shock on the oocyte also compromise the ability of the subsequent embryo to develop to the blastocyst stage (Roth et al., Reference Roth and Hansen2004a,Reference Roth and Hansenb; Nabenishi et al., Reference Nabenishi, Ohta, Nishimoto, Morita, Ashizawa and Tsuzuki2012; Rodriques et al., Reference Rodrigues, Ispada, Risolia, Rodrigues, Lima, Assumpção, Visintin and Paula-Lopes2016) although this consequence of heat shock has not always observed (de Castro e Paula & Hansen, Reference de Castro e Paula and Hansen2007; Cebrian-Serrano et al., 2013). In the present experiment, the per cent of cleaved embryos becoming blastocysts for oocytes cultured without melatonin was 26.7% for control oocytes and 22.0% for heat-shocked oocytes. Therefore, the primary defect caused by heat shock here was the competence of the oocyte to cleave after fertilization. Treatment of the oocyte with the prooxidant menadione also reduced per cent of oocytes that cleaved while not significantly affecting subsequent development of cleaved embryos. Previous experiments with heat shock would indicate that reduced competence for cleavage is due to disruption of nuclear maturation (Roth & Hansen, Reference Roth and Hansen2005; Nabenishi et al., Reference Nabenishi, Ohta, Nishimoto, Morita, Ashizawa and Tsuzuki2012; Sebrian-Serrano et al., 2013; Meiyu et al., Reference Meiyu, Liu and Roth2015) as well as disruption of mitochondrial function (Rodrigues et al., Reference Rodrigues, Ispada, Risolia, Rodrigues, Lima, Assumpção, Visintin and Paula-Lopes2016; Payton et al., Reference Payton, Rispoli, Nagle, Gondro, Saxton, Voy and Edwards2018). Induction of oocyte apoptosis is also very important: inhibition of apoptosis can block anti-developmental effects of heat shock (Roth & Hansen, Reference Roth and Hansen2004a, Reference Roth and Hansen2005). The observation that melatonin was more effective at blocking effects of heat shock on competence of cleaved embryos to become blastocysts than the effect of menadione probably reflects ROS-independent actions of menadione on cellular function. For example, menadione can block Siah2 ubiquitin ligase through actions not involving ROS (Shah et al., 2009).
Deleterious effects of both heat shock and menadione on per cent of oocytes that cleaved were reduced by melatonin. Moreover, there was a tendency for melatonin to increase the competence of embryos derived from heat-shocked oocytes to develop to the blastocyst stage. Earlier experiments to evaluate the thermoprotective effect of melatonin on the oocyte have been difficult to interpret either because of lack of control oocytes not exposed to heat shock (Li et al., Reference Li, Zhang, He, Zhu, Xu, Ma, Tao and Liu2015, Reference Li, Wang, Zhang, Yi, He, Wang, Tian, Yang, Song, He and Liu2016) or the high concentrations of melatonin (10 mM) required to protect oocytes from heat shock reduced oocyte competence in the absence of heat shock (Cebrian-Serrano et al., Reference Cebrian-Serrano, Salvador, Raga, Dinnyes and Silvestre2013). The experiments conducted here did not allow the determination of whether the cytoprotective effects of melatonin were mediated by reducing ROS production or through changes in cellular function mediated by activation of melatonin receptors. The former explanation seems more likely because addition of melatonin caused a large reduction in ROS generation, melatonin was protective against two different stresses (heat shock & menadione) that both increased ROS production and there were little effects of melatonin on oocyte competence in the absence of heat shock or menadione. There are reports of the existence of melatonin receptors or their mRNA in the bovine oocyte and cumulus cell (El-Raey et al., Reference El-Raey, Geshi, Somfai, Kaneda, Hirako, Abdel-Ghaffar, Sosa, El-Roos and Nagai2011; Tian et al., Reference Tian, Wang, He, Zhang, Tan, Reiter, Xu, Ji and Liu2014) and further studies are necessary to resolve the mechanism of action of the cytoprotective effects of melatonin on the oocyte.
An additional indication of the mechanism of action of melatonin is the effective concentration of the hormone. The concentration of melatonin used here, 1 µM, is much higher than the reported kD of membrane melatonin receptors, having values of ~30−225 pM (Poon et al., Reference Poon, Liu, Pang, Brown and Pang1994; Kobayashi et al., Reference Kobayashi, Itoh, Kondo, Okuma, Sato, Kanishi, Hamada, Kiguchi and Ishizuka2003; Liu et al., Reference Liu, Xu, Shi, Lu, Ma, Xu and Guo2013). In an earlier study, there was no thermoprotective effect of 1 pM or 1 nM melatonin on bovine oocytes exposed to heat shock (Cebrian-Serrano et al., Reference Cebrian-Serrano, Salvador, Raga, Dinnyes and Silvestre2013; Ahmed et al., Reference Ahmed, Dutta and Nashiruddullah2016).
There is a report that administration of melatonin to cows via subcutaneous implants can improve fertility of heat-stressed cows (Garcia-Ispierto et al., Reference Garcia-Ispierto, Abdelfatah and López-Gatius2013). In that study, concentrations of melatonin in peripheral blood of treated cows peaked at 60−70 pg/ml (i.e. 260−300 pM). Therefore, beneficial effects of melatonin in that study may have involved receptor-mediated actions of melatonin on one or more components of the reproductive system rather than a direct cytoprotective action of the molecule.
The finding that 1 µM melatonin reduced effects of heat shock and melatonin on the oocyte is in contrast with recent results with the bovine 2-cell embryo. Culture of embryos at this stage of development causes an increase in ROS production and a decrease in the proportion of embryos that develop to the blastocyst stage (Ortega et al., Reference Ortega, Rocha-Frigoni, Mingoti, Roth and Hansen2016). In that study, melatonin blocked the increase in ROS production caused by heat shock but did not rescue the ability of 2-cell embryos to develop to the blastocyst stage. Perhaps, heat shock causes deleterious changes in cellular function of the 2-cell embryo that are independent of ROS production (and of the protective action of melatonin), whereas ROS production is the major proximate cause of damage to the oocyte caused by heat shock. Consistent with this idea is the fact that heat shock disrupts developmental competence of the 2-cell embryo to a greater degree than the oocyte (Edwards & Hansen, Reference Edwards and Hansen1997) and that oxygen concentration used for culture is not an important determinant of the magnitude of effects of heat shock on the zygote or 2-cell embryo (Rivera & Hansen, Reference Rivera and Hansen2001; Sakatani et al., Reference Sakatani, Alvarez, Takahashi and Hansen2012). Experiments using caspase inhibitors (Roth & Hansen, Reference Roth and Hansen2004a) or sphingosine 1-phosphate (Roth & Hansen, Reference Roth and Hansen2004b) indicated that the induction of apoptosis by heat shock is a major cause of decreased developmental competence of the oocyte. Such a mechanism for damage by heat shock is not operational in the 2-cell embryo because apoptosis responses in the preimplantation embryo are blocked until the 8- to 16-cell stages (Hansen & Fear, Reference Hansen and Fear2011).
While it is clear that heat stress can reduce oocyte competence in vivo (Putney et al., Reference Putney, Drost and Thatcher1988), an important question is the importance of the direct effect of elevated temperature on the oocyte as a cause of compromised oocyte function. As stated in the previous paragraph, the oocyte is more resistant to disruption by heat shock than the early cleavage-stage embryo. Moreover, there is recent evidence in the cow that intrafollicular temperature of the ovulatory follicle is about 0.9 to 1.1°C lower than rectal temperature (López-Gatius & Hunter, Reference López-Gatius and Hunter2019a, Reference López-Gatius and Hunterb). Thus, the actual degree of heat shock at the level of the oocyte may be less than the temperature used here. Additionally, recent data indicate that follicular fluid contains molecules that can protect the oocyte from heat shock (Rodriques et al., 2019). Although melatonin is present in follicular fluid, at concentrations ranging from 10−11 to 10–9 M (Shi et al., Reference Shi, Tian, Zhou, Wang, Gao, Zhu, Zeng, Tian and Liu2009; Tong et al., Reference Tong, Sheng, Sun, Li, Li, Zhang and Chen2017), it is not responsible for the protective properties of follicular fluid because concentrations are too low to block effects of heat shock (Cebrian-Serrano et al., Reference Cebrian-Serrano, Salvador, Raga, Dinnyes and Silvestre2013; Ahmed et al., Reference Ahmed, Dutta and Nashiruddullah2016). At least some of the protective activity is associated with the exosome fraction of follicular fluid (Rodrigues et al., Reference Rodrigues, Tuna, Alli, Tribulo, Hansen, Koh and Paula-Lopes2019).
In the absence of heat shock or menadione. there was no effect of melatonin on competence of oocytes to cleave or of the resultant embryos to develop to the blastocyst stage. Similar results have been obtained by others (Farahavar et al., Reference Farahavar, Shahane, Kohram and Vahedi2010; Rodriques-Cunha et al., Reference Rodrigues-Cunha, Mesquita, Bressan, Collado, Balieiro, Schwarz, de Castro, Watanabe, Watanabe, de Alencar Coelho and Leal2016). The absence of beneficial effects of melatonin on performance of in vitro fertilization procedures is in contrast with studies in cattle (Zhao et al., Reference Zhao, Min, Du, Hao, Liu, Qin, Wang and Zhu2015; Yang et al., Reference Yang, Tao, Chai, Wu, Wang, Li, He, Xie, Ji, Dai, Yang and Liu2017; Pang et al., Reference Pang, Zhao, Sun, Jiang, Hao, Du and Zhu2018) and goats (Soto-Heras et al., Reference Soto-Heras, Roura, Catalá, Menéndez-Blanco, Izquierdo, Fouladi-Nashta and Paramio2018) in which melatonin improved one or more indices of oocyte function. Except for the study of Pang et al. (Reference Pang, Zhao, Sun, Jiang, Hao, Du and Zhu2018), the studies in which melatonin exhibited positive effects used oocytes that were compromised in some way, either being classified as being of poor quality based on morphological criteria (Zhao et al., Reference Zhao, Min, Du, Hao, Liu, Qin, Wang and Zhu2015; Yang et al., Reference Yang, Tao, Chai, Wu, Wang, Li, He, Xie, Ji, Dai, Yang and Liu2017) or being from immature animals (Soto-Heras et al., Reference Soto-Heras, Roura, Catalá, Menéndez-Blanco, Izquierdo, Fouladi-Nashta and Paramio2018). It may be, therefore, that melatonin can be a useful additive to oocyte maturation medium when oocyte competence is compromised.
In conclusion, melatonin reduced the production of ROS by maturing oocytes, especially when they were exposed to heat shock or menadione and protected oocytes from deleterious effects of both stresses on competence to cleave after coincubation with sperm. These results suggested that excessive production of ROS compromises oocyte function. Damage to the oocyte caused by ROS may, therefore, be important for effects of elevated temperature on the oocyte during maturation (Putney et al., Reference Putney, Drost and Thatcher1988), at least in cases in which oocyte temperature rises sufficiently to trigger increased ROS production.
Acknowledgements
The authors thank Florida Beef, Wauchula, FL, USA, for allowing us to procure ovaries and Eddie Cummings and Dr Paula Tribulo for technical assistance.
Financial support
This research was supported by Research Grant Award No. US-4719-14 from BARD (the United States−Israel Binational Agricultural Research and Development Fund) to PJ Hansen and Z Roth, by Fundação de Amparo à Pesquisa do Estado de São Paulo (grant no. 2015/20379-0 to Cláudia Lima Verde Leal and PhD scholarship no. 2017/04376-6 to Fernanda Cavallari de Castro) and by funds from the L.E. ‘Red’ Larson Endowment. The authors thank Florida Beef, Wauchula, Florida, for allowing us to procure ovaries and Eddie Cummings and Dr Paula Tribulo for technical assistance.
Conflicts of interest
The authors have no conflict to declare.
Ethical standards
Not applicable.