Introduction
Oocyte activation has been induced by various types of physical and chemical stimulation, including electric pulses (Prochazka et al., Reference Prochazka, Durnford, Fiser and Marcus1993), ethanol (Kubiak, Reference Kubiak1989), calcium ionophore (Ware et al., Reference Ware, Barnes, Maiki-Lauria and First1989), inhibitors of protein synthesis (Fulka et al., Reference Fulka, Leibfried-Rutledge and First1991) and puromycin (Nakasaka et al., Reference Nakasaka, Yamano, Hinokio, Nakagawa, Yoshizawa and Aono2000; Nakagawa et al., Reference Nakagawa, Yamano, Moride, Yamashita, Yoshizawa and Aono2001a). In humans, oocyte activation with calcium ionophore and puromycin has been reported to result in successful pregnancy in patients with repeated failed fertilization following intracytoplasmic sperm injection (ICSI) (Murase et al., Reference Murase, Araki, Mizuno, Kawaguchi, Naito, Yoshizawa and Araki2004). Roscovitine has also been used to arrest meiotic activation in various animals (Jimenez-Macedo et al., Reference Jimenez-Macedo, Izquierdo, Urdaneta, Anguita and Paramio2006; da Silvia Rascado et al., Reference da Silvia Rascado, Martins, Minto, de Sa Lorena and da Cruz Landim-Alvarenga2010), and oocyte activation by combined treatment with calcium ionophore and roscovitine has been reported in cats and mice (da Silvia Rascado et al., Reference da Silvia Rascado, Martins, Minto, de Sa Lorena and da Cruz Landim-Alvarenga2010, Iba et al., Reference Iba, Yano, Umeno, Hinokio, Kuwahara, Irahara, Yamano and Yasui2011).
Reproductive function in females is affected by obesity at various stages including ovulation, oocyte development, embryo development, endometrial development, embryo implantation and fetal development (Brewer & Balen, Reference Brewer and Balen2010). There are several lines of evidence for negative effects of excessive metabolites, such as glucose (Bermejo-Alvarez et al., Reference Bermejo-Alvarez, Roberts and Rosenfeld2012), lipids (Leroy et al., Reference Leroy, Van Hoeck, Clemente, Rizos, Gutierrez-Adan, Van Soom, Uytterhoeven and Bols2010; Wonnacott et al., Reference Wonnacott, Kwong, Hughes, Salter, Lea, Garnsworthy and Sinclair2010) and leptin (Arias-Alvarez et al., Reference Arias-Alvarez, Bermejo-Alvarez, Gutierrez-Adan, Rizos, Lorenzo and Lonergan2011), on oocyte and embryo development in animal models. In high fat diet (HFD)-induced mice, it has been reported that ovulation rate, embryo development, placental function, ovarian function and mitochondrial function in mice were affected by an HFD (Minge et al., Reference Minge, Bennett, Norman and Robker2008; Igosheva et al., Reference Igosheva, Abramov, Poston, Eckert, Fleming, Duchen and McConnell2010; Jungheim et al., Reference Jungheim, Schoeller, Marquard, Louden, Schaffer and Moley2010; Cardozo et al., Reference Cardozo, Pavone and Hirshfeld-Cytron2011). However, Bermejo-Alvarez et al. (Reference Bermejo-Alvarez, Roberts and Rosenfeld2012) reported that the number of oocytes in HFD-fed mice was the same as that in control diet-fed mice and that embryo development was normal in HFD-fed mice. In humans, inconsistent findings regarding the effects of obesity on oocytes and embryos have also been reported. Dokras et al. (Reference Dokras, Baredziak, Blaine, Syrop, Van Voorhis and Sparks2006) reported that retrieved oocytes and metaphase II oocytes in obese women were significantly fewer than those in normal-weight women despite the fact that the numbers of developing oocytes were the same. However, Carrell (Reference Carrell2001) reported that difference in the numbers of retrieved oocytes and metaphase II oocytes between obese women and normal-weight women were not statistically significant. It has been reported that the quality of embryos in obese women was significantly poorer than that in lean women (Carrell, Reference Carrell2001), but another group found this difference only in women less than 35 years of age (Metwally et al., Reference Metwally, Tuckerman, Laird, Ledger and Li2007). Several groups have also demonstrated that there was no significant correlation between embryo quality and BMI (Spandorfer et al., Reference Spandorfer, Kump, Goldschlag, Brodkin, Davis and Rosenwaks2004; Shalom-Paz et al., Reference Shalom-Paz, Marzal, Wiser, Almog, Reinblatt, Tulandi and Holzer2011).
Therefore, the effects of obesity on oocytes and the embryo have been controversial in animal models and humans. In addition, to the best of our knowledge, the influence of obesity on artificial activation of oocytes in mice with HFD-induced obesity has not been reported. The aim of the present study was to determine the effects of increased dietary intake and HFD on artificial oocyte activation by using puromycin or roscovitine.
Materials and methods
Induction of obesity and breeding
All animal experiments were conducted in accordance with the ethical standards of the Animal Care and Use Committee of the University of Tokushima Graduate School. Six-week-old female B6C3F1 mice (Japan SLC Inc., Shizuoka, Japan) were randomly divided into a control diet group (12 mice), increased dietary intake group (12 mice) and HFD group (12 mice). Mice were fed 35 g/cage/day and 120 g/cage/day of a low-dose irradiation diet (Oriental Yeast Co. Ltd, Tokyo, Japan) for 4 weeks as control diet group and increased dietary intake group, respectively. Mice in the HFD group were fed 110 g/cage/day of HFD-60 (Oriental Yeast Co. Ltd, Tokyo, Japan) for 4 weeks.
Oocytes
After 4 weeks, cumulus-enclosed oocytes were recovered from the oviducts of mice that had received an intraperitoneal injection of 10 IU of pregnant mare's serum gonadotropin (Sigma, St. Louis, MO, USA) followed 48 h later by 10 IU of human chorionic gonadotropin (Wako, Osaka, Japan) at 14 h prior to sacrifice by cervical dislocation. The cumulus masses were dispersed in 0.1% hyaluronidase (Sigma, St. Louis, MO, USA) in modified human tubal fluid (mHTF; Nippon Medical & Chemical Instruments Co., Ltd. Osaka, Japan) medium at 37°C in an atmosphere of 5% CO2 in air for 8–10 min. The cumulus-free oocytes were collected and rinsed twice in fresh mHTF medium prior to use. The numbers and morphological appearance of recovered oocytes were compared among the three groups.
Chemicals
Stock solutions of 1 mg/ml calcium ionophore A23187 (Sigma-Aldrich, Tokyo, Japan), 10 mg/ml puromycin (Sigma, St. Louis, MO, USA) and 10 mg/ml roscovitine (Sigma, St. Louis, MO, ISA) were stored at –20°C. Prior to each experiment, these chemicals were diluted with mHTF containing 0.4% bovine serum albumin (BSA) to the following concentrations: 5 μM calcium ionophore A23187, 10 μg/ml puromycin and 50 μM roscovitine.
Oocyte activation
Oocyte activation was performed in accordance with a previous report (Nakasaka et al., Reference Nakasaka, Yamano, Hinokio, Nakagawa, Yoshizawa and Aono2000). After cumulus-free oocytes had been treated with 5 μM calcium ionophore in mHTF medium for 5 mins, the oocytes were divided into the following three groups: (1) a no activation treatment group, in which oocytes were cultured in mHTF medium with 0.4% BSA for 4 h; (2) a calcium ionophore and puromycin group, in which oocytes were cultured in mHTF medium with 0.4% BSA containing 5 μM calcium ionophore and 10 μg/ml puromycin for 4 h; and (3) a calcium ionophore and roscovitine group, in which oocytes were cultured in mHTF medium with 0.4% BSA containing 5 μM calcium ionophore and 50 μM roscovitine for 4 h. Oocytes were cultured at 37°C in an atmosphere of 5% CO2 in air. Oocyte activation was observed at ×400 magnification under an inverted microscope (IMT2–31 Olympus Diaphot, Tokyo, Japan) equipped with Nomarski differential interference contrast. Activated oocytes were defined as oocytes with at least one pronucleus. The rates of oocyte activation were compared among control diet mice, increased dietary intake mice and HFD mice.
Assessment of blood glucose and lipid profiles
Blood samples for measurements of glucose and lipid profiles were taken from fasting mice on the day before the experiment. Samples obtained were frozen at −40°C until used for analysis. Serum levels of glucose, triglyceride, free fatty acid and total cholesterol were measured.
Statistical analysis
Rates of oocyte activation were evaluated using the chi-squared test. Differences in body weights and serum parameters among control diet mice, increased dietary intake mice and HFD mice were determined by analysis of variance (ANOVA). Results with P-values less than 0.05 were considered to be statistically significant.
Results
Body weights
As can be seen in Table 1, the mean body weight of HFD mice was significantly greater than the mean body weights of control diet mice and increased dietary intake mice (P < 0.05).
Table 1 Oocytes in control diet, increased dietary intake and HFD mice
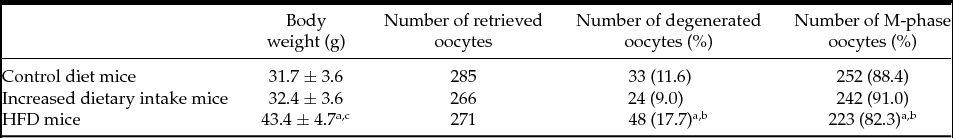
HFD: high fat diet. Body weight: mean ± standard deviation.
a P < 0.05 versus control diet mice. b P < 0.01 versus increased dietary intake mice. c P < 0.05 versus increased dietary intake mice.
Numbers of oocytes and proportions of metaphase II oocytes following superovulation
As can be seen in Table 1, total numbers of superovulated oocytes in control diet mice, increased dietary intake mice and HFD mice were 285, 266 and 271, respectively. There were no significant differences in total number of superovulated oocytes among the three groups. The proportions of metaphase II oocytes following superovulation in the three groups were 88.4% (252/285), 91.0% (242/266) and 82.3% (223/271), respectively. The proportions of degenerated oocytes following superovulation in the three groups were 11.6% (33/285), 9.0% (24/266) and 17.7% (48/271), respectively. The proportions of degenerated oocytes following superovulation in HFD mice were significantly higher than those in control diet mice and increased dietary intake mice (P = 0.04 and P = 0.003, respectively).
Serum levels of glucose, triglyceride, free fatty acid and total cholesterol
The mean glucose level in HFD mice was significantly higher than those in control diet mice and increased dietary intake mice (P = 0.019 and P = 0.032, respectively) (Table 2). Total cholesterol level in HFD mice was significantly higher than that in increased dietary intake mice (P = 0.008). Free fatty acid level in HFD mice was significantly lower than those in control diet mice and increased dietary intake mice (P = 0.002 and P = 0.017, respectively).
Table 2 Serum levels of glucose, triglyceride, free fatty acid and total cholesterol in control diet, increased dietary intake and HFD mice

HFD: high fat diet. Values are mean ± standard deviation.
a P < 0.05 versus control diet mice. b P < 0.05 versus control diet mice and increased dietary mice. c P < 0.01 versus increased dietary mice.
Appearance of activated oocytes
As can be seen in Table 3, oocytes with only calcium ionophore treatment were not activated during the 4-h incubation period. The rates of activation in superovulated oocytes treated with calcium ionophore and roscovitine were 90.3% (56/62) in control diet mice, 89.8% (44/49) in increased dietary intake mice and 67.9% (36/53) in HFD mice. The rate of activation in calcium ionophore and roscovitine-treated oocytes in HFD mice was significantly lower than the rates in control diet mice and increased dietary intake mice (P = 0.003 and P = 0.007, respectively). The rates of activation in superovulated oocytes treated with calcium ionophore and puromycin were 90.6% (58/64) in control diet mice, 94.0% (47/50) in increased dietary intake mice and 71.4% (40/56) in HFD mice. The rate of activation in calcium ionophore and puromycin-treated oocytes in HFD mice was significantly lower than the rates in control diet mice and increased dietary intake mice (P = 0.007 and P = 0.002, respectively). The degree of suppression of activation in roscovitine-treated oocytes and that in puromycin-treated oocytes in HFD mice were not significantly different.
Table 3 Proportions of oocytes activated in control diet, increased dietary intake and HFD mice

HFD: high fat diet,
a P < 0.01 versus control diet mice. b P < 0.01 versus increased dietary intake mice.
Discussion
In the present study, the rates of degenerated oocytes in HFD mice were significantly higher than that in control diet mice, although there was no significant difference in total numbers of retrieved oocytes. Results of previous studies on the effects of obesity on follicle development and ovulation are conflicting. It has been reported that ovarian follicles following superovulation were significantly more apoptotic and that mature oocytes following superovulation were smaller and fewer in obese mice than in control diet mice (Cardozo et al., Reference Cardozo, Pavone and Hirshfeld-Cytron2011; Jungheim et al., Reference Jungheim, Schoeller, Marquard, Louden, Schaffer and Moley2010). The oocytes following superovulation in mice with diet-induced obesity showed increases in mitochondrial DNA content, generation of reactive oxygen species and an oxidated redox state (Igosheva et al., Reference Igosheva, Abramov, Poston, Eckert, Fleming, Duchen and McConnell2010). However, another study showed that diet-induced obesity did not affect the number of oocytes that naturally ovulated (Bermejo-Alvarez et al., Reference Bermejo-Alvarez, Roberts and Rosenfeld2012). Minge et al. (Reference Minge, Bennett, Norman and Robker2008) reported that the natural ovulation rate in HFD mice was increased compared to that in control diet mice. A difference in the degrees of obesity due to diets including HFD in mice might be involved in the difference in these results. Also, the discrepancy of these results may be due to naturally ovulated oocytes and superovulated oocytes. In humans, the effect of obesity on oocyte maturity is also controversial. Retrieved oocytes and metaphase II oocytes in obese women were significantly fewer than those in normal-weight women despite the fact that the numbers of developing follicles were the same in the IVF cycles (Dokras et al., Reference Dokras, Baredziak, Blaine, Syrop, Van Voorhis and Sparks2006). Carrell (Reference Carrell2001) reported that the number of metaphase II oocytes retrieved in obese women undergoing IVF was decreased, but the difference was not statistically significant. A large prospective study did not demonstrate a weight-related reduction in the number and maturity of oocytes retrieved in the IVF-ICSI cycles (Bellver et al., Reference Bellver, Ayllon, Ferrando, Melo, Goyri, Pellicer, Remohi and Meseguer2010).
To date, the influence of HFD on oocyte activation has not been reported. We firstly found that the number of activated oocytes treated with calcium ionophore and roscovitine or puromycin in HFD mice was significantly smaller than the numbers in control diet mice and increased dietary intake mice, suggesting that HFD deteriorates induction of artificial oocyte activation. HFD may influence various reproductive stages including fertilization and embryo development. In previous studies, fertilization rates in naturally ovulated oocytes in HFD mice were shown to be similar to those in control diet mice (Robker, 2008; Cardozo et al., Reference Cardozo, Pavone and Hirshfeld-Cytron2011). It has been reported that naturally ovulated oocytes from HFD mice exhibited slower development of the embryo (Minge et al., Reference Minge, Bennett, Norman and Robker2008; Cardozo et al., Reference Cardozo, Pavone and Hirshfeld-Cytron2011). In humans, it has been reported that fertilization rates of the IVF cycles in overweight and obese women were lower than those in normal-weight women (Salha et al., Reference Salha, Dada and Sharma2001; van Swieten et al., Reference van Swieten, van der Leeuw-Harmsen, Bading and van der Linden2005), while other studies did not show a weight-related reduction in fertilization rate of the IVF cycles (Dokras et al., Reference Dokras, Baredziak, Blaine, Syrop, Van Voorhis and Sparks2006; Bellver et al., Reference Bellver, Ayllon, Ferrando, Melo, Goyri, Pellicer, Remohi and Meseguer2010). Also, results of studies regarding the effect of obesity on embryo quality were inconsistent. While Carrell (Reference Carrell2001) showed that the quality of the embryo was significantly decreased in obese women undergoing IVF, other studies showed that there were no significant differences in quality of the embryo among the BMI strata in patients undergoing in vitro maturation and IVF (Shalom-Paz et al., Reference Shalom-Paz, Marzal, Wiser, Almog, Reinblatt, Tulandi and Holzer2011; Spandorfer et al., Reference Spandorfer, Kump, Goldschlag, Brodkin, Davis and Rosenwaks2004).
The mechanism for suppression of oocyte activation by roscovitine and puromycin in HFD mice has not been clarified. The arrest of meiosis at metaphase II in mammals is maintained by high levels of M-phase-promoting factor (MPF) and a cytoplasmic factor that stabilizes MPF activity in metaphase II oocytes. Oocyte activation is characterized by an increase in pulsatile of intracellular calcium due to binding of spermatozoa with oocytes and by decreases in activities of MPF and MPF kinase. MPF is a heterodimer composed of a cdc2 kinase subunit and a regulatory cyclin B subunit. Roscovitine, which is a specific inhibitor of cyclin-dependent protein kinase, prevents p34cdc2 dephosphorylation and inhibits MPF kinase activity (Meijer & Kim, Reference Meijer and Kim1997). Conversely, puromycin, which is a protein synthesis inhibitor, is responsible for reducing cytostatic factor synthesis and thus indirectly decreasing MPF activity, although the action of puromycin in oocyte activation has not been fully clarified. Thus, both roscovitine and puromycin are associated with a decrease in MPF activity. In HFD mice, suppression of MPF activity may be attenuated by roscovitine or puromycin. Further study on the effect of roscovitine or puromycin on MPF activity in HFD mice may be needed.
We speculated that increases in circulating lipidemic factors might have adverse effects on degeneration of oocytes and oocyte activation in HFD mice. However, contrary to our expectation, serum triglyceride and free fatty acid levels in HFD mice were lower than those in the other two groups in this study. Our results are supported by the results of a previous study showing that the existence of ovaries, i.e. presence of ovarian steroid hormones, prevents aggravation of HFD-induced dyslipidemia in female mice (Ludgero-Correia et al., Reference Ludgero-Correia, Aguila, Mandarim-de-Lacerda and Faria2012). Thus, other unexamined factors, particularly omega-3 and omega-6 polyunsaturated fatty acids, induced by HFD might have been involved in the increase in degeneration of oocytes and decrease in oocyte activation in this study. Oocyte activation by roscovitine and puromycin might be involved in glucose metabolism rather than lipid metabolism. Further study on the difference in oocyte activation by insulin resistance may be needed.
If unfertilized oocytes after ICSI are properly activated, they may form two pronuclei with extrusion of the second polar body and then cleave and develop normally. It has been demonstrated that the combination of calcium ionophore and puromycin could effectively salvage unfertilized oocytes after ICSI (Nakagawa et al Reference Nakagawa, Yamano, Moride, Yamashita, Yoshizawa and Aono2001a; Lu et al., Reference Lu, Zhao, Gao, Li, Ma, Mullen, Critser and Chen2006) and that the use of this combination was an effective method for producing human haploid parthenogenones (Nakagawa, et al., Reference Nakagawa, Yamano, Nakasaka, Hinokio, Yoshizawa and Aono2001b). Murase et al. (Reference Murase, Araki, Mizuno, Kawaguchi, Naito, Yoshizawa and Araki2004) reported that a successful pregnancy outcome was achieved by oocyte activation with calcium ionophore and puromycin in patients with previous repeated failed fertilization following ICSI. Obesity due to intake of high fat food in women may influence oocyte activation by using puromycin at ICSI.
There are some limitations in this study. First, we could not follow up the parthenotes to the blastocyst stage. A study for further stages of oocytes may be needed. Second, to date, results of studies regarding the effects of omega-3 and omega-6 polyunsaturated fatty acids on ovarian function and oocyte quality have been inconsistent (Santos et al., Reference Santos, Bilby, Thatcher, Staples and Silvestre2008; Wonnacott et al., Reference Wonnacott, Kwong, Hughes, Salter, Lea, Garnsworthy and Sinclair2010), and their effects on artificial oocyte activation have not been reported. A study regarding artificial oocyte activation by using omega-3 and omega-6 polyunsaturated fatty acids is needed. In addition, the mechanism by which a HFD affects the process of oocyte activation is needed. Recently, Ge et al. (Reference Ge, Luo, Lin, Liang, Huang, Wei, Hou, Han, Schatten and Sun2013) reported that DNA methylation of imprinted genes in oocytes was not altered in HFD mice, while DNA methylation of metabolism-related genes was changed (Ge et al., Reference Ge, Luo, Lin, Liang, Huang, Wei, Hou, Han, Schatten and Sun2013).
In conclusion, HFD-induced obesity deteriorated induction of oocyte activation by roscovitine and puromycin in mice.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Statement of interest
The authors hereby that there are no conflicting interests.





