Introduction
Cooling protocols for fish embryos have been investigated for some species such as mrigala (Cirrhinus mrigala) and catla (Catla catla) by Ahammad et al. (Reference Ahammad, Bhattacharyya and Jana1998), rohu (Labeo rohita) by Ahammad et al. (Reference Ahammad, Bhattacharyya and Jana2003a) and pacu (Piaractus mesopotamicus) by Streit Jr. et al. (Reference Streit, Digmayer, Ribeiro, Sirol, Moraes and Galo2007). The cooling technique consists of submitting cryoprotected fish embryos at sub-zero temperatures followed by short storage periods, resulting in a decrease in enzymatic and cellular activity (Cloud et al., Reference Cloud, Armstrong, Wheeler, Kucera, Thorgaard, Tiersch and Mazik2000; Ahammad et al., Reference Ahammad, Bhattacharyya and Jana2003b). This biotechnological method allows several practical applications such as: transportation of embryos obtained from remote areas (Ahammad et al., Reference Ahammad, Bhattacharyya and Jana2003a); synchronising the development of embryos collected from different spawning events (Lahnsteiner, Reference Lahnsteiner2008); and optimises the use of hatchery facilities (Streit Jr. et al., Reference Streit, Digmayer, Ribeiro, Sirol, Moraes and Galo2007).
Moreover, the development of this technology may help to understand fish embryo sensitivity at low temperatures and the cryoprotectant agent (CPA) action on these embryos, as information concerning embryos cryopreservation of Neotropical freshwater fish has so far been extremely limited in the literature.
The cooling technique for fish embryos has shown good results when permeable CPAs are used in association with non-permeable and is indispensable when submitting the embryos to sub-zero temperatures (Beirão et al., Reference Beirão, Robles, Herráez, Sarasquete, Dinis and Cabrita2006; Fornari et al., Reference Fornari, Ribeiro, Streit, Vargas and Moraes2010). However, embryos from different fish species, at different developmental stages may tolerate and react to CPAs (concentration and exposure levels) in various ways (Zhang & Rawson, Reference Zhang and Rawson1995; Dinnyés et al., Reference Dinnyés, Urbhnyi, Baranyai and Magyary1998).
Rhinelepis aspera, locally called ‘cascudo preto’, is a rheophilic species as are most Brazilian native fish. This species undertakes long migration; its reproductive season takes place from October to January, presents external fecundation and has total spawning with no parental care. Cascudo preto has been considered as a species in high risk of extinction (Abilhoa & Duboc, Reference Abilhoa, Duboc, Mikich and Bérnils2004). According to Agostinho et al. (Reference Agostinho, Gomes, Suzuki, Carolsfed, Harvey, Baer and Ross2003) this species represented an important part of the total fish capture in the Paranapanema river during 1980 s, but is currently no longer present based on the capture statistics, mainly due to pollution, overfishing and environmental changes. Therefore, studies that may contribute to reproduction and preservation of this species are very important.
In the present study, 10 different cryoprotectant solutions were investigated in order to develop a cooling protocol for Rhinelepis aspera embryos stored at −8°C for 6 h.
Materials and methods
Broodstock care and egg production
The study was carried out in February 2008 at the Hydrology and Aquaculture Station – Duke Energy International, Salto Grande, São Paulo State (Brazil), in collaboration with both PeixeGen and Aquam research groups.
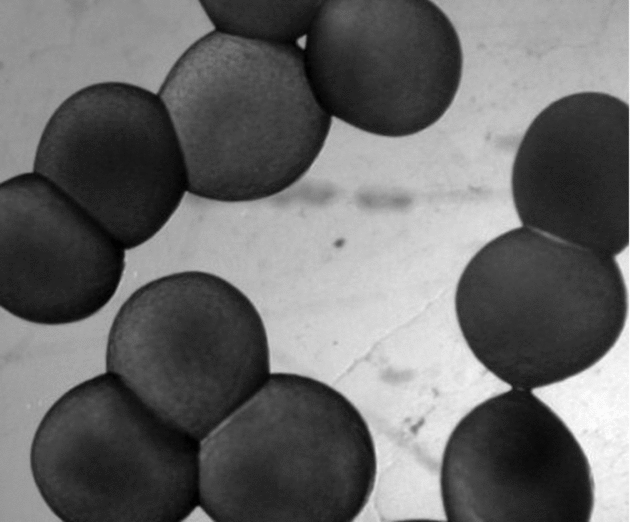
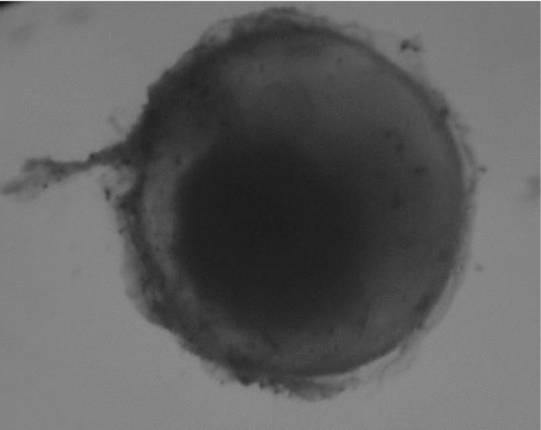
Cascudo preto (Rhinelepis aspera) broodstock were randomly sampled for the study. Twelve males and 10 females were injected intraperitoneally with a commercial carp pituitary crude extract (CPE) by a single injection that contained 2.5 and 5.5 mg/kg CPE, respectively. Both mature oocytes and fresh semen were stripped into the same container (dry method) by gentle abdominal massage and water was added to activate spermatozoa motility and to promote fertilization. Thereafter, eggs were treated with a solution that contained urea, salt and tannin, as suggested by Woynarovich & Horváth (Reference Woynarovich and Horváth1983) in order to reduce the eggs’ adherence (Fig. 1) and to facilitate embryos manipulation.

Figure 1 Rhinelepis aspera eggs presenting adherence. Digital image (7.2 megapixels) obtained using a stereomicroscope (×10 magnification).
Hatching and selection of embryos
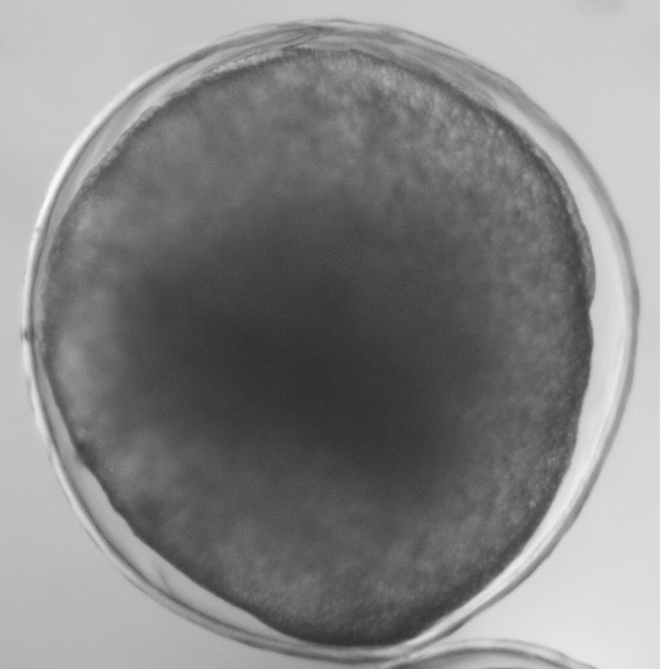
Eggs were incubated in 7-litre open-flow conical hatcheries at 27.5 ± 0.5°C and the sequence of events was assisted in order to follow embryonic development. Three random samples were collected after the blastoporous closing stage, 9 h post-fertilization (Fig. 2) to assess the fertilization rate, and 3300 healthy embryos (judged by chorion morphology) were selected for the experiments.

Figure 2 Rhinelepis aspera embryo at blastoporous closing stage (75% epiboly movement) 9 h post-fertilisation. Digital image (7.2 megapixels) obtained using a stereomicroscope (×30 magnification).
Cooling solutions and storage procedures
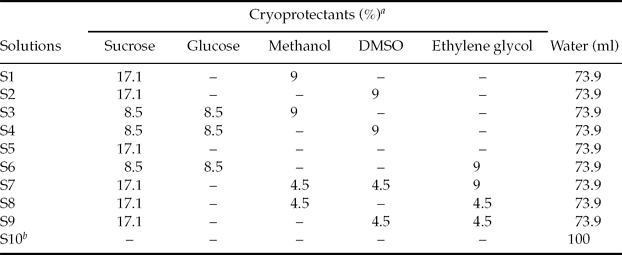
Cryoprotectant solutions were composed of a mixture of non-permeable cryoprotectants (sucrose and glucose) and permeable cryoprotectants (methanol, dimethyl sulphoxide (DMSO) and ethylene glycol) using several different combinations (Table 1).
Table 1 Composition of 10 cryoprotectant solutions used for the Rhinelepis aspera embryos cooling study

aValues are given as w/v basis.
bCryoprotectant action (CPA)-free treatment. DMSO, dimethyl sulphoxide.
Groups of 100 viable embryos were placed in 6-ml glass vials and exposed to nine different cryoprotectant solutions (Table 1); each cryoprotectant solution represented one treatment. A tenth treatment (CPA-free) was designed as control, i.e. embryos were exposed to the cooling protocol only in distilled water (Table 1).
The vials that contained embryos and cryoprotectant solutions were sealed, kept at room temperature for 2 min, and then cooled gradually by immersion in an ice-water bath at 15°C for 10 min; transferred to another ice-water bath at 5°C for 10 min and, finally, stored in a refrigerator at −8°C for 6 h.
After 6 h, the sealed vials were taken from the refrigerator and transferred to 3-litre open-flow conical hatcheries, acclimatized for 2 min, and then the vials were opened and embryos kept inside the hatcheries to complete embryonic development.
The individual vial, containing 100 embryos, represented one replicate for each cryoprotectant solution, and three replicates were used.
Control group (no treatment)
At the same time that embryos were collected and selected for cooling studies from hatcheries (9 h post-fertilisation), three samples (100 healthy embryos) were transferred directly to other hatcheries (27.5 ± 0.5°C) and the hatching performance was recorded. This hatching rate was used as a reference for comparison with the treated groups and to control the presence of natural causes of embryo mortality.
Hatching assessment and data analysis
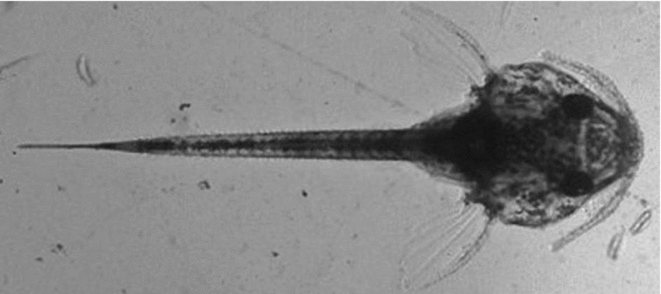
When embryonic development was completed (about 36 h post-fertilization), both control and treated groups were carefully removed from the hatcheries to determine the hatching rate counting live larvae (Fig. 3) and failed eggs (Fig. 4) under a stereomicroscope.
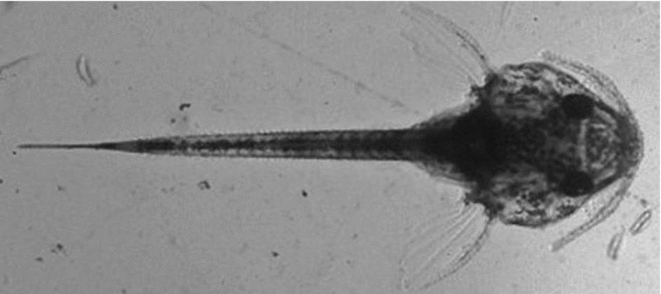
Figure 3 Newly hatched larva of Rhinelepis aspera after the cooling protocol. Digital image (7.2 megapixels) obtained with a stereomicroscope (×10 magnification).

Figure 4 Rhinelepis aspera failed egg. Digital image (7.2 megapixels) obtained with a stereomicroscope (×10 magnification).
The hatching rate was calculated as follows:
Hatching rate (%) = (Number of live larvae ÷ Total number of embryos) × 100
Differences in hatching rates among the cryoprotectant solutions were verified by the chi-squared test using PROC NPAR1WAY developed by SAS software (SAS Institute, Cary, NC, USA, 2003).
Results
Control group (no treatment)
The control group of embryos, which was selected and transferred directly to hatcheries without undergoing any cooling treatment, presented a hatching rate of 91% (data not shown; Table 2). This hatching rate was a very useful reference for comparison with treated groups.
Table 2 Hatching rate of Rhinelepis aspera after storage for 6 h at −8°C using 10 cryoprotectant solutions

a ,b,c Means followed by different letters are significantly different by the chi-squared test (p < 0.05). CPA, cryoprotectant action. DMSO, dimethyl sulphoxide.
Cooled groups
Rhinelepis aspera embryos were affected adversely by exposure to low temperatures. The viability of embryos cooled in S10 (CPA-free treatment) and even in most cryoprotectant solutions was not preserved after storage for 6 h at −8°C.
Although the results that showed high sensitivity of Rhinelepis aspera embryos at low temperature, positive effects of some cryoprotectant solutions could be verified. Hatching rate was observed in the groups cooled under S1, S7 and S8 conditions (Table 2), with significant differences among each other (p < 0.05).
Discussion
Only solutions that contained methanol associated with sucrose as non-permeable cryoprotectant provided some protection to Rhinelepis aspera embryos from cold damage. When glucose was used as the non-permeable cryoprotectant (S3, S4 and S6), eggs did not hatch. Similar results were found by Streit Jr. et al. (Reference Streit, Digmayer, Ribeiro, Sirol, Moraes and Galo2007), who performed toxicity tests with several CPAs for Piaractus mesopotamicus embryos. As an oligosaccharide, sucrose provides an additional effect on cellular protection compared with simple sugars such as glucose (Rall, Reference Rall1987).
The efficiency of methanol as a permeable cryoprotectant qualifies its use in development of this cooling protocol. Only the groups of embryos treated with solutions that contained methanol (S1, S7 and S8) presented some degree of viability after the cooling protocol, while the use of DMSO and ethylene glycol, isolated or combined, resulted in no hatching rate at all. DMSO was chosen due to its optimal capacity to penetrate into the cells (Cabrita et al., Reference Cabrita, Robles, Chereguini, Wallace and Herráez2003). It is usually recommended for more complex cells, such as the cellular mass formed during embryo development. Ethylene glycol has a low molecular weight compared with other cryoprotectants, which permits a fast passage through the cellular membranes (Voelkel & Hu, Reference Voelkel and Hu1992). However, the very fast passage of the cryoprotectant to the inside and outside of the cell may produce a too aggressive dynamic and may be lethal to the cell, which may have happened in this study.
The S1 group presented a good hatching rate (above 50%), although this hatching rate was lower than that found in the control group, not undergoing any cooling treatment (91%), which illustrates the damage caused by cooling. The failure in prevention of cellular cold damage, even in the S1 group, may be related to the exposure time of the embryo to cryoprotectant solutions and the temperature of storage. This relationship is defined as ‘solution/time equilibrium’.
The equilibrium time in the solution can be critical to the infusion of the cryoprotectant solution into the embryo. Széll & Shelton (Reference Széll and Shelton1986) observed that mice embryos exposed to glycerol at 20°C reached the necessary degree of dehydration for cryoprotection very quickly, while the osmotic equilibrium occurred only after a longer exposure time to the solution, when the cryoprotectant enters the embryonic cells. Moreover, Bertolini et al. (Reference Bertolini1994) in a study using Mus musculus embryos concluded that the cryoprotectant penetration inside the cell is enhanced at 20°C.
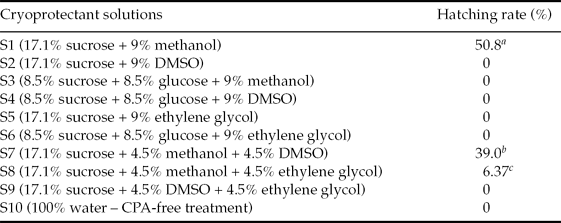
The results obtained in by S1 treatment were less efficient than those obtained by Streit Jr. et al. (Reference Streit, Digmayer, Ribeiro, Sirol, Moraes and Galo2007) with Piaractus mesopotamicus embryos, even using the same cryoprotectant solution, temperature and exposure time. This difference may be related to the specificity of the Rhinelepis aspera embryo. According to Cabrita et al. (Reference Cabrita, Robles, Chereguini, Wallace and Herráez2003) the effect of each cryoprotectant does not depend only on its chemical proprieties but also on the species of fish. In the Rhinelepis aspera embryo a marked feature is the reduced perivitelline layer (Fig. 5), which could have interfered with the dehydration process, either preventing intracellular water output or the cryoprotectant input.

Figure 5 Rhinelepis aspera embryo post-fertilization. Arrow indicates the reduced perivitelline layer. Digital image (7.2 megapixels) obtained using a stereomicroscope (×10 magnification).
The fact that methanol has a low toxicity and high permeability through fish embryos membranes certainly contributed to the good results obtained verified in S1 treatment, compared with other cryoprotectant solutions tested. Moreover, the cryoprotectant efficiency is based on the relationship among the permeation rates, which depend on its concentration and the exposure time of embryos to cryoprotectant solution (Bart, Reference Bart, Tiersch and Mazik2000).
In conclusion, cryoprotectants methanol (per-meable) and sucrose (non-permeable) were essential for the cooling protocols for Rhinelepis aspera embryos. The cryoprotectant solution composed of 17% sucrose + 9% methanol allowed a hatching rate of over 50%, even 6 h after storage at −8°C. Cryopreservation of Rhinelepis aspera embryos by cooling is reported here for the first time. Further studies are needed in order to extend the storage time and to improve the hatching rate for this species.