Introduction
Due to male or female factors or idiopathic reasons, many couples are facing infertility issues (Agarwal et al., Reference Agarwal, Durairajanayagam and Du Plessis2014). Thanks to assisted reproductive technologies (ART), such as IVF, many infertile couples can now have children (Schultz and Williams, Reference Schultz and Williams2002). However, observing a glimpse of pregnancy among infertile couples shows that even though ATR techniques have solved many of their problems, the success rates of these techniques are still unsatisfactory and even have some failure (Ben-Rafael et al., Reference Ben-Rafael, Benadiva, Ausmanas, Barber, Blasco, Flickinger and Mastroianni1987; Głabowski et al., Reference Głabowski, Kurzawa, Wiszniewska, Baczkowski, Marchlewicz and Brelik2005).
Various factors are involved in the occurrence of these disorders and their elimination requires further research in upgrading laboratory ART (Makarevich and Markkula, Reference Makarevich and Markkula2002).
At this time, the culture and maturity of immature oocytes in vitro has become an efficient method for production of adult oocytes (Desai et al., Reference Desai, Alex, Abdelhafez, Calabro, Goldfarb, Fleischman and Falcone2010; Nottola et al., Reference Nottola, Cecconi, Bianchi, Motta, Rossi, Continenza and Macchiarelli2011; Jafarzadeh et al., Reference Jafarzadeh, Nazarian, Ghaffari Novin, Shams Mofarahe, Eini and Piryaei2018; Adib et al., Reference Adib, Seifati, Dehghani Ashkezari, Akyash, Khoradmehr and Aflatoonian2020) In vitro culture (IVC) is an important phase in the growth of embryos that are to be implanted and result in a successful pregnancy. Preparation of an adequate medium that provides in vitro conditions that most resemble the complicated in vivo environment is one of the primary challenges for the growth and development of in vitro follicles and embryos. (Brower and Schultz, Reference Brower and Schultz1982; Picton et al., Reference Picton, Harris, Muruvi and Chambers2008; Miraki et al., Reference Miraki, Mokarizadeh, Banafshi, Assadollahi, Abdi, Roshani and Fathi2017). The IVC system has been regularly improved to achieve equivalent quality and development of embryos in vivo. However utilization of this technique is influenced by some factors including low rates (30–60%) of embryos reaching the blastocyst stage (Głabowski et al., Reference Głabowski, Kurzawa, Wiszniewska, Baczkowski, Marchlewicz and Brelik2005). Success in these techniques depends on various factors such as the condition of the culture medium (Makarevich and Markkula, Reference Makarevich and Markkula2002). Increasing interest in optimizing culture media to better supply and maintain culture conditions has led to the promotion of newer culture medium formulations that provide nutritional requirements and improve the state of the culture medium in embryo development (Desai et al., Reference Desai, Lawson and Goldfarb2000; Swain Reference Swain2010). Culture medium components play a decisive role in increasing the outcome of these techniques. Therefore, embryo culture media should be designed to be, as much as possible, similar to the natural environment (Richter, Reference Richter2008; Chronopoulou and Harper, Reference Chronopoulou and Harper2015).
Generally, embryos are produced using oocytes collected from ovaries, then transported to the fallopian tube and fertilized by sperm. The zygote develops in a protected environment in the female reproductive system, in which all materials essential for fetal development are available, including sustainable food sources and growth factors (Bavister, Reference Bavister2000; Paria et al., Reference Paria, Lim, Das, Reese and Dey2000; Desai et al., Reference Desai, Abdelhafez, Bedaiwy and Goldfarb2008; Taketsuru and Kaneko, Reference Taketsuru and Kaneko2016). There is evidence that growth factor receptors on embryonic cell surfaces are critical for embryo development. Also, it has been shown that the presence of growth factors in culture media plays an essential role in preimplantation embryo development (Richter, Reference Richter2008).
Granulosa cells produce growth factors secreted on oocytes and stimulate metabolic pathways crucial for ultimate oocyte growth and development (Eppig, Reference Eppig2018).
Previous studies have shown that the conditioned culture from granulosa cells has beneficial effects on fetal development and that mesenchymal stem cell-conditioned medium is suitable for growth of preantral follicles (Malekshah et al., Reference Malekshah, Moghaddam and Daraka2006; Ling et al., Reference Ling, Feng, Zhou, Gao, Wei and Tian2008). Furthermore, in 2017 we introduced ESCCM as a beneficial medium that supported follicle growth, oocyte maturation and embryo development (Miraki et al., Reference Miraki, Mokarizadeh, Banafshi, Assadollahi, Abdi, Roshani and Fathi2017).
Embryonic stem cells are an in vitro duplicate of embryonic cells, called the inner cell mass, at the blastocyst stage (Fathi et al., Reference Fathi, Altiraihi, Mowla and Movahedin2010). These cells are pluripotent cells that can differentiate in vivo into any cell type, similar to the blastomeres that produce embryos (Thomson et al., Reference Thomson, Itskovitz-Eldor, Shapiro, Waknitz, Swiergiel, Marshall and Jones1998; Amit et al., Reference Amit, Carpenter, Inokuma, Chiu, Harris, Waknitz, Itskovitz-Eldor and Thomson2000; Odorico et al., Reference Odorico, Kaufman and Thomson2001).
Embryonic stem cells produce biological secretions and activated proteins; research suggests their ability to provide an environment rich in mitogenic substances, growth factors, cytokines and chemokines. These proteins inhibit cancer cell proliferation and cardiovascular cell death, aid cardiomyocyte division, and promote angiogenesis (Giuffrida et al., Reference Giuffrida, Rogers, Nagy, Calogero, Brown and Casper2009; Fatma et al., Reference Fatma, Selby, Singla and Singla2010; LaFramboise et al., Reference LaFramboise, Petrosko, Krill-Burger, Morris, McCoy, Scalise, Malehorn, Guthrie, Becich and Dhir2010). Conditioned medium produced by embryonic stem cells has the ability to control the cell’s fate (Fuchs et al., Reference Fuchs, Tumbar and Guasch2004; Naveiras and Daley, Reference Naveiras and Daley2006).
Embryonic stem cells secrete various growth factors including epidermal growth factor (EGF), insulin-like growth factors 1 and 2 (IGF-I/II), stem cell factor (SCF), fibroblast growth factor (FGF), and transforming growth factor (TGF) (Guo et al., Reference Guo, Graham-Evans and Broxmeyer2006; Bendall et al., Reference Bendall, Hughes, Campbell, Stewart, Pittock, Liu, Bonneil, Thibault, Bhatia and Lajoie2009; Fathi et al., Reference Fathi, Altiraihi, Mowla and Movahedin2010). There is evidence that growth factors such as IGF-I, TGFβ, leukaemia inhibitory factor (LIF), and EGF improve oocyte maturation (Das et al., Reference Das, Stout, Hensleigh, Tagatz, Phipps and Leung1991; Gómez et al., Reference Gómez, Tarin and Pellicer1993; De La Fuente et al., Reference De La Fuente, O’Brien and Eppig1999; Wånggren et al., Reference Wånggren, Lalitkumar, Hambiliki, Ståbi, Gemzell-Danielsson and Stavreus-Evers2007; Thongkittidilok et al., Reference Thongkittidilok, Tharasanit, Sananmuang, Buarpung and Techakumphu2014; Meiyu et al., Reference Meiyu, Liu and Roth2015; Arat et al., Reference Arat, Caputcu, Cevik, Akkoc, Cetinkaya and Bagis2016; Richani and Gilchrist, Reference Richani and Gilchrist2018). Therefore, evaluation of the effect of ESCCM on follicle and embryo development seems to be a logical approach. In this study, we investigated the effect of ESCCM on the development of mice embryos in vitro. We found that ESCCM cannot pass embryos from the 2-cell stage, but is appropriate for fetal growth from the 8-cell stage.
Materials and methods
Mouse embryonic stem cell culture and preparation of conditioned medium
ESCGM contains Dulbecco’s modified Eagle’s medium with supplements including 100 U/ml penicillin, 50 µg/ ml streptomycin (Gibco), 1 mM sodium pyruvate (Gibco), 15% fetal bovine serum (Gibco), 1% non-essential amino acids (Gibco), 0.1 mM β-mercaptoethanol (Sigma) and 10 ng/ml LIF (ESGRO-LIF, Gibco) and was used to culture embryonic stem cells. To prepare ESCCM, embryonic stem cells were cultured in ESCGM. When cultured embryonic stem cells reached 70–80% confluency (c. 48 h later), supernatant from the cell culture was collected. Approximately 15 ml of prepared ESCCM were placed into tubes and centrifuged at 3000 rpm for 4–6 min to remove live and dead cells present in the culture medium. Then, the supernatant was filtered through a 0.22-μm membrane and used immediately.
Animals
All 30 male and 280 female NMRI 4–8-week-old mice were housed and bred at the Central Animal House of Kurdistan University of Medical Sciences. Animals were maintained on a 12-h/12-h light/dark (LD) cycle, at a temperature range of 22–24°C, and given access to food and water ad libitum.
Superovulation and obtaining naturally fertilized embryos of unfertilized oocytes
To induce oocyte development, 5 IU pregnant mare serum gonadotropin (PMSG) was injected intraperitoneally (i.p.) into each mouse. To induce superovulation, 48 h after PMSG injection, 5 HCG units were injected i.p. Subsequently, to obtain naturally fertilized embryos, these mice were placed overnight for mating with male mice and that had previously been kept separately. The following day, mice with a positive plug were separated from other mice and used to generate 2pn, 2-cell, and 8-cell embryos. To obtain unfertilized oocytes for use in IVF, 14 h after HCG injection the unmated female mice were killed, ovaries were separated and then put into a sterile dish. Afterward, oocytes were released by tearing the uterine ampulla and transferred to HTF. The oocytes displaying distinct first polar bodies were used for IVF.
Obtaining 2pn, 2-cel and 8-cell embryos
To collect 2pn embryos, at the 0.5 day mice were killed and the fallopian tube was removed from the genital tract and stored under mineral oil. Then, embryos were extracted from the uterine tube. Mechanically, the ampulla was torn, and embryos that were surrounded by granulosa cells were transferred to HTF containing hyaluronidase. After c. 15 min, the granulosa cells were removed. Next, embryos were washed into HTF droplets. Finally, quality was checked under a stereomicroscope, healthy 2pn embryos were isolated and randomly divided into three groups. For 2-cell and 8-cell stage embryos, all steps of the process were similar to that used to obtain 2pn embryos, except that mice were killed on the 1.5 and 2.5 days after mating, and embryos were extracted by flushing the uterine tube with an insulin syringe containing medium.
In vitro fertilization
Sperm were collected from the cauda epididymis of mature NMRI male mice (6–8-week-old) and then transferred to HTF for 1–1.5 h at 37°C for capacitation. Oocytes were transferred to HTF in three different dishes and sperm were added. After 5 h, fertilized oocytes were washed several times, transferred to KSOM, ESCCM or ESCGM and the development stages were followed up daily.
Embryo transfer
Pseudopregnant mice were prepared as recipients by sterile mating of fertile female NMRI mice to vasectomized males. The following morning, the presence or absence of vaginal plugs was examined. Females that had a vaginal plug were used as pseudopregnant recipients for embryo transfer.
Selected recipient mice were isolated from the other mice, and on day 3 of pseudopregnancy (2.5 days postcoital), they were anesthetized with an i.p. injection of ketamine (80 mg/kg body weight) and xylazine (20 mg/kg body weight). Then, the hair of the dorsal skin was shaved, and a 1-cm long incision was generated parallel to the dorsal midline to expose the oviduct and uterus. As Nagy and colleagues described in 2003 (Nagy et al., Reference Nagy, Gertsenstein, Vintersten and Behringer2003), an average of 10 embryos that developed to the morula or blastocyst stages were removed using a mouse pipette and transferred to each oviduct of the recipient mice. Finally, on day 19.5 of pregnancy, recipients underwent natural delivery or caesarean section; the numbers of live pups were recorded, and the final analysis was made.
Experiment design
In this study, three groups of NMRI mice were isolated: The first group included 395 embryos fertilized in vitro, the second group consisted of 200 embryos fertilized in vivo isolated in the 2pn stage, and the third group contained 320 embryos fertilized in vivo isolated in the 8-cell stage. Embryos from each group were cultured in three different media: ESCCM, ESCGM or KSOM (positive control group) (Table 1). The growth stages were evaluated at 24 h and up to 120 h using a stereomicroscope, and the numbers of 2-cell and 8-cell, morula, and blastocyst embryos were recorded.
Table 1. Numbers of embryos in each group and in different media

Results
Embryo development after in vitro fertilization
In the first group, 395 oocytes obtained from the uterine ampulla of mice 12 h after injection of HCG were used for in vitro fertilization. After 24 h, the success rate of in vitro fertilization was determined by counting the number of 2-cell embryos.
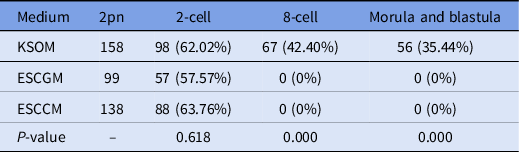
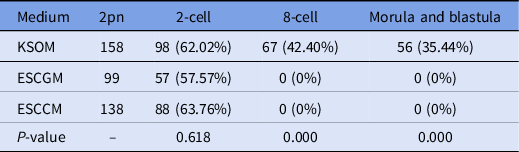
The results of embryo transfer from in vitro fertilization to KSOM, ESCCM or ESCGM showed that 62.02% of embryos in KSOM as the control environment were able to reach the 2-cell stage, while 42.40%, and 35.44% reached the 8-cell and blastocyst stages, respectively (Table 2). However, while 57.57% of embryos from the ESCGM and 63.76% of embryos from the ESCCM culture medium were able to reach the 2-cell stage (P = 0.618), none of them were able to pass through the 2-cell stage, and so were blocked (Fig. 1A) or their division had stopped (0%). Differences were significant at these stages (P = 0.000) (Table 2).
Table 2. Investigation of embryo development after in vitro fertilization
ESCCM, embryonic stem cell-conditioned medium; ESCGM, embryonic stem cell growth medium; KSOM, potassium-enriched simplex optimized medium.
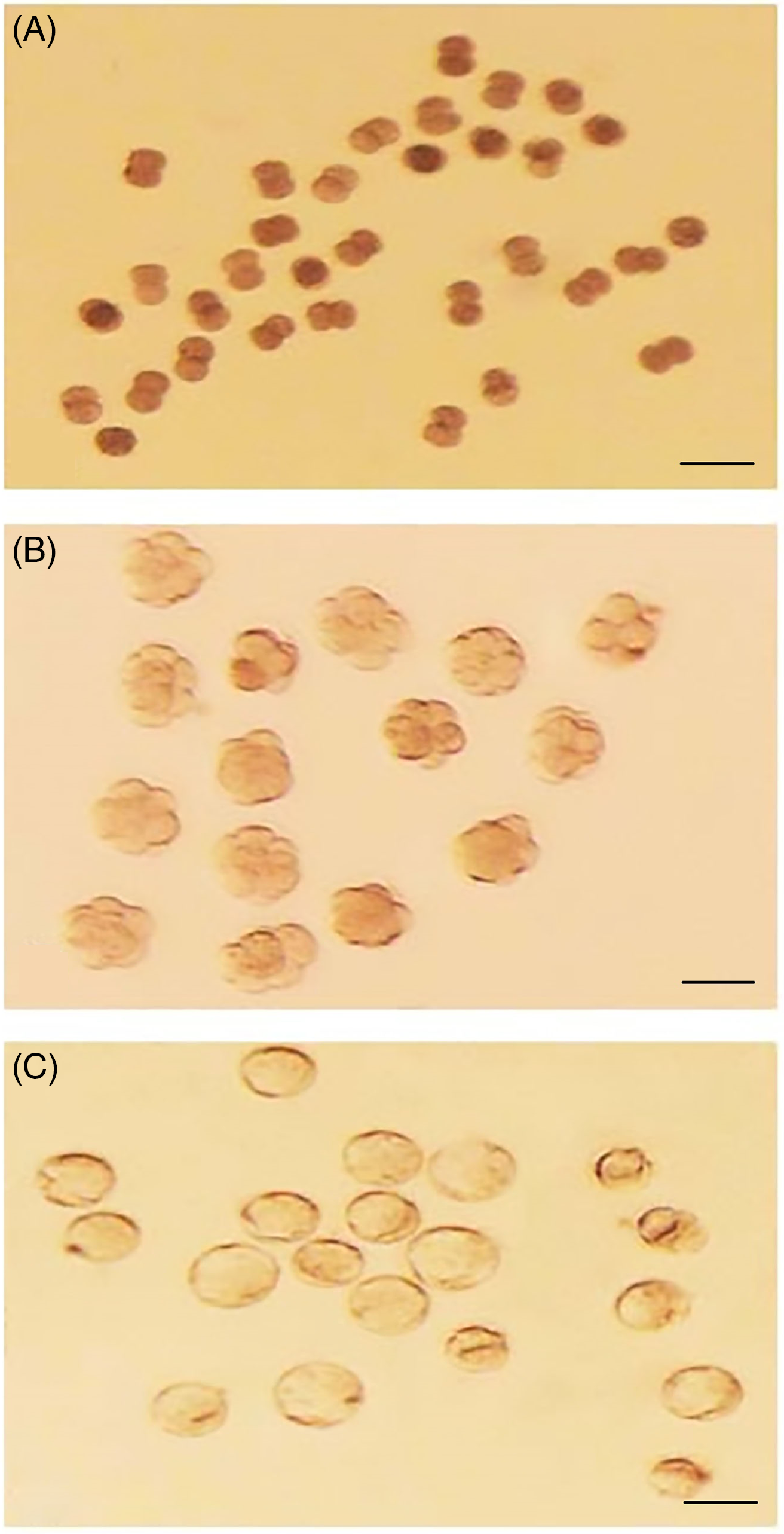
Figure 1. (A) Two-cell block embryos obtained from fertilized oocytes in HTF and cultured in ESCCM. (B) Eight-cell embryos obtained from fertilized oocytes in HTF and cultured in KSOM. (C) Blastocyst embryos obtained from 8-cell embryos cultured in ESCGM. Bars, 50 μm.
In vitro culture of naturally fertilized 2pn-stage embryos
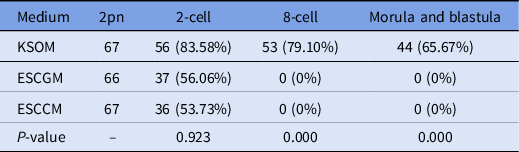
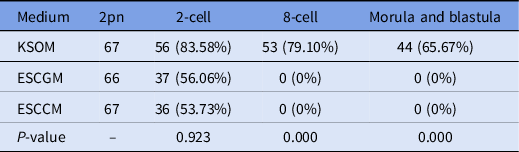
Here, 200 naturally fertilized embryos were obtained from uterine ampules of positive vaginal plug mice. Embryos were divided randomly into three groups as described in Table 3. The numbers of 2-cell, 8-cell, morula, and blastulae were recorded. The results obtained from the development of the naturally fertilized embryo were similar to that of embryos from in vitro fertilization. In detail, 83.58% of embryos transferred to the KSOM environment were able to reach the 2-cell stage, 79.10% reached the 8-cell stage, and 65.67% could reach the blastocyst stage. However, as shown in Table 3, 37 embryos (56/06%) in ESCGM and 36 embryos (53.73%) in ESCCM were able to reach only the 2-cell stage, and none of them was able to pass through the 2-cell stage, and was blocked (0%). Although there was no significant difference between ESCCM and ESCGM for reaching the 2-cell stage (P = 0.923), the difference compared with KSOM was significant in the development of embryos to later stages after the 2-cell stage (P = 0.000).
Table 3. Investigation of embryo development after natural fertilization, 2-cell, 8-cell. morula and blastula stages
ESCCM, embryonic stem cell-conditioned medium; ESCGM, embryonic stem cell growth medium; KSOM, potassium-enriched simplex optimized medium.
In vitro culture of naturally fertilized 8-cell stage embryos
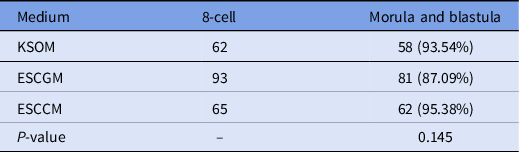
In total, 220 8-cell-stage embryos derived from natural fertilization were obtained from the uterus of mice and divided randomly into three groups, then cultured in KSOM, ESCCM (Fig. 1B) or ESCGM. Embryos that reached the morula and blastocyst stages were counted. The results showed that 93.54% of embryos transferred to KSOM were able to reach the blastocyst stage. As shown in Table 4, there was no significant difference between the numbers of embryos that reached the morula and blastula stages in ESCGM (87.09%; Fig. 1C) and ESCCM (95.38%) (P = 0.145).
Table 4. Investigation of embryo development after natural fertilization, 8-cell. morula and blastula stages

Embryo development and birth rate
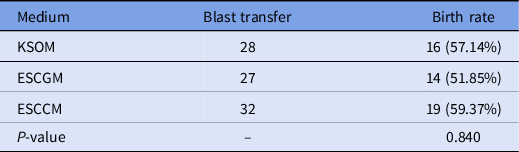
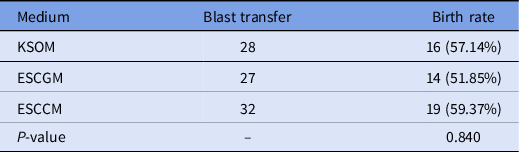
To investigate the viability of embryos after 2 days of culture in KSOM, ESCCM or ESCGM, morula and blastocyst embryos were isolated and transferred to uteri of pseudopregnant mice. There was no significant difference between the rate of success in implantation and the birth of embryos cultured in KSOM (57.14%), ESCCM (59.37%) or ESCGM (51.85%) (Table 5).
Table 5. Results of birth rate after morula and blastocyst embryo transfer obtained from KSOM, ESCGM or ESCCM media
ESCCM, embryonic stem cell-conditioned medium; ESCGM, embryonic stem cell growth medium; KSOM, potassium-enriched simplex optimized medium.
Statistical analysis
For each of the developmental stage categories, such as 2-cell and 8-cell stages of embryo to blastocyst stages after natural and in vitro fertilization states, developmental competence to the blastocyst stage in ESCGM, ESCCM or KSOM groups was calculated and a comparison was made. Data were analyzed according to the three-sample test for equality of proportions with continuity correction. Rates of live newborn mice in the study groups were analyzed using the two-sample proportion test and chi-squared test with R v.3.1.0 software. A P-value ≤ 0.05 was considered significant.
Discussion
In this study, we evaluated the efficiency of ESCGM and ESCCM compared with KSOM (as the control group) for the development of embryos during in vitro and natural fertilization at different stages. Our results showed that ESCCM and ESCGM could not pass the embryo after the 2-cell stage, but they were suitable for development of the embryo from the 8-cell stage to blastocyst. No significant difference between the success rate in implantation and birth of embryos cultured in KSOM, ESCCM or ESCGM was observed.
Reproductive tracts in both mice and humans produce several growth factors such as IGF-1, EGF, VEGF, PDGF (Österlund et al., Reference Österlund, Wramsby and Pousette1996; Smotrich et al., Reference Smotrich, Stillman, Widra, Gindoff, Kaplan, Graubert and Johnson1996; Lighten et al., Reference Lighten, Hardy, Winston and Moore1997; Möller et al., Reference Möller, Rasmussen, Lindblom and Olovsson2001).
Choosing the right culture medium for preantral follicles is important. Therefore, finding the requirements for follicle growth in culture medium can help to reduce the limitations of infertility treatment. Improvements in the culture medium has a significant effect on obtaining the desired oocyte for use in research and even on infertility. In recent years, much attention has been paid to conditional environments such as environments rich in auxiliary materials in biology and this has raised new hope for their use in cell and embryo culture. The results of studies so far have shown that several growth factors are secreted by embryonic stem cells, including EGF, FGF, SCF, LIF and VEGF (Guo et al., Reference Guo, Graham-Evans and Broxmeyer2006; Bendall et al., Reference Bendall, Hughes, Campbell, Stewart, Pittock, Liu, Bonneil, Thibault, Bhatia and Lajoie2009).
Several experiments have shown that growth factors such as EGF, Activine, IGF-I, and TGF have been effective in supporting oocyte maturation (Feng et al., Reference Feng, Catt and Knecht1988; Gómez et al., Reference Gómez, Tarin and Pellicer1993; Lorenzo et al., Reference Lorenzo, Illera, Silvan, Munro, Illera and Illera1997; Alak et al., Reference Alak, Coskun, Friedman, Kennard, Kim and Seifer1998; Pawshe et al., Reference Pawshe, Rao and Totey1998; De La Fuente et al., Reference De La Fuente, O’Brien and Eppig1999). We have recently found that ESCCM can successfully support different stages of embryo development (Miraki et al., Reference Miraki, Mokarizadeh, Banafshi, Assadollahi, Abdi, Roshani and Fathi2017). Previous studies have shown that some growth factors, such as insulin and EGF, have led to the stimulation of metabolic and synthesis activity in the early stages of embryos (Heyner et al., Reference Heyner, Smith and Schultz1989; Beebe and Kaye, Reference Beebe and Kaye1991; Harvey and Kaye, Reference Harvey and Kaye1991; Sakkas and Trounson, Reference Sakkas and Trounson1991). Gelber and colleagues in 2011 showed that ESCCM was not as successful as KSOM in supporting embryonic development at the 2-cell stage, but after the 8-cell stage, ESCCM is more efficient (Gelber et al., Reference Gelber, Tamura, Alarcon and Marikawa2011). The results of this study were consistent with the results we obtained. ESCGM contains more complex compounds compared with KSOM and includes multiple vitamins, antioxidants and growth factors such as BMP4 and LIF. ESCGM could support the development of embryonic cells for long periods of time (Smith et al., Reference Smith, Heath, Donaldson, Wong, Moreau, Stahl and Rogers1988; Williams et al., Reference Williams, Hilton, Pease, Willson, Stewart, Gearing, Wagner, Metcalf, Nicola and Gough1988; Ying et al., Reference Ying, Nichols, Chambers and Smith2003).
It has also been shown that when embryo development stops at the 2-cell stage, it can be possible to pass from this stage by adding lactate. It has also been demonstrated that pyruvate is used relatively as much as glucose as the primary source of energy. The development of mouse embryos from the zygote stage to the 2-cell stage is completely dependent on pyruvate, while glucose is more likely to support embryo development in the 4-cell and 8-cell stages (Brinster, Reference Brinster1965; Brinster and Thomson, Reference Brinster and Thomson1966). Therefore, one of the suggested factors that may result in blocking the 2-cell stage is the high amount of glucose in ESCGM and ESCCM (17.5 mM vs 0.2 mM). It can be concluded that glucose is a critical factor in supporting embryo development, especially during the pre-8-cell stages (Biggers et al., Reference Biggers, Whittingham and Donahue1967; Conaghan et al., Reference Conaghan, Handyside, Winston and Leese1993; Kim et al., Reference Kim, Funahashi, Niwa and Okuda1993).
Another factor that might have impaired the development of 2-cell stage embryos is the absence of EDTA in ESCGM and ESCCM. Several studies have shown that adding EDTA into embryo culture medium can help embryos to pass the 2-cell stage, although the way EDTA works has not been well defined (Abramczuk et al., Reference Abramczuk, Solter and Koprowski1977; Gardner and Lane, Reference Gardner and Lane1996).
LIF may play a special role in the relationship between mother and fetus before implantation because the presence of LIF for implantation of mouse embryos is essential (Stewart et al., Reference Stewart, Kaspar, Brunet, Bhatt, Gadi, Köntgen and Abbondanzo1992) and impaired LIF adjustment has been suggested as a factor for infertility (Dimitriadis et al., Reference Dimitriadis, Stoikos, Stafford-Bell, Clark, Paiva, Kovacs and Salamonsen2006). From different studies focused on the effects of LIF on the maturation of oocytes, cumulus cells in mice and humans, LIF enhanced the results of cleavage, embryo development and birth rate (De Matos et al., Reference De Matos, Miller, Scott, Tran, Kagan, Nataraja, Clark and Palmer2008).
In the present study, although ESCGM and ESCCM possessed limited abilities to enable embryos to reach the 2-cell stage, neither ESCGM nor ESCCM was able to pass the embryos from the 2-cell stage. Therefore, it can be concluded that there is probably no factor in these culture media to overcome the block at the 2-cell stage, alternatively there are factors in these culture media that block the next stage of embryo development; or maybe both occur at the same time.
Different results were observed for embryos after the 8-cell stage. Several 8-cell stage embryos were separated from the uterus and transferred to control medium culture, ESCGM, or ESCCM. These were able to develop to the blastocyst stage. This finding shows that conditioned medium can support embryo development to reach morula and blastocyst stages. This part of the study showed that material secreted from embryonic stem cells and conditioned media support embryo development very well. Although the results of this study may not alone prove the superiority of ESCGM and ESCCM compared with KSOM, in support of embryonic development in the preimplantation stages, it can be concluded that culture media with different compositions compared with KSOM are needed to support the development of embryos at preimplantation stages.
The findings of this study concluded that ESCCM and ESCGM could not pass embryos after the 2-cell stage, but were suitable for development of the embryo from the 8-cell stage to blastocyst. Therefore, we believe needs and requirements of embryos are completely different at different stages of development, i.e. from the start to the time of implantation.
Author contributions
Saber Miraki, Fardin Fathi and Mohammad Ghasem Golmohammadi conceived, designed, and carried out the experiments, processed the experimental data, performed the analysis, drafted the manuscript and critically revised the manuscript. Asrin Rashidi, Omid Banafshi and Masoud Alasvand helped in process the experimental data, performed the analysis and drafted the manuscript, and critically revised the manuscript.
Financial support
This research was financially supported by the Kurdistan University of Medical Sciences.
Conflict of interest
The authors declare that there is no conflict of interests.
Ethical standards
All the protocols and procedures of the study were approved by the Kurdistan University of Medical Sciences Animal Ethics Committee (permit no. 1394/260).
Data availability
The datasets generated and/or analyzed during the current study are available.