Introduction
Spermatogenesis is a biological process that occurs within the seminiferous epithelium and leads to the transformation of spermatogonia into spermatozoa, in close association with Sertoli cells (Hess & De Franca, Reference Hess, De Franca and Cheng2008). During spermatogenesis, and mainly during transit through male and female genital tracts, sperm may be subjected to environmental toxins that may result in structural, metabolic and DNA damage (Aitken & Bennetts, Reference Aitken, Bennetts, De Jonge and Barratt2006; Sakkas & Alvarez, Reference Sakkas and Alvarez2010; González-Marín et al., Reference González-Marín, Gosálvez and Roy2012).
Semen analysis is one of the principal methods used in the assessment of sperm function (World Health Organization, Reference World Health Organization2010), and serves as a sensitive biological marker of exposure to toxins (Telisman et al., Reference Telisman, Colak, Pizenta, Jurasovic and Cvitkovic2007; Mendiola et al., Reference Mendiola, Torres-Cantero, Moreno-Grau, Ten, Roca, Moreno-Grau and Bernabeu2008). As this analysis does not provide information on the integrity of the genetic material, several methods have been developed to monitor lesions in DNA. Of these methods, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL) is considered to be a sensitive diagnostic tool for detecting DNA fragmentation in spermatozoa. This method is based on the specific binding of terminal deoxynucleotidyl transferase (TdT) to T-OH ends of DNA with a subsequent synthesis of a polydeoxynucleotide polymer (Gavrieli et al., Reference Gavrieli, Sherman and Ben-Sasson1992; Sergerie et al., Reference Sergerie, Laforest, Boulanger, Bissonnette and Bleau2005a,Reference Sergerie, Laforest, Bujan, Bissonnette and Bleaub; Ramalho-Santos et al., Reference Ramalho-Santos, Amaral, Sousa, Rodrigues, Martins, Baptista, Mota, Tavares, Amaral, Gamboa, Mendez-Vilas and Diaz2007).
Lead is a heavy metal that enters in the body through the skin, intestines or lungs, with the most significant route being through the respiratory tract. In the general population, the main source of exposure results from air pollution associated with petroleum use, cigarettes, foods and drinks. Lead exposure can also occur in workers who use lead acid batteries, paints or varnishes (Levin & Goldberg, Reference Levin and Goldberg2000; TNO Strategy, Technology and Policy, 2005; Agency for Toxic Substances and Disease Registry, 2007). Occupational and environmental exposure to lead with blood lead levels above 0.40 μg/ml may cause a decrease in sperm quality, including reduction in sperm volume, count, motility, vitality, membrane integrity and normal morphology, as well as increased DNA damage and reduced sperm chromatin condensation and fertility (World Health Organization, Reference World Health Organization1980; United Nations Environment Programme, 2003). These changes are associated with lead exposure to environmental factors (Benoff et al., Reference Benoff, Jacob and Hurley2000a; Telisman et al., Reference Telisman, Cvitkovic, Jurasovic, Pizent, Gavella and Rocic2000, Reference Telisman, Colak, Pizenta, Jurasovic and Cvitkovic2007; De Rosa et al., Reference De Rosa, Zarrilli, Paesano, Carbone, Boggia, Petretta, Maisto, Cimmino, Puca, Colao and Lombardi2003; Pant et al., Reference Pant, Upadhyay, Pandey, Mathur, Saxena and Srivastava2003; Hernandez-Ochoa et al., Reference Hernández-Ochoa, García-Vargas, López-Carrillo, Rubio-Andrade, Morán-Martínez, Cebrián and Quintanilla-Vega2005; Hamoud et al., Reference Hammoud, Carrell, Gibson, Sanderson, Parker-Jones and Peterson2010; Li et al., Reference Li, Zhong, Jiang, Wang, Zuo and Sha2012) and occupational factors (Fisher-Fischbein et al., Reference Fisher-Fischbein, Fischbein, Melnick and Bardin1987; Levin & Goldberg, Reference Levin and Goldberg2000; Naha et al., Reference Naha, Bhar, Mukherjee and Chowdhury2005; TNO Strategy, Technology and Policy, 2005; Naha & Chowdhury, Reference Naha and Chowdhury2006; Hsu et al., Reference Hsu, Chang, Guo, Liu and Shih2009).
Lead compounds initiate oxidative stress, producing reactive oxygen species (ROS), which cause lipid peroxidation of the sperm membrane; this membrane is particularly sensitive as it is enriched in polyunsatured fatty acids. Increased levels of ROS also alter oxidative phosphorylation and P450 metabolism. These processes also disturb calcium signalling mechanisms and are involved in membrane mitochondrial damage, apoptosis induction, decreased sperm maturation, capacitation and acrosome reaction capabilities (Maneesh & Jayalekshmi, Reference Maneesh and Jayalekshmi2006; Sakkas & Alvarez, Reference Sakkas and Alvarez2010; Bejarano et al., Reference Bejarano, Espino, Paredes, Ortiz, Lozano, Pariente, Rodriguez and Bashamboo2012; González-Marín et al., Reference González-Marín, Gosálvez and Roy2012). The production of 4-hydroxynonenal (HNE), the main product of lipid peroxidation, decreases the levels of antioxidants such as glutathione (GSH) and oxidized glutathione (GSSG), reducing the removal of free radicals and thus enhancing sperm damage. Furthermore, lead has a high affinity for sulfhydryl groups (–SH) and binds to many enzymes, inactivating them and making them unavailable as antioxidants, such as GSH, glutathione reductase and glutathione peroxidase (GPX). The major source of ROS in spermatozoa is membrane NADPH oxidase, which produces the superoxide anion (O2−). This anion is converted to hydrogen peroxide (H2O2) by superoxide dismutase (SOD). H2O2 is then removed by the action of GPX that requires NADPH. NADPH is mainly formed in the pentose phosphate/hexose monophosphate pathways, using glucose-6-phosphate and glucose-6-phosphate dehydrogenase (G6PDH; de Lamirande & Gagnon, Reference de Lamirande and Gagnon1992; Griveau & Le Lannou, Reference Griveau and Le Lannou1997; Ye et al., Reference Ye, Fu, Zhu, Ni, Lu, Kuang, Yang and Shu1999; Liu et al., Reference Liu, Kato, Akhand, Hayakawa, Suzuki, Miyata, Kurokawa, Hotta, Ishikawa and Nakashima2000; Aitken & Krausz, Reference Aitken and Krausz2001; Kasperczyk et al. Reference Kasperczyk, Birkner, Kasperczyk and Zalejska-Fiolka2004, Reference Kasperczyk, Kasperczyk, Horak, Ostałowska, Grucka-Mamczar, Romuk, Olejek and Birkner2008; Patrick, Reference Patrick2006).
Lead causes sperm DNA damage by the action of ROS that induce DNA fragmentation with the occurrence of DNA strand breaks. Sperm DNA damage also occurs by disturbance of the chromatin structure. This damage is mediated by replacement of zinc, an essential element for DNA stability. The stabilization of sperm chromatin depends on the formation of disulfide (S–S) bonds between the cysteine residues of protamines during sperm maturation, and this action requires zinc. Lead has a high affinity for sulfhydryl groups, such as those present in protamines, and interacts with human protamine-2 (HP2), a zinc-containing protein. Protamines are key proteins that protect the genetic material by sequestering metals that would otherwise promote DNA fragmentation. Lead interacts with HP2 and reduces binding of protamines to DNA and thus alters chromatin stability. This alteration is either at compaction, as needed for DNA protection, or at decondensation, as needed after fertilization. In addition, abnormal chromatin structure increases the risk of DNA damage, and concomitantly exposure of the genome to mutations (Benoff et al., Reference Benoff, Jacob and Hurley2000a, Reference Benoff, Cooper, Centola, Jacob, Hershlag and Hurley2000b, Reference Benoff, Centola, Millan, Napolitano, Marmar and Hurley2003; Quintanilla-Vega et al., Reference Quintanilla-Vega, Hoover, Bal, Silbergeld, Waalkes and Anderson2000a, Reference Quintanilla-Vega, Hoover, Bal, Silbergeld, Waalkes and Anderson2000b; Sallmén, Reference Sallmén2001; Hernández-Ochoa et al., Reference Hernández-Ochoa, García-Vargas, López-Carrillo, Rubio-Andrade, Morán-Martínez, Cebrián and Quintanilla-Vega2005; Telisman et al., Reference Telisman, Colak, Pizenta, Jurasovic and Cvitkovic2007).
As lead induces sperm DNA fragmentation, as well as morphological, motility and structural changes, exposure to lead has been associated with deficient embryonic development, decreased implantation and pregnancy rates, and increased miscarriage rates. It can also cause fetal mutations and increases the risk of cancer in offspring (Benoff et al., Reference Benoff, Jacob and Hurley2000a; Aitken & Krausz, Reference Aitken and Krausz2001; Sallmén, Reference Sallmén2001; Olsen et al., Reference Olsen, Lindeman, Wiger, Duale and Brunborg2005; Cocuzza et al., Reference Cocuzza, Sikka, Athayde and Agarwal2007; Delbès et al., Reference Delbès, Hales and Robaire2010; Sakkas & Alvarez, Reference Sakkas and Alvarez2010; González-Marín et al., Reference González-Marín, Gosálvez and Roy2012).
The effects of lead on sperm parameters in vitro have been evaluated previously by only five studies and indicated reduced total motility (Kanwar et al., Reference Kanwar, Chadha, Batla, Sanyal and Sandhu1988: lead chloride), decreased total motility and fertility (Benoff et al., Reference Benoff, Cooper, Centola, Jacob, Hershlag and Hurley2000b, Reference Benoff, Centola, Millan, Napolitano, Marmar and Hurley2003: lead acetate; Huang et al., Reference Huang, Tseng and Lin2001: lead nitrate), and inhibition of sperm metabolism (Ghaffari & Motlagh, Reference Ghaffari and Motlagh2011: lead chloride). The present work aimed to study the effect of in vitro exposure to lead chloride (PbCl2) on the quality of all sperm parameters (motility, vitality, hypoosmotic swelling test and morphology) as well as on sperm DNA fragmentation in normozoospermic men, with multiple correlations. Our results suggested that lead may affect male fertility; the possible mechanisms of lead effects are discussed.
Materials and methods
Patients and sample collection
Under informed and signed consent, semen samples were obtained from men who performed spermiogram analysis. The selected group consisted of 31 men, with a mean age of 35.9 ± 6.2, that presented normal concentration, motility, morphology, viscosity, liquefaction time, pH, and absence of immature forms and leukocytes. Sperm parameters (motility, vitality, hypoosmotic swelling test and morphology) were evaluated in accordance with World Health Organization (WHO) Guidelines (World Health Organization, Reference World Health Organization2010). All samples were collected by masturbation after 2–4 days of abstinence, in an appropriate sterile container. Samples provided for the study were treated after 1 h of transportation.
Study design
For each patient, semen was centrifuged (1500 rpm, 5 min) to remove the seminal fluid, and the pellet was diluted in sperm preparation medium (SPM; Origio, Jyllinge, Denmark) to obtain a concentration of spermatozoa per tube of 7.5 million/ml. Sperm samples (230 μl) were incubated at room temperature (RT), for two different time periods, 4 h or 8 h, with 5 μl of PbCl2 (Merck, Hohenbrunn, Germany) at two concentrations, 15 μg/ml or 30 μg/ml, from previously prepared saline stock solutions of 700 μg/ml and 1400 μg/ml, respectively. For each incubation time, sperm samples were also incubated with SPM only (control group). After each incubation sperm motility, vitality and hypoosmotic swelling tests were analyzed and smears were prepared for morphology and DNA fragmentation.
Sperm analysis
For sperm motility, 10 μl of sperm samples were placed on a glass slide and covered with a coverslip. The numbers of rapid progressive sperm, slow progressive sperm, in situ motile sperm and immotile sperm were scored in the same field. For vitality assessment, 50 μl of sperm samples were added to 50 μl of a 0.5% eosin-Y solution (Merck, Darmstadt, Germany) in 40 ml saline solution, and a drop was placed on a slide for observation. The numbers of stained (dead) and unstained (vital) cells were registered (Fig. 1 A). For the hypoosmotic swelling test, 50 μl of sperm samples were added to 500 μl of a hypo-osmotic solution (Sigma Aldrich, St. Louis, USA) and incubated at 37ºC for 30 min. After incubation, a drop was placed on a glass slide. For each experiment, 100 spermatozoa were counted with a manual laboratory counter (Digisystem Laboratory Instruments Inc., Taipei, Taiwan) using a light microscope (CX21FS1; Olympus, Tokyo, Japan) under ×400 magnification (Fig. 1 B). For morphology analysis, a sperm smear was prepared on a slide, air-dried, fixed in 96% v/v ethanol and stained with Papanicolaou stain (Merck; Sigma-Aldrich). One hundred spermatozoa were counted at ×1000 magnification and scored as normal or abnormal sperm (including head, neck/mid-piece and tail). Staining and analysis were performed in accordance with WHO guidelines (World Health Organization, Reference World Health Organization2010).

Figure 1 (A) Vitality test. The dead spermatozoon presents a red eosin-stained head (arrow). (B) Hypoosmotic swelling test. The reactive live sperm presents a coiled tail (arrow). (C) Presence of coiled tails (arrows) under morphological analysis. (D, E) TUNEL assay by fluorescence microscopy. (D) Sperm without DNA damage. (E) Sperm with DNA damage. (a) DAPI (blue fluorescence); (b) TUNEL-negative (dark fluorescence) and TUNEL-positive (green fluorescence); (c) merged image.
Determination of sperm DNA fragmentation by TUNEL
The TdT-mediated dUTP nick-end labelling (TUNEL) assay was performed using the In Situ Cell Death Detection Kit (Roche Diagnostics, Mannheim, Germany). Adhesion microscope slides were used (Marienfeld, Lauda-Königshofen, Germany). Air-dried smeared slides were fixed with 4% paraformaldehyde (Merck) in phosphate-buffered saline (PBS; Sigma-Aldrich) 1 h at RT. Slides were then washed in PBS and then permeabilized with 0.1% Triton-X (Sigma-Aldrich) in 0.1% sodium citrate (Sigma-Aldrich) for 2 min at 4ºC. After washing twice with PBS for 5 min, the labelling reaction was performed by incubation with 50 μl of labelling solution (provided by the kit) containing TdT enzyme in a humidified chamber, for 1 h at 37°C in the dark. After incubation, the slides were washed four times in PBS and then counterstained with 4′,6-diamidoino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, USA). In total, 200 spermatozoa for each sample were evaluated by fluorescence microscopy, using a Leitz DMRBE microscope (Leica, Wetzlar, Germany). The number of cells with green fluorescence (positive) was expressed as a percentage of the total sample stained with DAPI.
Statistical analysis
Statistical analysis was performed with the software STATISTICA (Version 11, Statsoft, USA). A two-way analysis of variance (ANOVA) was applied for each parameter measured, considering the effects of time (4 h and 8 h) and PbCl2 dose (15 ppm and 30 ppm). The percentages were arcsine transformed prior to analysis, and provided homogenous variances in all cases (as checked with the Levene's test), except for one parameter (rapid progressive motility). In this case, a log transformation was used to grant homogeneity of variances (inferences were equivalent to those based with the arcsine data set). Post-hoc analysis (comparisons between independent groups) was made with the Scheffe's test. For the vitality parameter, a Dunnett's test was also used to compare each exposed group with the control (as the Scheffe's test did not disclose intergroup differences after a significant ANOVA). A P-value < 0.05 was considered to be statistically significant.
Results
Sperm motility
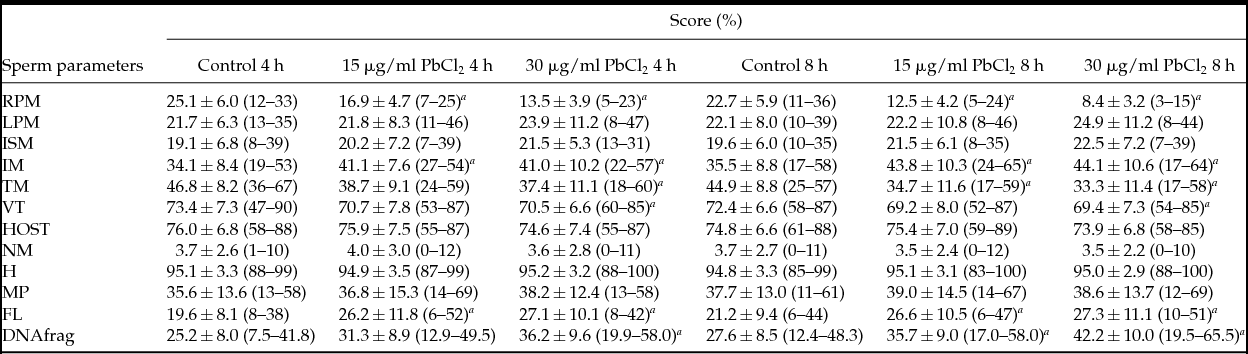
Results showed that PbCl2 caused a significant decrease in rapid progressive motility, when comparing either both incubation times (F(1.180) = 40.582, P < 0.001) or concentrations (F(2.180) = 99.697, P < 0.001), as well as between groups (F(2.180) = 5.748, P = 0.004). In comparison with controls, at both times of exposure a significant inhibition in groups exposed to 15 μg/ml PbCl2 (P < 0.001) or 30 μg/ml PbCl2 (P < 0.001) was observed, whereas for both concentrations significant differences were observed at 8 h (P < 0.001) but not at 4 h (P = 0.2236) (Table 1). For slow progressive motility, no significant differences were observed regarding both incubation times (F(1.180) = 0.201, P = 0.654) or concentrations (F(2.180) = 1.366, P = 0.258), as well as between groups (F(2.180) = 0.022, P = 0.978). The same finding was observed for in situ motility relative to both incubation times (F(1.180) = 0.952, P = 0.331) or concentrations (F(2.180) = 2.67, P = 0.072), as well as between groups (F(2.180) = 0.056, P = 0.945) (Table 1). For immotile sperm no significant differences were found at both incubation times (F(1.180) = 3.05, P = 0.082), but a significant increase was observed at different concentrations (F(2.180) = 14.016, P < 0.001). Comparisons between groups did not show significant differences (F(2.180) = 0.128, P = 0.880). At both incubation times a significant increase was observed in groups submitted to 15 μg/ml PbCl2 (P < 0.001) or 30 μg/ml PbCl2 (P < 0.001), whereas no significant differences were found in respect to concentrations (P = 0.998) regarding the different times of exposure (Table 1). For total motility, a significant decrease was observed for both incubation times (F(1.180) = 4.82, P = 0.029) or concentrations (F(2.180) = 19.33, P < 0.001), but not between groups (F(2.180) = 0.18, P = 0.831). For the time of exposure, at 4 h a significant decrease was observed to the group exposed to 30 μg/ml PbCl2 (P = 0.0244) but not to the group submitted to 15 μg/ml PbCl2 (P = 0.076), whereas after 8 h a significant decrease was found at both concentrations, 15 μg/ml PbCl2 (P = 0.012) or 30 μg/ml PbCl2 (P = 0.002). In contrast, for both concentrations, no significant differences were found for incubation times, 4 h (P = 0.999) or 8 h (P = 0.0998) (Table 1).
Table 1 Effect of lead chloride on sperm parameters and DNA fragmentation
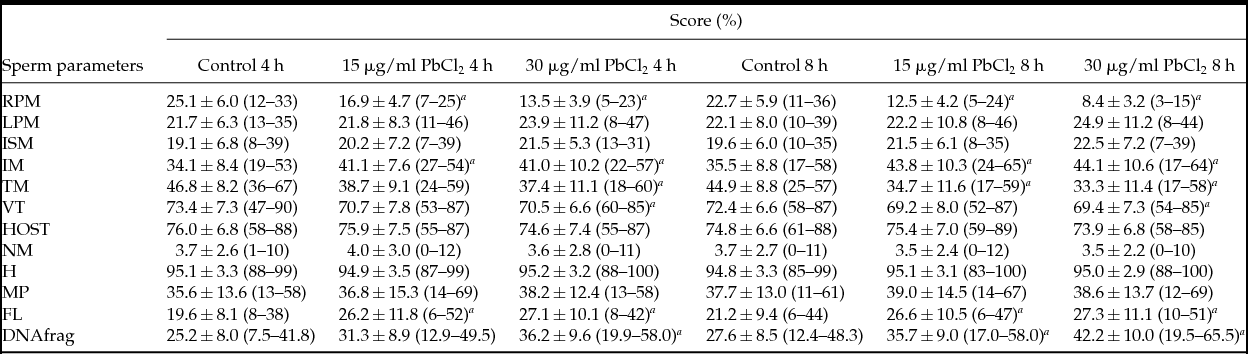
a Statistical significant difference between control group and groups exposed to lead chloride (P < 0.05).
Data are expressed as mean ± standard deviation.
DNAfrag: DNA fragmentation; FL: flagellum abnormalities; H: sperm head abnormalities; HOST: hypoosmotic swelling test; IM: immotile sperm; ISM: in situ motility; LPM: low progressive motility; MP: mid-piece abnormalities; NM: normal morphology; RPM: rapid progressive motility; TM: total motility (RPM + LPM); VT: vitality.
Sperm vitality and hypoosmotic swelling tests
For the vitality test, a significant decrease was observed for both concentrations (F(2.180) = 3.27, P = 0.038) after ANOVA. However, after Scheffe's test, such an effect was not found. No significant differences were observed at both incubation times (F(1.180) = 1.38, P = 0.241) or between groups (F(2.180) = 0.02, P = 0.985). Using the Dunnett's test, at both concentrations a significant inhibition to group exposed to 30 μg/ml PbCl2 was observed (P = 0.048) but not at 15 μg/ml PbCl2 (P = 0.057) (Table 1). For the hypoosmotic swelling test, no significant differences were found after when comparing incubation times (F(1.180) = 0.7, P = 0.404) or concentration (F(2.180) = 0.67, P = 0.511), or between groups (F(2.180) = 0.04, P = 0.961) (Table 1).
Sperm morphology
Compared with normal morphology, no significant differences were observed at both incubation times (F(1.180) = 0.2244, P = 0.636) or concentrations (F(2.180) = 0.0707, P = 0.932), as well as between groups (F(2.180) = 0.1545, P = 0.857). The same finding was observed for sperm head abnormalities, when both incubation times (F(1.180) = 0.0, P = 0.892) or concentrations (F(2.180) = 0.0, P = 0.954) were compared or between groups (F(2.180) = 0.1, P = 0.927) and, for mid-piece abnormalities, to both incubation times (F(1.180) = 0.594, P = 0.442) and concentrations used (F(2.180) = 0.267, P = 0.766) or between groups (F(2.180) = 0.079, P = 0.924) (Table 1). In contrast, for sperm tail abnormalities, although no significant differences were found when comparing both incubation times (F(1.180) = 0.25, P = 0.616), a significant increase was observed for concentrations (F(2.180) = 8.08, P < 0.001). Comparisons between groups also did not show significant changes (F(2.180) = 0.09, P = 0.916). For both concentrations, a significant increase in groups exposed to 15 μg/ml PbCl2 (P = 0.006) or 30 μg/ml PbCl2 was observed (P = 0.001), whereas for both exposure times no significant differences were observed (P = 0.916) (Table 1). Additionally, coiled tails were counted in five patients and this anomaly was the one more frequently found. A significant increase was observed (P = 0.003) in the percentage of coiled tails in the group exposed to 30 μg/ml PbCl2 after 8 h of incubation (Fig. 1C).
Sperm DNA fragmentation
For sperm DNA fragmentation, a significant increase was observed for both incubation times (F(1.180) = 10.34, P = 0.002) and concentrations (F(2.180) = 30.98, P < 0.001). No significant differences were observed between groups (F(2.180) = 0.68, P = 0.508). After 4 h of incubation no significant changes were observed in the group exposed to 15 μg/ml PbCl2 (P = 0.244), whereas significant differences were found for the 30 μg/ml PbCl2 group (P < 0.001). After 8 h of incubation, a significant increase was found for both groups submitted to 15 μg/ml PbCl2 (P = 0.039) or 30 μg/ml PbCl2 (P < 0.001). For both concentrations, no significant differences were observed at 4 h (P = 0.475) or 8 h (P = 0.135) (Table 1 and Fig. 1 D,E).
Discussion
Environmental and occupational studies on lead exposure have shown that toxic plasma levels range from about 0.2–1.5 μg/ml. However, these low levels cannot be used in in vitro experiments with sperm to obtain and study any possible toxic effects. Subsequent studies have used concentrations of 50 μg/ml PbCl2 (Kanwar et al., Reference Kanwar, Chadha, Batla, Sanyal and Sandhu1988), 80 μg/ml PbCl2 (Ghaffari & Motlagh, Reference Ghaffari and Motlagh2011), 50 μg/ml lead acetate (Benoff et al., Reference Benoff, Cooper, Centola, Jacob, Hershlag and Hurley2000b, Reference Benoff, Centola, Millan, Napolitano, Marmar and Hurley2003), or 50–500 μg/ml of lead nitrate (Huang et al. Reference Huang, Tseng and Lin2001). We thus chose intermediate concentration levels of 15 μg/ml and 30 μg/ml and intermediate times of exposure of 4 h and 8 h. These results obtained in vitro are thus not biologically or environmentally relevant, but are important to explain the effects observed in the general population and to help to elucidate the mechanisms of lead toxicity. For this reason, as high lead blood levels influence the reproductive and fertility capabilities, in vitro results have suggested that the effects observed in lead-exposed people could be related to the disruption of the hypothalamus–hypophysis–gonad axis and not by direct action on sperm (Huang et al., Reference Huang, Tseng and Lin2001; Ghaffari & Motlagh, Reference Ghaffari and Motlagh2011).
Sperm motility
The present results showed that sperm exposure to PbCl2 significantly decreased sperm total and rapid progressive motility and significantly increased the percentage of immotile sperm. Other authors have presented similar results for total sperm motility after in vitro incubation with lead nitrate (Huang et al., Reference Huang, Tseng and Lin2001), lead acetate (Benoff et al., Reference Benoff, Centola, Millan, Napolitano, Marmar and Hurley2003) or lead chloride (Kanwar et al., Reference Kanwar, Chadha, Batla, Sanyal and Sandhu1988). Decreased motility was also observed by others for environmental exposure (Benoff et al., Reference Benoff, Jacob and Hurley2000a; De Rosa et al., Reference De Rosa, Zarrilli, Paesano, Carbone, Boggia, Petretta, Maisto, Cimmino, Puca, Colao and Lombardi2003; Pant et al., Reference Pant, Upadhyay, Pandey, Mathur, Saxena and Srivastava2003; Telisman et al., Reference Telisman, Cvitkovic, Jurasovic, Pizent, Gavella and Rocic2000; Hernández-Ochoa et al., Reference Hernández-Ochoa, García-Vargas, López-Carrillo, Rubio-Andrade, Morán-Martínez, Cebrián and Quintanilla-Vega2005; Hammoud et al., Reference Hammoud, Carrell, Gibson, Sanderson, Parker-Jones and Peterson2010; Li et al., Reference Li, Zhong, Jiang, Wang, Zuo and Sha2012) or occupational exposure (Fisher-Fischbein et al., Reference Fisher-Fischbein, Fischbein, Melnick and Bardin1987; Naha et al., Reference Naha, Bhar, Mukherjee and Chowdhury2005; TNO Strategy, Technology and Policy, 2005; Naha & Chowdhury, Reference Naha and Chowdhury2006).
Decreased sperm motility can occur by several mechanisms. Lead compounds can generate ROS. ROS attack the double bonds of polyunsaturated fatty acids and initiate peroxidation, leading to a loss of fluidity and function of sperm membranes. Lipid peroxidation releases HNE, which damage DNA and decreases GSH and GSSG levels, thus reducing antioxidant defences. High levels of ROS also decrease the activity of other antioxidant enzymes such as SOD, catalase, G6PDH or glutathione-S-transferase. The inhibition of G6PDH determines a decrease in NADPH levels followed by a decrease in GSH and, consequently, an increase in H2O2 that can attack membranes (Griveau & Le Lannou, Reference Griveau and Le Lannou1997; Liu et al., Reference Liu, Kato, Akhand, Hayakawa, Suzuki, Miyata, Kurokawa, Hotta, Ishikawa and Nakashima2000; Aitken & Krausz, Reference Aitken and Krausz2001; Kasperczyk et al. Reference Kasperczyk, Birkner, Kasperczyk and Zalejska-Fiolka2004, Reference Kasperczyk, Kasperczyk, Horak, Ostałowska, Grucka-Mamczar, Romuk, Olejek and Birkner2008; Maneesh & Jayalekshmi, Reference Maneesh and Jayalekshmi2006; Sakkas & Alvarez, Reference Sakkas and Alvarez2010; Bejarano et al., Reference Bejarano, Espino, Paredes, Ortiz, Lozano, Pariente, Rodriguez and Bashamboo2012). Decline in sperm motility may also occur due to a decrease in axoneme protein phosphorilation, reducing ATP production, as glyceraldehyde-3-phosphate dehydrogenase, a key enzyme in glycolysis, which is the main pathway for energy production of sperm motility, resides in the fibrous sheath (de Lamirande & Gagnon, Reference de Lamirande and Gagnon1992, Gagnon & de Lamirande, Reference Gagnon, de Lamirande, De Jonge and Barratt2006; Nascimento et al., Reference Nascimento, Shi, Tam, Chandsawangbhuwana, Durrant, Botvinick and Berns2008). Lead chloride was also shown to inhibit sperm motility by interfering with key enzymes of carbohydrate metabolism and energy production (Kanwar et al., Reference Kanwar, Chadha, Batla, Sanyal and Sandhu1988). Similarly, Ghaffari & Motlagh (Reference Ghaffari and Motlagh2011) found that lead reduces in vitro the activity of creatine kinase, which is important for energy homeostasis, by replacing the magnesium ion present in this enzyme through oxidation of –SH groups. More recently, proteomic studies have shown that sperm have the ability to generate ATP from membrane phospholipids, which would be compromised by membrane damage (Amaral et al., Reference Amaral, Castillo, Estanyol, Ballescà, Ramalho-Santos and Oliva2013). Lead can also replace calcium (Ca2+) in calmodulin, causing a conformational change with subsequent inhibition of phosphorylation of tyrosine residues of the tail proteins, thus reducing motility, as well as on Ca2+ signalling and axonemal microtubules (Gagnon & de Lamirande, Reference Gagnon, de Lamirande, De Jonge and Barratt2006; Bejarano et al., Reference Bejarano, Espino, Paredes, Ortiz, Lozano, Pariente, Rodriguez and Bashamboo2012).
Sperm vitality and membrane integrity
For sperm vitality, we observed a significant decrease in the group exposed to 30 μg/ml PbCl2. Decreased vitality was also observed by others after environmental exposure (Telisman et al., Reference Telisman, Cvitkovic, Jurasovic, Pizent, Gavella and Rocic2000; De Rosa et al., Reference De Rosa, Zarrilli, Paesano, Carbone, Boggia, Petretta, Maisto, Cimmino, Puca, Colao and Lombardi2003; Hernández-Ochoa et al., Reference Hernández-Ochoa, García-Vargas, López-Carrillo, Rubio-Andrade, Morán-Martínez, Cebrián and Quintanilla-Vega2005) or occupational exposure (Naha et al., Reference Naha, Bhar, Mukherjee and Chowdhury2005). These effects have not been studied previously in vitro. Vitality is related to sperm membrane integrity, and thus the above described mechanisms could also explain these observations.
Sperm morphology
We only found a significant increase in flagellum abnormalities, namely in the percentage of coiled tails. Decreased normal morphology was observed after environmental exposure (Telisman et al., Reference Telisman, Cvitkovic, Jurasovic, Pizent, Gavella and Rocic2000, Reference Telisman, Colak, Pizenta, Jurasovic and Cvitkovic2007; Hernández-Ochoa et al., Reference Hernández-Ochoa, García-Vargas, López-Carrillo, Rubio-Andrade, Morán-Martínez, Cebrián and Quintanilla-Vega2005) or occupational exposure (Fisher-Fischbein et al., Reference Fisher-Fischbein, Fischbein, Melnick and Bardin1987; TNO Strategy, Technology and Policy, 2005; Naha & Chowdhury, Reference Naha and Chowdhury2006; Hsu et al., Reference Hsu, Chang, Guo, Liu and Shih2009). These effects have not been studied previously in vitro. As for putative mechanisms, in a multicentre study, authors observed a significant increase in sperm morphological abnormalities, including coiled tails, and this fact was related to several situations such as occupational exposure to toxins (Auger et al., Reference Auger, Eustache, Andersen, Irvine, Jørgensen, Skakkebæk, Suominen, Toppari, Vierula and Jouannet2001; Yeung et al., Reference Yeung, Tüttelmann, Bergmann, Nordhoff, Vorona and Cooper2009). The presence of coiled tails may be due to detachment of the cell membrane from the flagellum skeleton. This event may occur due to the oxidative action on proteins of the outer dense fibres and fibrous sheath, which are rich in –SH groups and for which Pb has a high affinity (Patrick, Reference Patrick2006; Yeung et al., Reference Yeung, Tüttelmann, Bergmann, Nordhoff, Vorona and Cooper2009).
Sperm DNA fragmentation
The present results demonstrated that PbCl2 causes a significant increase in sperm DNA fragmentation. Increased sperm DNA fragmentation was also observed in environmental exposure (Hernández-Ochoa et al., Reference Hernández-Ochoa, García-Vargas, López-Carrillo, Rubio-Andrade, Morán-Martínez, Cebrián and Quintanilla-Vega2005) and occupational exposure (Hsu et al., Reference Hsu, Chang, Guo, Liu and Shih2009). These effects have not been studied previously in vitro. Several mechanisms are responsible for lead action on sperm DNA. ROS cause oxidative modifications on DNA bases. Hydroxyl radicals react with guanosine to form 8-oxo-2′-desoxyguanosine (8OHdG), which causes point mutations that can lead to carcinogenesis and apoptosis. ROS can also mediate mutations in mitochondrial DNA. Free radicals attack the integrity of the sperm nuclear genetic material by inducing DNA breaks, modifications in DNA bases and cross-linking of chromatin. When ROS target membrane lipids, they initiate lipid peroxidation, a chain reaction that produces multiple breakdown molecules such as malonaldehyde (MDA) and HNE. Metabolites of HNE cause DNA adducts that then cause double-stranded breaks and alter the DNA structure (Liu et al., Reference Liu, Kato, Akhand, Hayakawa, Suzuki, Miyata, Kurokawa, Hotta, Ishikawa and Nakashima2000; Xu et al., Reference Xu, Shen, Zhu, Chua, Wang, Chia and Ong2003; Maneesh & Jayalekshmi, Reference Maneesh and Jayalekshmi2006; Cocuzza et al., Reference Cocuzza, Sikka, Athayde and Agarwal2007; Sakkas & Alvarez, Reference Sakkas and Alvarez2010; Bejarano et al., Reference Bejarano, Espino, Paredes, Ortiz, Lozano, Pariente, Rodriguez and Bashamboo2012; G González-Marín et al., Reference González-Marín, Gosálvez and Roy2012). Another factor responsible for sperm structural DNA changes is that lead affects the binding of DNA to HP2. Protamines bind to the outer surface of the DNA helix, interacting with DNA phosphate residues. As lead has affinity for these residues, it interferes with DNA binding sites, altering the protamine–DNA complex. This action disturbs the process of chromatin condensation, needed for gene protection in the sperm cell, as well as for DNA decondensation in the ooplasm for formation of the male pronucleus (Quintanilla-Vega et al., Reference Quintanilla-Vega, Hoover, Bal, Silbergeld, Waalkes and Anderson2000a, Reference Quintanilla-Vega, Hoover, Bal, Silbergeld, Waalkes and Anderson2000b; Aitken & Krausz, Reference Aitken and Krausz2001).
In conclusion, our data showed that, in vitro, PbCl2 significantly adversely affects semen parameters (motility, vitality and morphology) and increases sperm DNA fragmentation. The mechanisms involved in these changes are probably related to the fact that lead causes membrane peroxidation and formation of free radicals, interfering with energy production, axoneme function and DNA compaction, and also triggering DNA fragmentation. All these effects are expected to affect male infertility, thereby further underlining the urgent need for increased population prevention.
Acknowledgements
We would like to acknowledge Elsa Oliveira, 1st Class Technical Specialist of Pathology, Cytology and Thanatology in the Area of Diagnosis and Therapy (ICBAS-UP, UMIB) for microscopy assistance.
Conflict of interest statement
None declared.