Introduction
Sperm-mediated gene transfer (SMGT) is based on the intrinsic ability of sperm cells to bind and internalize exogenous DNA and transfer it to the oocyte during fertilization (Lavitrano et al., Reference Lavitrano, French, Zani, Frati and Spadafora1989, Reference Lavitrano, Forni, Bacci, Di Stefano, Varzi, Wang and Seren1992, Reference Lavitrano, Busnelli, Cerrito, Giovannoni, Manzini and Vargiolu1997; Francolini et al., Reference Francolini, Lavitrano, Lamia, French, Frati, Cotelli and Spadafora1993; Zani et al., Reference Zani, Lavitrano, French, Lulli, Maione, Sperandio and Spadafora1995). The capacity of sperm cells to bind exogenous DNA was first described by Brackett et al. (Reference Brackett, Baranska, Sawicki and Koprowski1971), and a growing interest in these results was generated after Lavitrano et al. (Reference Lavitrano, French, Zani, Frati and Spadafora1989) reported the production of transgenic mice using this technique.
Transgenesis is a great tool that makes possible the generation of genetically modified animals for use in medicine, biotechnology and basic research. The most frequently used methods for generating transgenic animals are DNA microinjection, somatic cell nuclear transfer (SCNT) and viral vectors (Kues & Niemann, Reference Kues and Niemann2004). However, these methods are technically demanding, costly and possibly affected by manipulation of embryos (Lavitrano et al., Reference Lavitrano, Camaioni, Fazio, Dolci, Farace and Spadafora2006). The main advantage of SMGT is its simplicity and reduced manipulation of embryos, but the main drawbacks are the low reproducibility of published results and degree of variation in the expression of exogenous DNA (Wall, Reference Wall2002). Currently, some authors have indicated that the efficiency of SMGT is lower than that of pronuclear microinjection, SCNT and chimera production in domestic animals. However, if SMGT can be optimized, this technology could become a powerful tool for the efficient and economic production of domestic transgenic animals (Smith & Spadafora, 2005).
The effectiveness of SMGT depends on sperm viability and motility, particularly progressive motility, which is related to the fertilization potential and ability of sperm to bind and internalize exogenous DNA (Suarez & Dai, Reference Suarez and Dai1992; Lavitrano et al., Reference Lavitrano, Camaioni, Fazio, Dolci, Farace and Spadafora2006). The appropriate time to start co-incubation of sperm and exogenous DNA is during the beginning of capacitation. Furthermore, the co-incubation medium must be free of calcium to delay the capacitation process and avoid the likelihood of exogenous DNA damage by endonucleases (Lavitrano et al., Reference Lavitrano, Maione, Forte, Francolini, Sperandio, Testi and Spadafora2003). The effect of DNA on mammalian sperm is controversial, with some studies indicating that the binding of exogenous DNA to sperm does not usually interfere with physiological sperm parameters, such as motility (Chan et al., Reference Chan, Luetjens and Schatten2000). However, other studies have shown that binding exogenous DNA reduces sperm viability and that live sperm with bound DNA are immotile as a result of endonuclease activation, DNA fragmentation, and subsequent cell death. This reaction could correspond to a natural protection process that prevents the transmission of exogenous DNA to the offspring (Maione et al., Reference Maione, Pittoggi, Achene, Lorenzini and Spadafora1998; Spadafora, Reference Spadafora1998; Anzar & Buhr, Reference Anzar and Buhr2006). However, in the current literature, there is a large number of studies indicating that to increase the percentage of transgenic embryos by SMGT via intracytoplasmic sperm injection (ICSI), either the sperm membranes must be damaged to facilitate interaction with DNA or sperm must be transfected to facilitate the incorporation of exogenous DNA into their nuclei (Perry et al., Reference Perry, Wakayama, Kishikawa, Kasai, Okabe, Toyoda and Yanagimachi1999; Moreira et al., Reference Moreira, Giraldo, Cozar, Pozueta, Jimenez, Montoliu and Gutierrez-Adan2004; Osada et al., Reference Osada, Toyoda, Moisyadi, Akutsu, Hattori, Sakaki and Yanagimachi2005; Li et al., Reference Li, Mizutani, Ono and Wakayama2010).
Regarding less aggressive transfection treatments, several different strategies have been developed to increase the percentage of transgenic embryos after SMGT in combination with artificial insemination (AI), conventional in vitro fertilization (IVF) and ICSI. The different methodologies include the following:
-
(i) REMI (restriction enzyme-mediated insertion), which is a process consisting of the transfection of sperm using liposomes containing a linearized plasmid and the restriction enzymes used for linearization. The integration of foreign DNA is mediated by restriction enzymes, unlike in SMGT, in which DNA integration is achieved by the cellular machinery (Shemesh et al., Reference Shemesh, Gurevich, Harel-Markowitz, Benvenisti, Shore and Stram2000; Sparrow et al., Reference Sparrow, Latinkic and Mohun2000; Harel-Markowitz et al., Reference Harel-Markowitz, Gurevich, Shore, Katz, Stram and Shemesh2009).
-
(ii) Electroporation consists of the electroporation of sperm to increase DNA capture. However, the efficiency of this technique in combination with IVF has shown only low rates of fertilization and poor embryo development, with transgene integration in embryos but no expression reported (Rieth et al., Reference Rieth, Pothier and Sirard2000).
-
(iii) Liposomes are used to pretreat DNA, which is then incubated with sperm. This strategy has proven to be highly efficient in pigs for the production of transgenic embryos by ICSI; however, offspring were not obtained after transfer of such embryos, nor were transgenic embryos after IVF (Lai et al., Reference Lai, Sun, Wu, Murphy, Kuhholzer, Park, Bonk, Day and Prather2001).
-
(iv) Linker-based sperm-mediated gene transfer (LB-SMGT) is a process that uses a linker protein, a monoclonal antibody (mAb C) that facilitates binding between sperm and foreign DNA. The use of this technique has been successful in producing pigs and transgenic mice by AI and IVF, respectively (Chang et al., Reference Chang, Qian, Jiang, Liu, Wu, Chen, Lai, Lo, Hsiao, Brown, Bolen, Huang, Ho, Shih, Yao, Lin, Chen, Wu, Lin, Xu and Wang2002).
-
(v) The recombinase A enzyme has been used to facilitate random transgene integration of exogenous DNA in sperm. This procedure was able to generate transgenic pig embryos and piglets using ICSI, but not by IVF (Garcia-Vazquez et al., Reference Garcia-Vazquez, Ruiz, Matas, Izquierdo-Rico, Grullon, De Ondiz, Vieira, Aviles-Lopez, Gutierrez-Adan and Gadea2010).
Taken together, these data highlight the need to develop and/or optimize a strategy to facilitate the incorporation of exogenous DNA into sperm without affecting its fertilization and embryo developmental potential to increase the efficiency of SMGT. Transfection is a method that makes possible the introduction of foreign DNA into animal cells, and there is currently a wide range of compounds that have been reported to increase the transfection efficiency in eukaryotic cells. Lipofectamine (Lipofectamine®LTX-Plus™, Life Technologies, CA, USA), for instance, is a liposome-based transfection formulation that is specifically designed for gene expression studies in hard-to-transfect and sensitive cell lines, whereas TurboFect (TurboFect®, Thermo Scientific, MA, USA), unlike the lipid-based transfection method, uses a cationic polymer that forms compact and stable complexes with DNA, preventing its degradation and facilitating efficient delivery to eukaryotic cells. Interestingly, neither transfection compound has been tested in SMGT. SuperFect (SuperFect®, Qiagen, Hilden, Germany), on the other hand, is a reagent based on activated dendrimer technology that was developed to have a high transfection efficiency in a broad range of cell lines. This compound has been successfully used for the production of coagulation factor VIII in transgenic mice generated by AI-SMGT (Yin et al., Reference Yin, Zhang, Shi, Xie, Wang and Wang2009), but it has not been used in bovine species. Thus, in the present study, we assessed the effects of these sperm transfection methods on various sperm quality and functional parameters and evaluated the efficacy of the incorporation of exogenous DNA into sperm. The results will allow a prediction of the most appropriate reproductive technique to be used in SMGT (IVF-SMGT or ICSI-SMGT) based on the fertilization capacity of transfected sperm.
Materials and methods
Unless stated otherwise, all chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA).
Transgene construction and labelling
Plasmid DNA was used to co-incubate and transfect sperm. The plasmid used was pCAG-HcRed (5520 bp), a gift from Connie Cepko (Addgene plasmid #11152). For evaluations by flow cytometry and fluorescence microscopy, the plasmid was labelled with fluorescein isothiocyanate-12-dUTP (Thermo Fisher Scientific, Inc., MA, USA) using the Nick Translation System (Thermo Fisher Scientific, Inc., Wyman St Waltham, MA, USA) according to the manufacturer's instructions. In the latter case, the labelled plasmid was assessed each time by fluorescence microscopy after co-incubation with sperm.
Preparation, incubation and transfection of spermatozoa with exogenous DNA
Frozen semen from a bull with proven in vivo and in vitro fertility was used (Alta Genetics, Inc., Alberta, Canada) after selection by a Percoll gradient (Parrish et al., Reference Parrish, Krogenaes and Susko-Parrish1995). Sperm were washed and manipulated in Ca2+- and Mg2+-free HBSS medium (Thermo Fisher Scientific, Inc., Wyman St Waltham, MA, USA) supplemented with 0.1% of polyvinyl alcohol (PVA). Fractions of 1 × 106 sperm were incubated for 0.5, 1, 2 or 4 h at 38.5°C with 0.5 µg of pCAG-HcRed only (control DNA) or with complexes of pCAG-HcRed (exogenous DNA) and 3 µl of Lipofectamine, 2 µl of SuperFect, or 3 µl of TurboFect according to the instructions of each respective manufacturer.
Evaluation of sperm using fluorescence microscopy
To acquire z-stacks and evaluate the co-incubation and transfection times of sperm, co-incubated and transfected sperm were analysed by fluorescence confocal laser-scanning microscopy (Olympus FluoView FV1000 Olympus, Japan). Briefly, 10 µl of treated sperm was deposited on a slide with a drop of Dako Fluorescence Mounting Medium (Agilent Technologies Company, CA, USA), covered with a coverslip and analysed.
Analysis of seminal parameters by flow cytometry
Evaluation of sperm viability and binding of plasmid DNA
To determine the number of live and dead sperm with bound exogenous DNA and the amount of exogenous DNA bound to live sperm, sperm co-incubated or transfected with FITC-labelled pCAG-HcRed were treated for 10 min with 18 µM of propidium iodide (PI) at 38.5°C in darkness. These were washed once with calcium- and magnesium-free Dulbecco's phosphate-buffered saline (DPDS) (Thermo Fisher Scientific, Inc., Wyman St Waltham, MA, USA) and then analysed by flow cytometry (FACS CANTO II, BD Biosciences, San Jose, CA, USA).
Evaluation of the acrosome
Acrosome membrane integrity was assessed by staining with 0.3 µg/ml FITC-conjugated peanut agglutinin (PNA) and 18 µM PI for 10 min at 38.5°C in darkness. Sperm were washed once with DPBS and analysed immediately.
Evaluation of DNA integrity
To evaluate DNA integrity, a terminal deoxynucleotidyl transferase dUTP nick-end labelling (TUNEL) assay was performed using an In Situ Cell Death Detection Kit with Fluorescein (Roche Biochemical, Indianapolis, IN, USA) according to the manufacturer's instructions for cell suspension. Briefly, the samples were fixed for 1 h at 4°C in phosphate-buffered saline (PBS) with 1% bovine serum albumin (BSA) (w/v) (pH 7.4) and permeabilized with 0.2% Triton X-100 with 0.1% sodium citrate for 1 h at room temperature. Then, permeabilized spermatozoa were incubated with the TUNEL reaction mixture in darkness at 38.5°C for 1 h. To verify cell permeabilization, samples were counterstained with 18 µM PI for the last 5 min of incubation.
Evaluation of mitochondrial membrane potential (∆Ψm)
The tetramethylrhodamine methyl ester perchlorate (TMRM) fluorescent probe was prepared as a stock solution at 10 mM in dimethyl sulfoxide (DMSO), aliquoted and stored at −20°C. A working solution was freshly prepared each time by diluting the stock solution with DPBS to a concentration of 250 µM. Sperm were incubated with 1.2 µL of TMRM for 30 min at 37°C in darkness. Sperm were washed once by centrifugation at 200 g for 5 min and finally re-suspended in DPBS for flow cytometry.
Evaluation of reactive oxygen species (ROS)
Sperm were incubated for 20 min in Ca2+- and Mg2+-free HBSS medium containing 10 μM of 5-(and-6)-carboxy-2,7′-dihydrofluorescein diacetate (CH2DFFDA) (Thermo Fisher Scientific, Inc., MA, USA) and then incubated for 10 min in medium containing 18 µM PI. Sperm were washed once with DPBS and analysed immediately by flow cytometry. Sperm incubated with 2% H2O2 for 15 min before the assay with CH2DFFDA were used as a positive control.
DNase protection assay
In the DNase protection assay, sperm were transfected and co-incubated with FITC-labelled pCAG-HcRed, as described above. After treatments, the samples were washed three times using Ca2+- and Mg2+-free PBS medium and centrifuged at 1200 rpm for 5 min. After this, a portion of the pellet was incubated at 37°C for 30 min in DPBS medium containing 20 units of DNase I (Thermo Fisher Scientific, Inc., MA, USA) and 1× DNase I Buffer. Once DNase treatment was completed, sperm were washed twice using DPBS and centrifuged at 1200 rpm for 5 min. Then, together with the corresponding control treatments that did not contain DNase I, all samples were immediately analysed by flow cytometry.
The different sperm parameters analysed in the present study were evaluated on a total of 30,000 sperm in three replicates (10,000 each). The data were provided on a logarithmic scale and analysed using Cell-Quest Pro Software (BD Biosciences).
Analysis of sperm motility
Total and progressive sperm motility were evaluated using the integrated sperm analysis system (ISAS®, Proiser, Valencia, Spain) by depositing an aliquot of 2 µl of sperm on a D4C16 slide (ISAS®, Proiser, Valencia, Spain). All materials used to manipulate the sperm during the motility analysis were brought to 37°C beforehand. In each treatment, motility was evaluated in duplicate in five fields with approximately 200 sperm per field. This experiment was replicated three times.
Statistical analysis
Descriptive statistics were calculated based on the average and standard deviation calculated for each of the analysed variables using GraphPad Prism v. 6.00 for Windows (GraphPad Software, La Jolla, California, USA). The differences between treatments were analysed using analysis of variance (ANOVA) after arcsine transformation of the proportional data. To identify the differences between the groups, Tukey's post hoc test was performed with a significance level of P < 0.05.
Results
Location of DNA in co-incubated and transfected sperm
Exogenous DNA incorporation pattern in sperm was determined by fluorescence confocal laser-scanning microscopy and z-stack acquisitions. In addition, this experiment was able to determine the conditions and minimum time necessary for sperm to bind exogenous DNA after each treatment. The results showed that at all of the times evaluated (0.5, 1, 2 or 4 h) and with all treatments (control DNA, Lipofectamine, SuperFect and TurboFect), exogenous DNA was incorporated in the upper half of the sperm tail, and a lower amount was found heterogeneously in different regions of the sperm head (only visible in fluorescence fields), except for sperm transfected with TurboFect, in which the amount of DNA incorporated in the sperm head was greater and more uniform [visible in merged differential interference contrast (DIC) and fluorescence fields] (Fig. 1).

Figure 1 Location of DNA in co-incubated and transfected sperm. Sperm treated with 0.5 μg of FITC-labelled DNA for 30 min. Sperm co-incubated with DNA (a) and sperm transfected in the presence of Lipofectamine (b), SuperFect (c) and TurboFect (d). Confocal z-stack of merged DIC and fluorescence fields. Projection reconstructed from 12 confocal optical sections, each was 0.5 μm thick.
Effect of co-incubation and transfection method on sperm viability and the binding of exogenous DNA
The effects of co-incubation and transfection using Lipofectamine, SuperFect and TurboFect as well as the transfection time (0.5, 1, 2 or 4 h, respectively) on sperm viability and the differential capacity of transfected sperm (live or dead) to incorporate DNA were determined. The results showed that 100% sperm bound exogenous DNA after incubation with it alone (control DNA) and also after incubation with DNA-transfection complexes (data not shown). It was also observed that the transfection time (0.5, 1, 2 or 4 h) using SuperFect and simple co-incubation with DNA (control DNA), did not affect the proportion of live sperm that bound exogenous DNA (SuperFect: 85.4, 86.5, 77.9 and 81.7%; control DNA: 74.9, 79.4, 79.2 and 79.9%, respectively). A similar result was observed for Lipofectamine (86.4, 81.2, and 85.7%) and TurboFect (77.6, 74.8, and 76.6%) for 0.5, 1 or 2 h, respectively. However, the last two treatments reduced the number of live sperm with bound exogenous DNA when transfection was carried out for 4 h (73.4 and 62.9% for Lipofectamine and TurboFect, respectively) compared with shorter treatment times (Fig. 2).
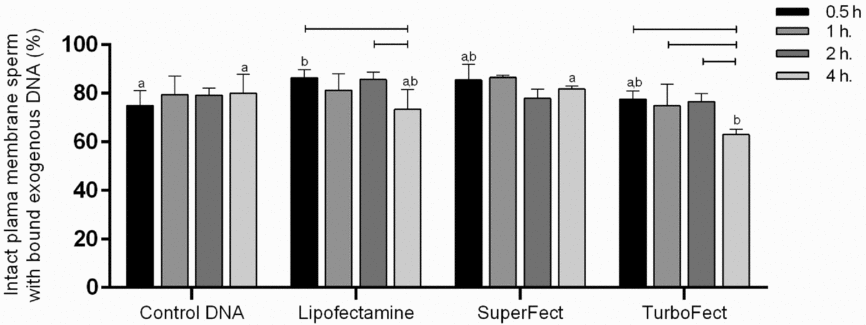
Figure 2 Effect of transfection, co-incubation with DNA and treatment time on sperm viability and the capture/incorporation of exogenous DNA labelled with fluorescein isothiocyanate (FITC). a,b,cDifferent letters between treatments within a determined time and lines within treatment on the bars indicate significant differences (P < 0.05). ANOVA was used for analysis. P-values are 0.044, 0.007 and 0.012 for Treatment, Time and Interaction, respectively.
We also observed that transfection for 4 h using TurboFect generated a smaller (P < 0.05) proportion of live sperm with bound exogenous DNA (62.9%) than transfection for the same period of time using SuperFect (81.7%) and control DNA (79.9%). Meanwhile, transfection for 0.5 h using Lipofectamine increased (P < 0.05) the percentage of live sperm that had bound exogenous DNA (86.4%) compared with co-incubation with DNA (control DNA) for the same period (74.9%) (Fig. 2).
Effect of transfection method on the amount of exogenous DNA bound to the sperm
The effect of co-incubation and transfection using Lipofectamine, TurboFect and SuperFect for different times (0.5, 1, 2 or 4 h) on the amount of DNA bound by sperm after transfection was determined. The results showed that the transfection time using Lipofectamine, TurboFect, and co-incubation with DNA (control DNA) did not affect the amount of exogenous DNA present in sperm (143.3, 141.7, 155.3 and 150.3; 209.3, 204.7, 224 and 200.3; and 146.0, 154.3, 161.3 and 171.7 AU, respectively). However, transfection using SuperFect for 4 h promoted higher (P < 0.05) sperm DNA binding (221.0 AU) than shorter transfection times (174.7 and 173.3 AU for 0.5 or 1 h, respectively) (Fig. 3). Additionally, we observed that transfection using TurboFect for 0.5 or 1 h promoted (P < 0.05) sperm DNA binding more than transfection using Lipofectamine (143.3 and 141.7 AU, respectively) and co-incubation with DNA (146.0 and 154.3 AU, respectively) for the same period of time. We also observed that transfection for 2 or 4 h using SuperFect and TurboFect promoted DNA binding (195.0 and 221 and 224.0 and 200.3 AU, respectively) more than Lipofectamine (155.3 and 150.3 AU, respectively). In addition, transfection using TurboFect and SuperFect for 2 and 4 h promoted DNA binding (224.0 and 221 AU, respectively) compared with control DNA (161.3 and 171.7 AU, respectively) (Fig. 3).

Figure 3 Amount of FITC-labelled DNA bound or incorporated into live sperm transfected and co-incubated with exogenous DNA. a,b,cDifferent letters between treatments within a determined time and lines within treatment on the bars indicate significant differences (P < 0.05), respectively. ANOVA was used for analysis. P-values are 0.0003, 0.011 and 0.146 for Treatment, Time and Interaction, respectively.
Effect of transfection method on sperm motility
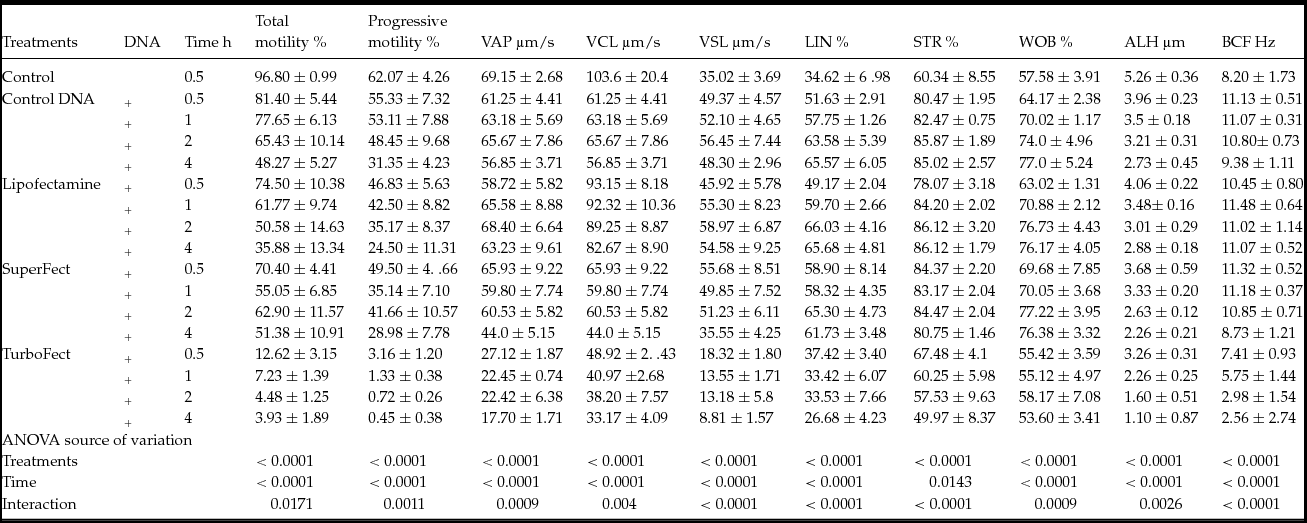
The effect of the transfection agents (Lipofectamine, SuperFect and TurboFect) and transfection time (0.5, 1, 2 or 4 h) on motility parameters was determined. The results revealed that DNA, transfection agents and transfection time had negative impacts (P < 0.05) on these parameters (Table 1). In addition, it was noted that transfection using TurboFect most significantly affected the motility parameters (Table 1).
Table 1 Average values of sperm motility parameters after transfection and co-incubation with exogenous DNA for 0.5, 1, 2 or 4 h
Effect of transfection on the incorporation of exogenous DNA
DNase protection assays were performed to evaluate whether the fluorescence signal observed in the cytometry assessments corresponded to FITC-labelled DNA bound to the surface of sperm or to DNA incorporated into sperm. The results showed that, after treating transfected sperm and control DNA with 20 units of DNase, all sperm had less (P < 0.05) DNA; however, sperm continued to carry a large (P < 0.05) amount of DNA as the mean fluorescence intensity was much greater than that of untreated sperm (autofluorescence 28.0 AU) (Fig. 4).

Figure 4 DNase protection assays. Mean fluorescence intensity of sperm transfected and co-incubated with DNA in the presence and absence of DNase I. a–eDifferent letters above the bars indicate significant differences (P < 0.05). ANOVA was used for analysis. P-value > 0.0001.
Effect of transfection method on the acrosome, DNA integrity, mitochondrial membrane potential (∆Ψm) and ROS level
A study of the effect of transfection time showed that, in general, longer transfection times did not promote greater sperm DNA binding and negatively affected the motility parameters. It was therefore determined that 30 min was sufficient for sperm transfection in further SMGT experiments. Consequently, the effects of co-incubation and transfection of sperm using Lipofectamine, SuperFect and TurboFect for 30 min on the functional sperm parameters, including the state of the acrosome, DNA fragmentation level, mitochondrial membrane potential (∆Ψm) and oxidative stress level (ROS), were determined. The results showed that transfection and co-incubation with DNA affected (P < 0.05) the integrity of the acrosome compared to the control and that treatment with SuperFect or TurboFect resulted in a smaller (P < 0.05) proportion of live spermatozoa with an intact acrosome than treatment with control DNA or Lipofectamine. A large (P < 0.05) percentage of spermatozoa with high ∆ΨM was observed in the Lipofectamine, SuperFect and control DNA treatment groups compared to the TurboFect treatment group. However, no differences in the integrity of DNA and levels of ROS were observed among the groups, including the controls, (Table 2).
Table 2 Effect of transfection and co-incubation with exogenous DNA for 30 min on the acrosomal membrane and DNA integrity, mitochondrial membrane potential (∆Ψm) and the ROS levels
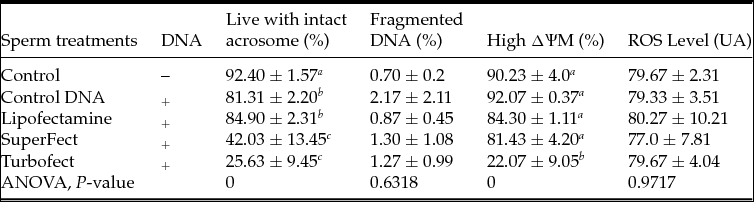
a,b,c The data followed by different letters in the same column are significantly different (P < 0.05).
Discussion
For the success of SMGT, exogenous DNA must be efficiently captured by spermatozoon and retain its functional properties and fertilization potential (Canovas et al., Reference Canovas, Gutierrez-Adan and Gadea2010). Several studies indicate that bovine sperm are able to bind exogenous DNA and that the binding efficiency is higher in frozen/thawed sperm than in fresh sperm, presumably because the cryopreservation procedure alters the plasma membrane, facilitating binding and incorporation of exogenous DNA. However, viability and motility significantly decreased in sperm that have bound exogenous DNA (Anzar & Buhr, Reference Anzar and Buhr2006). Previous studies have reported bovine spermatozoa binding to exogenous DNA either naturally or by using transfection methods, both of which only have a moderately negative effect on sperm viability and motility that does not affect its in vitro fertilization potential (Alderson et al., Reference Alderson, Wilson, Laible, Pfeffer and L'Huillier2006; Canovas et al., Reference Canovas, Gutierrez-Adan and Gadea2010; Campos et al., Reference Campos, de Leon, Komninou, Dellagostin, Deschamps, Seixas and Collares2011). However, none of these studies has reported successful transgene expression in embryos generated by IVF-SMGT (Alderson et al., Reference Alderson, Wilson, Laible, Pfeffer and L'Huillier2006; Canovas et al., Reference Canovas, Gutierrez-Adan and Gadea2010; Campos et al., Reference Campos, de Leon, Komninou, Dellagostin, Deschamps, Seixas and Collares2011).
Among of the main disadvantages of SMGT are the poor efficiency and low reproducibility among laboratories and species (Feitosa et al., Reference Feitosa, Mendes, Milazzotto, Rocha, Martins, Simoes, Paula-Lopes, Visintin and Assumpcao2010; Eghbalsaied et al., Reference Eghbalsaied, Ghaedi, Laible, Hosseini, Forouzanfar, Hajian, Oback, Nasr-Esfahani and Oback2013). This situation is reflected by the low generation of transgenic animals despite the potential advantages and simplicity of SMGT (Osada et al., Reference Osada, Toyoda, Moisyadi, Akutsu, Hattori, Sakaki and Yanagimachi2005). Thus, there is clearly a need to optimize a strategy that promotes the incorporation of exogenous DNA into sperm without affecting their fertilization potential to promote the production of transgenic embryos and animals by sperm-mediated gene transfer via intracytoplasmic sperm injection (ICSI-SMGT) or by in vitro fertilization (IVF-SMGT). In bovines, there are several studies describing the capacity of spermatozoa to spontaneously bind exogenous DNA (Anzar & Buhr, Reference Anzar and Buhr2006; Canovas et al., Reference Canovas, Gutierrez-Adan and Gadea2010; Feitosa et al., Reference Feitosa, Mendes, Milazzotto, Rocha, Martins, Simoes, Paula-Lopes, Visintin and Assumpcao2010), and although transgenic embryos have been produced by ICSI-SMGT (Hoelker et al., Reference Hoelker, Mekchay, Schneider, Bracket, Tesfaye, Jennen, Tholen, Gilles, Rings, Griese and Schellander2007; Bevacqua et al., Reference Bevacqua, Pereyra-Bonnet, Fernandez-Martin and Salamone2010), the efficiency and reproducibility continue to be poor and generation of bovine transgenic offspring has yet to be demonstrated.
The present study envisaged a strategy to optimize and improve the incorporation of exogenous DNA by sperm transfection. To do this, exogenous DNA transfection was evaluated in bovine sperm using different commercial reagents that promote an increase in cell transfection rates, including Lipofectamine, TurboFect and SuperFect. Our results demonstrate that bovine sperm bind exogenous DNA spontaneously, confirming the results of some previous studies (Anzar & Buhr, Reference Anzar and Buhr2006; Canovas et al., Reference Canovas, Gutierrez-Adan and Gadea2010; Feitosa et al., Reference Feitosa, Mendes, Milazzotto, Rocha, Martins, Simoes, Paula-Lopes, Visintin and Assumpcao2010; Cavalcanti et al., Reference Cavalcanti, Milazzotto, Simoes, Nichi, de Oliveira Barros, Visintin and Assumpcao2016), and 100% of sperm bound exogenous DNA after transfection, including control DNA. These results are in contrast to those reported by other authors (Anzar & Buhr, Reference Anzar and Buhr2006; Canovas et al., Reference Canovas, Gutierrez-Adan and Gadea2010), who describe that only a portion of sperm bind exogenous DNA after co-incubation (49 and 29%, respectively). The differences observed could be a result of the assessment method used since it has been described that the detection of proteins by flow cytometry, the technique used in the present study, can be at least 10 times more sensitive than detection by conventional fluorescence microscopy (Bolanos et al., Reference Bolanos, Bodon, Jimenez, Garcia-Mayol, Lavergne and Diaz1988; Jenson et al., Reference Jenson, Grant, Ench, Heard, Thomas, Hilsenbeck and Moyer1998; Soboleski et al., Reference Soboleski, Oaks and Halford2005). Alternatively, the differences could be related to the different components present in the media since it has been described that media with Ca2+ and BSA accelerate the sperm capacitation processes (Visconti et al., Reference Visconti, Stewart-Savage, Blasco, Battaglia, Miranda, Kopf and Tezon1999; Aguila et al., Reference Aguila, Arias, Vargas, Zambrano and Felmer2015), which is unfavourable for binding exogenous DNA by sperm, which occurs in the early stages of capacitation (Lavitrano et al., Reference Lavitrano, Maione, Forte, Francolini, Sperandio, Testi and Spadafora2003). Additionally, the medium used for co-incubation must be free of calcium as this prevents endonucleases from damaging the exogenous DNA (Lavitrano et al., Reference Lavitrano, Maione, Forte, Francolini, Sperandio, Testi and Spadafora2003).
The proportion of live sperm that bound exogenous DNA (74.9%) is the same as the proportion of all sperm that were alive since we observed that all sperm bound DNA. This finding is unlike the results described by Canovas et al. (Reference Canovas, Gutierrez-Adan and Gadea2010), who obtained only 13.8% of live sperm with bound exogenous DNA despite observing high viability in treated sperm (78%). Meanwhile, in a study by Anzar & Buhr (Reference Anzar and Buhr2006), in spite of the low initial viability (32%), the authors observed that 46% of live sperm bound exogenous DNA. Additionally, our fluorescence confocal laser-scanning microscopy and z-stack analyses confirmed that sperm co-incubated with DNA and transfected sperm both preferably incorporate DNA in the upper half of the sperm tail and regions of the head. These data are in contrast with those described by Eghbalsaied et al. (Reference Eghbalsaied, Ghaedi, Laible, Hosseini, Forouzanfar, Hajian, Oback, Nasr-Esfahani and Oback2013) and Anzar & Buhr (Reference Anzar and Buhr2006), who observed a dotted DNA-binding pattern in the sperm head and uniform DNA-binding in the postacrosomal region.
When the motility of DNA-treated sperm was evaluated, we observed that the time of co-incubation with DNA and the transfection treatments negatively affected the total and progressive motility in all treatments. This effect was much more evident with TurboFect. Nevertheless, the results showed that the total motility observed in the control DNA was greater (81 and 78% for 30 min or 1 h, respectively) than that observed by Canovas et al. (Reference Canovas, Gutierrez-Adan and Gadea2010) (63%) and Campos et al. (Reference Campos, de Leon, Komninou, Dellagostin, Deschamps, Seixas and Collares2011) (60%) for similar treatment times. By contrast, the progressive motility was slightly lower (55 and 53% for 30 min or 1 h, respectively) than that observed by Canovas et al. (Reference Canovas, Gutierrez-Adan and Gadea2010) (60%). The differences observed between these two studies could be related to the greater DNA concentration used (5 and 10 µg), a factor that is known to negatively affect sperm parameters (Smith, Reference Smith2012). However, we and the two studies mentioned above observed motile sperm after DNA treatment, in contrast with the observations by Anzar & Buhr (Reference Anzar and Buhr2006). To determine whether the decrease in motility observed in control DNA, Lipofectamine and SuperFect has an effect on sperm fertilization capacity, IVF studies are needed, as it has been demonstrated recently in bovines that sperm with low progressive motility (<65%) can produce high cleavage rates and an acceptable proportion of embryos at the blastocyst stage (81% and 24%, respectively) after IVF (Li et al., Reference Li, Kalo, Zeron and Roth2016). Conversely, it is important to note that transfection with TurboFect, despite strongly affecting motility, did not affect the fertilization capacity of sperm after ICSI. Furthermore, TurboFect could increase the efficiency of ICSI-SMGT because sperm subjected to this treatment captured a larger amount of DNA at 30 min, and a low number of live sperm with intact acrosome membrane, which promotes the efficiency of ICSI (Arias et al., Reference Arias, Sanchez, Risopatron, Perez and Felmer2014).
In comparison with ICSI (Bevacqua et al., Reference Bevacqua, Pereyra-Bonnet, Fernandez-Martin and Salamone2010; Eghbalsaied et al., Reference Eghbalsaied, Ghaedi, Laible, Hosseini, Forouzanfar, Hajian, Oback, Nasr-Esfahani and Oback2013), IVF has been much less efficient in the production of bovine transgenic embryos via SMGT (Hoelker et al., Reference Hoelker, Mekchay, Schneider, Bracket, Tesfaye, Jennen, Tholen, Gilles, Rings, Griese and Schellander2007; Campos et al., Reference Campos, de Leon, Komninou, Dellagostin, Deschamps, Seixas and Collares2011; Eghbalsaied et al., Reference Eghbalsaied, Ghaedi, Laible, Hosseini, Forouzanfar, Hajian, Oback, Nasr-Esfahani and Oback2013; Cavalcanti et al., Reference Cavalcanti, Milazzotto, Simoes, Nichi, de Oliveira Barros, Visintin and Assumpcao2016). Our results indicate that 30 min are sufficient for bovine sperm to incorporate DNA after co-incubation with exogenous DNA and transfection. Additionally, a longer incubation time negatively affects sperm motility. Therefore, to evaluate the effect of DNA and the transfection treatments on sperm functionality in the spermatozoa treated during this time, we performed a series of additional evaluations that revealed that SuperFect and TurboFect negatively affected the integrity of the acrosome and sperm plasma membranes more than Lipofectamine and the DNA control treatment. In fact, acrosome integrity was affected in all treatments, including with control DNA, compared with the control that was not incubated with DNA. Interestingly, the results observed in this experiment were inconsistent with the nuclease activation mechanisms (apoptotic-like processes) proposed in the mouse model as a sperm protection mechanism against the massive intrusion of foreign DNA and subsequent transmission to the offspring (Maione et al., Reference Maione, Lavitrano, Spadafora and Kiessling1997; Smith, Reference Smith2002). This situation is because the TUNEL assay showed that the transfection treatments and co-incubation with exogenous DNA did not affect sperm DNA integrity. Observations of the control DNA group agree with the data reported by Feitosa et al. (Reference Feitosa, Mendes, Milazzotto, Rocha, Martins, Simoes, Paula-Lopes, Visintin and Assumpcao2010), who also did not observe differences in the DNA integrity after incubating sperm with DNA for 1 or 2 h. Nevertheless, these results are in contrast to those reported by Canovas et al. (Reference Canovas, Gutierrez-Adan and Gadea2010), who observed differences between the control without DNA (2.3%) and control DNA (4.4%). The proportion of sperm with DNA damage in the present study, however, was very low (<2.17%) and similar to the results described by Canovas et al. (Reference Canovas, Gutierrez-Adan and Gadea2010) and Feitosa et al. (Reference Feitosa, Mendes, Milazzotto, Rocha, Martins, Simoes, Paula-Lopes, Visintin and Assumpcao2010), as these levels are very much below the percentage described to affect sperm fertilization capacity in the bovine species (Takeda et al., Reference Takeda, Uchiyama, Kinukawa, Tagami, Kaneda and Watanabe2015).
One reason that could explain the lack of an effect of exogenous DNA on DNA fragmentation in bovine species could be the unique type of protamines (type I) present in the sperm chromatin since several studies have confirmed that DNA fragmentation is more related to type II protamines (Carrell et al., Reference Carrell, Emery and Hammoud2007). This effect could also explain the greater resistance of bovine spermatozoa to apoptotic-like processes compared with spermatozoa of other species, such as the mouse (Feitosa et al., Reference Feitosa, Mendes, Milazzotto, Rocha, Martins, Simoes, Paula-Lopes, Visintin and Assumpcao2010). Additionally, our data show that transfection using TurboFect causes a sharp reduction in the proportion of sperm with a high ∆Ψm (22%) compared with the other treatments (>81%), which could explain the low motility observed in this treatment (Kasai et al., Reference Kasai, Ogawa, Mizuno, Nagai, Uchida, Ohta, Fujie, Suzuki, Hirata and Hoshi2002; Paoli et al., Reference Paoli, Gallo, Rizzo, Baldi, Francavilla, Lenzi, Lombardo and Gandini2011). However, we did not observe any differences in the oxidative stress levels (ROS) in sperm treated with TurboFect. Given that the reduction in ∆Ψm has been related to an early stage of apoptosis that precedes to DNA fragmentation, ROS production and finally an increase in membrane permeability (Kroemer et al., Reference Kroemer, Zamzami and Susin1997), it would also be interesting to evaluate TurboFect for in vitro production of embryos by ICSI-SMGT, considering that, despite its low ∆Ψm, we did not observe any changes in the ROS level or in the fragmentation of DNA at 30 min. We did not observe any increase in membrane permeability either after at least 2 h of treatment, and only after 4 h of incubation did the membrane permeability increase, with 37.1% of sperm dead. These data, together with those described above, indicate that TurboFect could indeed be a good alternative for ICSI-SMGT.
As previously discussed, several methods have been assessed to improve exogenous DNA capture by sperm in different species, including bovine. These include electroporation (Rieth et al., Reference Rieth, Pothier and Sirard2000), treatments with Triton X-100 (Perry et al., Reference Perry, Wakayama, Kishikawa, Kasai, Okabe, Toyoda and Yanagimachi1999), liposomes (Eghbalsaied et al., Reference Eghbalsaied, Ghaedi, Laible, Hosseini, Forouzanfar, Hajian, Oback, Nasr-Esfahani and Oback2013; Lai et al., Reference Lai, Sun, Wu, Murphy, Kuhholzer, Park, Bonk, Day and Prather2001), DMSO (Eghbalsaied et al., Reference Eghbalsaied, Ghaedi, Laible, Hosseini, Forouzanfar, Hajian, Oback, Nasr-Esfahani and Oback2013) and chemical agents (Li et al., Reference Li, Mizutani, Ono and Wakayama2010). Although exogenous DNA has been described in some studies to be incorporated into spermatozoa at a high rate, its use in SMGT continues to be inefficient and variable in the different species evaluated (Eghbalsaied et al., Reference Eghbalsaied, Ghaedi, Laible, Hosseini, Forouzanfar, Hajian, Oback, Nasr-Esfahani and Oback2013). In the present study, to confirm that the exogenous DNA is incorporated into treated sperm, we incubated sperm that had bound exogenous DNA with DNase and observed that both transfected sperm and sperm co-incubated with DNA retained exogenous DNA after exposure to the enzyme. This result is in contrast to those of a similar experiment described by Eghbalsaied et al. (Reference Eghbalsaied, Ghaedi, Laible, Hosseini, Forouzanfar, Hajian, Oback, Nasr-Esfahani and Oback2013), as after the treatment with DNase, although we observed a significant reduction in the level of DNA, treated sperm retained a larger amount of DNA than the control without exogenous DNA. These results indicate that all treatments, including with control DNA, may result in DNA incorporation into sperm. This finding is in agreement with the mechanism proposed by Lavitrano et al. (Reference Lavitrano, Busnelli, Cerrito, Giovannoni, Manzini and Vargiolu1997), which includes the participation of DNA-binding proteins (DBPs), class II major histocompatibility complex (MHC) and CD4 molecules (reviewed by Lavitrano et al., Reference Lavitrano, Camaioni, Fazio, Dolci, Farace and Spadafora2006). In addition, our transfection results, particularly those observed with Lipofectamine, agree with those reported by Campos et al. (Reference Campos, de Leon, Komninou, Dellagostin, Deschamps, Seixas and Collares2011), who, using qPCR, also observed no differences in the amount of exogenous DNA present in the control DNA and sperm transfected with Lipofectamine.
In conclusion, our results confirm the capacity of bovine sperm to spontaneously bind and incorporate exogenous DNA and show that their interaction with exogenous DNA does not increase either ROS levels or DNA damage. Additionally, we showed that sperm transfection procedures using liposomes (Lipofectamine) enable sperm to capture DNA without compromising sperm viability or motility to any large extent; therefore, this method would be more suitable for producing embryos by IVF-SMGT. Meanwhile, transfection using cationic polymers (Turbofect) increases the amount of exogenous DNA present in sperm and although this compounds negatively affected some sperm parameters, including motility and acrosome integrity, paradoxically could be more beneficial for ICSI-SMGT procedures. Future studies are still required to confirm the effect of these treatments on transgenic embryo/animal production by ICSI-SMGT and/or IVF-SMGT.
Financial support
This research was supported by CONICYT, Chile grant FONDECYT 11130724
Conflict of interest
The authors declare that they have no conflict of interest.








