Introduction
The native Mediterranean brown trout (Salmo trutta macrostigma) population of the Biferno river in the Molise region of Italy, is declining as a result of river pollution, poorly regulated fishing activities and the introduction of allochthonous strains for recreational purposes, causing genetic introgression.
Such is the situation that Salmo trutta macrostigma has been listed as critically threatened and endangered by the International Union for Conservation of Nature (IUCN). Currently, the Mediterranean brown trout appears in the Italian IUCN Red List as ‘critically endangered’ under the scientific name Salmo cettii (Bianco et al., Reference Bianco, Caputo, Ferrito, Lorenzoni, Nonnis Marzano, Stefani, Sabatini, Tancioni, Rondinini, Battistoni, Peronace and Teofili2013). In the present paper, we use the more widely cited name Salmo trutta macrostigma for this taxon.
Sperm cryopreservation is a valuable tool for the conservation of endangered fish species (Roldan & Garde, Reference Roldan, Garde and Gomendio2004). The procedure has effectively been described as safe for the ex situ preservation of biodiversity (Martínez-Páramo et al., Reference Martínez-Páramo, Pérez-Cerezales, Gómez-Romano, Blanco, Sánchez and Herráez2009) by facilitating the storage of gametes in a gene bank (Gausen, Reference Gausen, Cloud and Thorgaard1993; Akçay et al., Reference Akçay, Bozkurt, Seçer and Tekin2004; Martínez-Páramo et al., Reference Martínez-Páramo, Pérez-Cerezales, Gómez-Romano, Blanco, Sánchez and Herráez2009; Bozkurt et al., Reference Bozkurt, Yavas, Karaca and Katkov2012). Indeed, a semen cryobank of endangered fish species would serve to preserve representative samples and to further reconstruct the original strain, population or variety after the environmental restoration (Martínez-Páramo et al., Reference Martínez-Páramo, Pérez-Cerezales, Gómez-Romano, Blanco, Sánchez and Herráez2009).
During the semen cryopreservation procedure, however, fish sperm cells, as observed for other animal species (Holt, Reference Holt2000; Bailey et al., Reference Bailey, Bilodeau and Cormier2000; Johnson et al., Reference Johnson, Weitze, Fiser and Maxwell2000; Iaffaldano et al., Reference Iaffaldano, Romagnoli, Manchisi and Rosato2011, Reference Iaffaldano, Di Iorio and Rosato2012), are subjected to cold stress which reduces their quality (Maldjian et al., Reference Maldjian, Pizzi, Gliozzi, Cerolini, Penny and Noble2005; Müller et al., Reference Müller, Müller, Pincemy, Kurz and Labbé2008; Bozkurt et al., Reference Bozkurt, Yavas, Karaca and Katkov2012; Cabrita et al., Reference Cabrita, Martínez-Páramo, Gavaia, Riesco, Valcarce, Sarasquete, Herráez and Robles2014). In turn, reduced sperm quality compromises fertilization ability (Gwo & Arnold, Reference Gwo and Arnold1992; Rana, Reference Rana, Bromage and Roberts1995), and larval survival (Suquet et al., Reference Suquet, Dreanno, Petton, Normant, Omnes and Billard1998).
Sperm cryopreservation has been widely investigated by several authors (Lahnsteiner, Reference Lahnsteiner2000; Billard & Zhang, Reference Billard, Zhang, Watson and Holt2001; Chao & Liao, Reference Chao and Liao2001; Tiersch et al., Reference Tiersch, Yang, Jenkins and Dong2007) with negative effects observed on spermatozoa structure and functionality, especially in salmonids (Cabrita et al., Reference Cabrita, Sarasquete, Martínez-Páramo, Robles, Beirão, Pérez-Cerezales and Herráez2010). Thus semen cryopreservation protocols developed for many fish species have shown variable success. The success of semen cryopreservation depends on interplay among many factors that influence sperm cryosurvival (Dziewulska & Domagała, Reference Dziewulska and Domagała2013). These factors include freezing medium composition, the cryoprotectant and its concentration, and freezing and thawing rate (Bozkurt et al., Reference Bozkurt, Akçay, Tekin and Seçer2005, Reference Bozkurt, Yavas, Karaca and Katkov2012; Mansour et al., Reference Mansour, Richardson and McNiven2006; Yavas et al., Reference Yavas, Bozkurt and Yıldız2014). The choice of freezing rate is certainly among the most important factors for an effective semen freezing protocol. Accordingly, the objectives of this study were to determine the effects of different freezing rates on post-thaw semen quality of Mediterranean brown trout from the Biferno river, and to assess the most effective freezing rate identified in terms of the in vivo reproductive performance of semen cryopreserved in this manner.
Materials and methods
Chemicals
The LIVE/DEAD Sperm Viability Kit was purchased from Molecular Probes, Inc. (Eugene, OR, USA) and all other chemicals used in this study were purchased from Sigma, Chemical Co. (Milan, Italy).
Animals
Specimens of Salmo trutta macrostigma were captured from the Biferno river at Bojano springs (Molise region) by electro-fishing. Twenty-five Mediterranean brown trout specimens were identified according to their phenotypic characteristics (Gibertoni et al., Reference Gibertoni, Jelli and Bracchi1998; Jelli & Gibertoni, Reference Jelli and Gibertoni1999; Penserini et al., Reference Penserini, Nonnis Marzano, Gandolfi, Maldini, Marconato and Gibertoni2006). Fish (twenty males and 5 females) were aged as 2+ to 5+ years. This sampling location was selected as it is a highly attractive spawning site for native trout population after upstream migration. Genetic analysis, still in progress, showed that samples captured at Bojano springs during spawning season, have low levels of introgression (about 0.15%) by allochthonous brown trout.
Semen and egg collection
Trout semen and eggs were collected during spawning in February. Abdomens and urogenital papilla were dried before stripping to avoid contamination of semen and eggs with urine, mucus and blood cells. Twenty males semen was collected by gentle abdominal massage. Each male was stripped once only and the total amount of expressible milt was collected individually. Ejaculates of different males were pooled (four ejaculates/pool) to avoid the effects of individual differences. In total, five pools were used. The semen pools were kept in a portable fridge (4ºC) before cryopreservation. Eggs were gathered from five mature females also by gentle abdominal massage. Eggs used were well rounded and transparent.
Experiment 1. Effects of different freezing rates on post-thaw semen quality
Sperm cryopreservation
Semen pools were transported from the river to the laboratory in a portable refrigerator at 4°C. Once at the laboratory, aliquots taken from each semen pool were immediately assessed for fresh semen quality as described below.
Semen was diluted 1:3 (semen:extender) in freezing extender containing 300 mM glucose, 10% egg yolk and 10% DMSO (dimethyl sulfoxide). We used this freezing extender because it has been associated with excellent in vivo reproductive performance in rainbow trout (Tekin et al., Reference Tekin, Seçer, Akçay and Bozkurt2003).
Each diluted semen pool was divided into three subsamples and kept at 4°C, packaged in 0.25 ml plastic straws that were sealed with polyvinyl alcohol (PVA). The straws grouped by treatment (freezing rate) were equilibrated for 10 min at 4°C, and then frozen by exposure to liquid nitrogen vapour at different heights above the liquid nitrogen surface (1, 5 or 10 cm) for 10 min to give three different freezing rates. During these 10 min, the temperature of the straws placed at 1 cm from the liquid nitrogen fell from +4°C to −140°C, at 5 cm from +4°C to −125°C and at 10 cm from +4°C to −90°C, indicating a slower freezing rate as the distance from the liquid nitrogen increases. Temperatures were monitored by a temperature sensor (Ascon M1). Subsequently, the straws were plunged into liquid nitrogen for storage at −196°C. Semen samples were thawed by immersing the straws in a water bath at 30°C for 10 s.
Sperm quality
Sperm quality parameters, as sperm viability (%), sperm motility (%) and spermatozoa movement duration (s), were determined in both fresh and frozen semen.
Sperm motility was subjectively evaluated. About 5 μl of fresh or frozen sperm were placed on a glass microscope slide and 10 μl of activation solution (0.3% NaCl) were added to the fresh semen, while frozen/thawed semen was activated with 10 μl of 1% NaHCO3. Sperm motility was expressed as the percentage of motile spermatozoa observed under ×40 magnification. Sperm were defined as motile if they showed forward movements whereas simply vibrating sperm were deemed immobile. Observations were made at room temperature (15–20°C).
The duration of sperm movement was measured using a sensitive chronometer that was started simultaneously with the addition of activation solution.
Sperm viability was assessed using the LIVE/DEAD Sperm Viability Kit containing the fluorescent stains SYBR-14 and propidium iodide (PI). This procedure was performed on 1 μl of fresh or thawed semen, which was added to 40 μl of immobilizing medium (80 mM NaCl, 40 mM KCl, 0.1 mM CaCl2, 30 mM Tris–HCl, pH 9.2) (v/v). Subsequently, 2.5 μl of SYBR-14 working solution (diluted 1:100 in DMSO) were added to the cell suspension. After 10 min of incubation at room temperature in the dark, 3 μL of working PI solution (PI solution diluted 1:100 in phosphate-buffered saline (PBS) diluent) were added to the cell suspension. Spermatozoa were incubated for a further 10 min under the same conditions. Next, 10 μl of this suspension were placed on microscope slides, covered with coverslips and examined at ×1000 magnification using a ×100 oil immersion objective under epifluorescence illumination. For each sample, approximately 200 spermatozoa were examined in duplicate aliquots. SYBR-14, a membrane permeant DNA stain, only stains live spermatozoa producing green fluorescence of the nuclei. Propidium iodide stains the nuclei of membrane-damaged cells red. Thus, spermatozoa showing green fluorescence are scored as alive and those showing red fluorescence as dead. The percentage of viable spermatozoa was calculated as the number of green cells ×100 divided by the total number of sperm counted.
Experiment 2. In vivo reproductive capacity of semen cryopreserved using the best freezing rate
This experiment was an artificial fertilization trial in which we compared results using fresh semen or thawed semen cryopreserved at the freezing rate identified as best in Experiment 1. Eggs were pooled from five females. Fertilization took place in four dry plastic dishes (two each for fresh and frozen semen) using the dry fertilization technique. Portions of ca. 100 eggs (10 g) were placed in each dish and then fertilized with fresh or frozen/thawed semen at a proportion of approximately 0.5 × 106 sperm/egg, as reported in Billard (Reference Billard1977) for optimum fertilization success. Eggs and sperm cells were gently mixed for 10 s. After fertilization, 25 ml of 0.3% sodium chloride was added to the sperm–egg mixture as fertilization solution and the mixture left for 45 min for eggs swelling. Subsequently, fertilized eggs were rinsed with hatchery water (10°C) and placed in a refrigerator at 5°C. Unfertilized and dead eggs were counted and removed continuously. After 50–60 days at 5°C, eggs reached the eyed-egg stage. Embryos started to hatch on days 90–95 after fertilization. We calculated fertilization or hatching rate as the number of eyed or hatched eggs divided by eggs initial number × 100, respectively.
Statistical analysis
Sperm quality variables (motility percentage and duration, viability) between treatments were compared by ANOVA (analysis of variance) followed by Scheffe's comparison test. Fertilization and hatching rates were compared between fresh and frozen semen using a t-test for independent samples. Significance was set at P < 0.05. All statistical tests were performed using the software package SPSS (SPSS 15.0 for Windows, 2006; SPSS, Chicago, Illinois, USA).
Results
Semen quality variables recorded for fresh semen (Table 1) indicated its good initial quality. Over 70% of the sperm population were motile and viable.
Table 1 Sperm quality variables recorded in freshly collected trout semen (N = 5)

Effects of different freezing rate on motility of frozen/thawed trout sperm are shown in Fig. 1. A significant effect of freezing rate was observed on sperm motility. This determined that higher sperm motility values were recorded when semen was frozen 5 cm above liquid nitrogen surface than at the other heights (P < 0.05). Lowest sperm motility was observed when loaded straws were frozen 1 cm above liquid nitrogen surface.
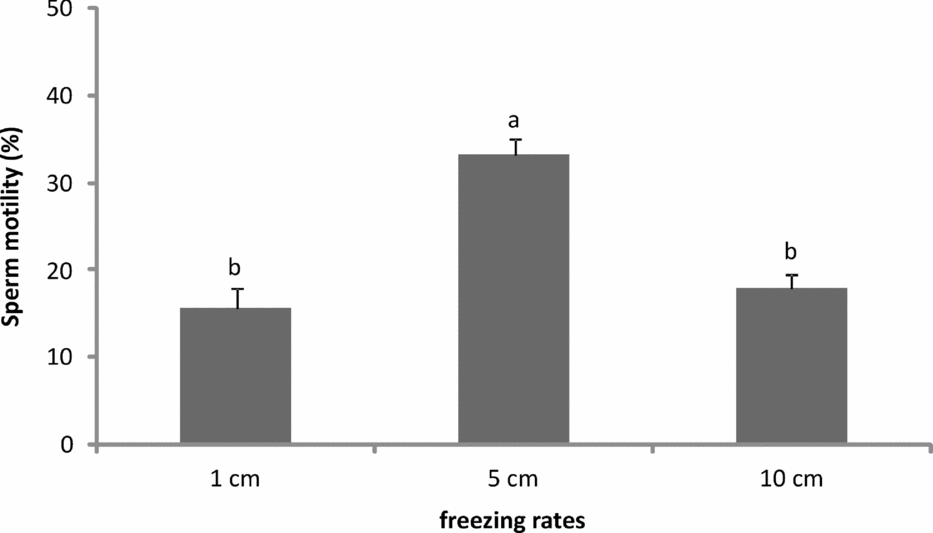
Figure 1 Post-thaw motility (%) of brown trout sperm cryopreserved at different freezing rates. Bars represent mean percentages ± standard error of the mean (SEM). a,bDifferent lowercase letters on bars indicate a significant difference (P < 0.05). Freezing rates (distance from liquid nitrogen).
A similar trend was observed for duration of motility (Fig. 2). Thus, higher post-thaw motility duration(s) values (P < 0.05) were observed when semen was frozen 5 cm above liquid nitrogen than at the other heights tested. In addition, lower motility duration values were recorded for semen cryopreserved at 1 cm, while intermediate values were recorded for 10 cm. Motility duration values for 10 cm were significantly higher (P < 0.05) than ones observed for 1 cm.

Figure 2 Post-thaw motility duration (s) recorded in brown trout sperm frozen at different freezing rates. Bars represent mean percentages ± standard error of the mean (SEM). a–cDifferent lowercase letters on bars indicate a significant difference (P < 0.05). Freezing rates (distance from liquid nitrogen).
Best sperm viability values were shown by semen frozen 5 cm above the liquid nitrogen surface (P < 0.05) (Fig. 3). No significant differences were detected between 1 cm and 10 cm.

Figure 3 Post-thaw viability (%) recorded in brown trout sperm frozen at different freezing rates. Bars represent mean percentages ± standard error of the mean (SEM). a,bDifferent lowercase letters on bars indicate a significant difference (P < 0.05). Freezing rate (distance from liquid nitrogen).
Fertilization and hatching rates recorded for cryopreserved and fresh semen are provided in Table 2. Data were significantly lower in the frozen semen group compared with ones calculated using fresh semen (25.4% and 22.5% versus 87.8% and 75.5%, respectively).
Table 2 Fertilization and hatching rates recorded after artificial fertilization with fresh or frozen brown trout semen

a,b Values with different superscript letters differ significantly (P < 0.05).
Discussion
This study was designed to identify the most effective freezing rate for semen cryopreservation in wild specimens of the Mediterranean brown trout (Salmo trutta macrostigma) native population of the Biferno river. Subsequently, the best freezing rate identified in vitro was compared with fresh semen in field trials. Until now, no literature data exist on the effects of different freezing rates on semen cryopreservation in native Mediterranean brown trout or other Italian autochthonous freshwater fishes. In vitro results clearly revealed a significant effect of freezing rate on cryopreserved semen quality. The freezing rate corresponding to a straw position 5 cm above the liquid nitrogen surface gave rise to the better sperm motility and viability values. This result means that this height of 5 cm was better able to protect the sperm cells from intracellular ice crystal formation during the semen cryopreservation process.
The velocity of cooling has been identified as crucial in that inaccurate cooling rates can negatively affect sperm survival, motility, plasma membrane integrity and mitochondrial function (Henry et al., Reference Henry, Noiles, Gao, Mazur and Critser1993). When cooling is slow, there is sufficient time for intracellular water efflux and balanced dehydration. However, if cooling is too slow, damage may occur through cell exposure to high concentrations of intracellular solutes. Extreme cell dehydration leads to shrinkage below the minimum cell volume necessary to maintain its cytoskeleton, genome-related structures, and ultimately cell viability (Mazur, Reference Mazur1984). Conversely, if cooling rates are too fast, external ice can induce intracellular ice formation and potential plasma membrane rupture and damage to intracellular organelles. In addition, mechanical damage is possible due to extracellular ice compression and close proximity of frozen cells can result in cell deformation and membrane damage (Fujikawa & Miura, Reference Fujikawa and Miura1986).
Another emerging point of our research was that despite observing post-thaw sperm motility/viability recovery rates of about 50% in vitro, this did not mean an adequate fertilizing ability in vivo. In effect, the fertilization and hatching rates obtained for semen frozen at the best freezing rate were substantially lower than ones recorded for fresh semen. These recovery rates were only 29 and 30% respectively. This lack of consistency between in vitro and in vivo results could be attributed to undetected sublethal cryodamage, which could destabilize the sperm membrane and thereby affect spermatozoa function and viability at later times (Drokin et al., Reference Drokin, Stein and Bartseherer1998). This loss of function is extremely important for freshwater fish spermatozoa whose motility, activation, and ability to fertilize the egg require their dilution in water or hypoosmotic solutions (Cabrita et al., Reference Cabrita, Alvarez, Anel, Rana and Herraez1998). These media constitute a hazardous environment for the cells, which, even under ideal conditions, remain motile for only around 1 min. Our in vivo results were similar to those reported by Labbé and Maisse (Reference Labbé and Maisse2001) for frozen brown trout semen. Better in vivo results were reported by Lahnsteiner et al. (Reference Lahnsteiner, Weismann and Patzner1997), Gopalakrishnan et al. (Reference Gopalakrishnan, Thakur, Ponniah, Kumar and Dayal1999) and Sarvi et al. (Reference Sarvi, Niksirat, Amiri, Mirtorabi, Rafiee and Bakhtiyari2006). Due to variability in the biological material used and multiplicity of preservation procedures, it has not been possible to reproduce either the quality or fertilizing capacity of cryopreserved semen. Susceptibility to semen cryopreservation varies among fish species (Cabrita et al., Reference Cabrita, Sarasquete, Martínez-Páramo, Robles, Beirão, Pérez-Cerezales and Herráez2010), and subpopulations (Martínez-Páramo et al., Reference Martínez-Páramo, Pérez-Cerezales, Gómez-Romano, Blanco, Sánchez and Herráez2009). In this regard, Martínez-Páramo et al. (Reference Martínez-Páramo, Pérez-Cerezales, Gómez-Romano, Blanco, Sánchez and Herráez2009) observed different fertilization rates using frozen semen from two brown trout subpopulations inhabiting different rivers in the same basin. This finding indicates that even different populations of a single species may require individualized semen cryopreservation protocols. The semen cryopreservation procedure induces considerable damage to cell membranes (plasma, nuclear, mitochondrial membranes) (Lahnsteiner et al., Reference Lahnsteiner, Weismann and Patzner1992, Reference Lahnsteiner, Berger, Weismann and Patzner1996b; Conget et al., Reference Conget, Fernandez, Herrera and Minguell1996; Drokin et al., Reference Drokin, Stein and Bartseherer1998; Zhang et al., Reference Zhang, Liu, Xu, Wang, Sawant, Li and Chen2003), mitochondria and chromatin structure (Cabrita et al., Reference Cabrita, Sarasquete, Martínez-Páramo, Robles, Beirão, Pérez-Cerezales and Herráez2010). Cell damage can be produced by mechanical forces due to ice crystal formation both within the cells and the external medium, by osmotic stress or oxidative stress (Cabrita et al., Reference Cabrita, Sarasquete, Martínez-Páramo, Robles, Beirão, Pérez-Cerezales and Herráez2010). Salmonids present particular susceptibility to semen cryopreservation damage owing to the special characteristics of their spermatozoa such as a short duration of motility, low ATP production, high sensitivity to osmotic stress, and a large number of spermatozoa required to fertilize one egg (Martínez-Páramo et al., Reference Martínez-Páramo, Pérez-Cerezales, Gómez-Romano, Blanco, Sánchez and Herráez2009).
Our results served to identify the optimal freezing rate for a sperm cryopreservation protocol for the Mediterranean brown trout. In contrast, the in vivo results obtained suggest a need for further work designed to improve the semen cryopreservation protocol by studying other factors involved in the procedure. Finding an efficient freezing protocol will allow for the introduction of a sperm cryobank to recover the native population of Mediterranean brown trout (Salmo trutta macrostigma) in the Biferno river. Indeed, cryobanking of fish sperm has several benefits over breeding in captivity in terms of costs, labour and safety. Thus, thousands of sperm samples from different generations can be kept in a minimum of space, without the risk of losses caused by disease or genetic drift over time. Moreover, moving and management of frozen samples are relatively simple, allowing greater flexibility in designing recovery programmes (Martínez-Páramo et al., Reference Martínez-Páramo, Pérez-Cerezales, Gómez-Romano, Blanco, Sánchez and Herráez2009).
In conclusion, our results identified the best freezing rate for a semen cryopreservation protocol for autochthonous trout.
An effective freezing protocol will allow for the creation of a sperm cryobank that, in combination with an extensive genetic study, a survey on migratory behavior and recovery of the altered habitat are crucial for the restoration of the native population of Mediterranean brown trout (Salmo trutta macrostigma) in the Biferno river. However, to develop this protocol, further factors such as the cryoprotectant and its concentrations, equilibration time and thawing rate will need to be optimized.
Acknowledgements
Authors are grateful to Ana Burton for editorial assistance and the PhD student Marsia Miranda for help with bibliographic research.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.







