Introduction
In vitro production (IVP) of bovine embryos is a reproductive technique that allows improving the genetic gain by reducing the interval between generations and increasing offspring of individuals of high genetic merit (Andrade et al., Reference Andrade, Oliveira, Lima, Santos Filho and Pina2002). However, advances in IVP are compromised due to only 25–40% of in vitro maturated oocytes reaching competence, while rates of 60–80% are observed in vivo (Farin et al., Reference Farin, Rodriguez, Alexander, Hockney, Herrick and Kennedy-Stoskopf2007). This difference is attributed to differences in the maturation process in vitro and in vivo (Combelles et al., Reference Combelles, Cekleniak, Racowsky and Albertini2002). During maturation, oocytes should complete meiosis, chromosome segregation and cytoplasmic rearrangement (Ferreira et al., Reference Ferreira, Vireque, Adona, Meirelles, Ferriani and Navarro2009) to achieve competence and reach the blastocyst stage (Luciano et al., Reference Luciano, Franciosi, Modina and Lodde2011). Despite in vitro maturation (IVM) offering support to nuclear maturation, cytoplasmic maturation is often compromised (Combelles et al., Reference Combelles, Cekleniak, Racowsky and Albertini2002).
Oocyte maturation is regulated by different processes including phosphorylation and dephosphorylation of proteins and cellular signalling molecules (Thach, Reference Thach1992; Colgan et al., Reference Colgan, Murthy, Prives and Manley1996; Gavin and Schorderet-Slatkine, Reference Gavin and Schorderet-Slatkine1997). Moreover, ATP concentration is crucial for formation of the meiotic spindle (Zhang et al., Reference Zhang, Wu, Lu, Guo and Ma2006), calcium oscillation (Dumollard et al., Reference Dumollard, Duchen and Sardet2006) and polar body extrusion during oocyte maturation (Stojkovic et al., Reference Stojkovic, Zhang and Crespi2001; Hao et al., Reference Hao, Liu, Wu, Wan, Cui, Chen and Zeng2009). Intracellular levels of ATP are associated with mitochondrial reorganization and total cell number in blastocysts. Embryos with low ATP concentrations have a slower development and have less cell numbers compared with embryos with higher ATP levels (Stojkovic et al., Reference Stojkovic, Zhang and Crespi2001). Therefore, the use of supplements to improve mitochondrial metabolism and ATP levels during IVM represents an alternative to obtain better efficiency during IVP.
Butafosfan is an organic phosphorus molecule that has been used as a metabolic modulator in dairy cows (Pereira et al., Reference Pereira, Silveira, Montagner, Schneider, Schmitt, Rabassa, Pfeifer, Del Pino, Pulga and Correa2013), and may represent an alternative to improve in vitro oocyte competence. Some field reports have suggested beneficial results from butafosfan injection in cattle- and horse-assisted reproduction programmes, although no scientific evidence can be found in the published literature. When associated with cyanocobalamin, butafosfan increased the number of small follicles in cows (Lima et al., Reference Lima, Pereira, Maffi, Santos, Martin, Del Pino, Leal, Brauner and Correa2017). Phosphorus is also essential for synthesis of nucleotides related to hormonal signalling (Cunningham, Reference Cunningham2002) and for growth, differentiation and cellular integrity (Berg et al., Reference Berg, Tymoczko and Stryer2006). ATP concentration is higher in muscle and liver tissue of rats treated with butafosfan (Hasi Su-rong et al. (Reference Hasi Su-rong, Xiao-yan and Bei-lei2004). Based on these findings, the aim of this study was to evaluate the addition of increasing doses of butafosfan during oocyte maturation in vitro on the initial embryo development in cattle.
Materials and methods
Collection and selection of cumulus–oocyte complexes (COCs)
Bovine ovaries were collected from a local slaughterhouse and transported to the laboratory in NaCl 0.9% solution with gentamicin 0.5% at 30ºC. COCs were aspirated from follicles (3–8 mm in diameter) and COC grades 1 and 2 were selected according to Leibfried and First (Reference Leibfried and First1979). All culture media were purchased commercially (Biotecnologia Animal®, Brasília, DF, Brazil).
Experimental design and in vitro maturation (IVM)
Selected COCs were distributed randomly into four groups (60 COCs/group) according to butafosfan supplementation during IVM, in total six replicates (n = 1400 COCs). Butafosfan concentrations were determined through the available phosphorus blood levels of animals receiving butafosfan (Bayer Saúde Animal®, São Paulo, SP, Brazil) according to the manufacturer’s recommendations (unpublished data). COCs were matured in 500 μl IVM medium (TCM199 supplemented with 0.2 mM sodium pyruvate, 0.2 µl/ml FSH, 75 µl/ml amikacin, 0.1 µl/ml estradiol and 10% of fetal bovine serum) supplemented with 0 mg/ml, 0.05 mg/ml, 0.1 mg/ml or 0.2 mg/ml of butafosfan (Bayer Saúde Animal®). IVM was performed at 39°C in an atmosphere of 5% CO2 in air for 24 h.
In vitro fertilization and in vitro culture
In vitro fertilization (IVF) was performed with semen obtained from the same ejaculate from a Bos taurus bull. Semen was thawed at 35°C for 30 s and sperm selection was performed using a mini Percoll density gradient method (Parrish et al., Reference Parrish, Krogenaes and Susko-Parrish1995). COCs (50 oocytes/group) were inseminated with sperm at the concentration of 1 × 106 cells/ml. IVF occurred in 400-µl drops of TALP-IVF medium supplemented with 6 mg/ml bovine serum albumin (BSA)-FAF, 0.2 mM sodium pyruvate, 30 µg/ml heparin, 20 µM penicillamine, 10 µM hypotaurine, 1 µM epinephrine and 75 µg/ml amikacin, at 39°C in an atmosphere of 5% CO2 in air for 20 h.
After IVF, presumptive zygotes were denuded from cumulus cells by repeated pipetting. Denuded oocytes were transferred for 200-µl drops of in vitro culture (IVC) medium (SOFaa supplemented with 5% fetal bovine serum, 2.7 mM of myo-inositol, 0.2 mM sodium pyruvate, 5 mg/ml BSA and 75 µg/ml amikacin) under mineral oil. The culture was conducted at 39°C in an atmosphere of 5% CO2 in air for 7 days after IVF (Day 0). After 72 h (Day 3) and 120 h (Day 5), 50% of the medium for IVC was replaced with fresh medium. The cleavage rate was evaluated at Day 2 and the blastocysts rate was recorded at Day 7 based on the number of inseminated oocytes.
Analysis of nuclear maturation
After IVM, five COCs/group were denuded by repeated pipetting, washed three times with phosphate-buffered saline (PBS) and fixed for 15 min in 4% paraformaldehyde. Then, a solution of PBS with 0.5% Triton X-100 was used for short-term storage. These oocytes were stained in PBS supplemented with 15 µl/ml Hoechst 33342 stain (Sigma-Aldrich®, St. Louis, MO, USA), fixed in slides and evaluated using an inverted fluorescence microscope IX 71 (Olympus®, Shinjuku-ku, Tokyo, Japan), with ultraviolet (UV) light filters of 330–385 nm. Oocytes with polar body extrusion were classified as maturated, indicating the metaphase II stage, while those with other chromatin configurations were classified as non-maturated. In total 120 oocytes were analyzed (30 oocytes/group).
Analysis of gene expression
After IVM, 15 COCs/group were denuded by repeated pipetting. Oocytes were removed from the drop and cumulus cells were recovered by centrifugation. Oocytes and cumulus cells were transferred separately to microtubes containing 100 μl TRIzol (Invitrogen®, Carlsbad, CA, USA), homogenized and stored at −70°C. In total six replicates were performed, 360 oocytes in total for RNA extraction. In addition, at Day 7, all embryos were also collected and stored in 100 μl TRIzol (Invitrogen®) at −70°C, six replicates in total.
Total RNA was extracted from cumulus cells, oocytes and embryos using TRIzol reagent (Invitrogen®), according to the manufacturer’s protocol. RNA concentration was measured on a spectrophotometer (NanoVue, Healthcare®, Amersham, UK) the 260/280 nm ratio of absorbance was determined. Reverse transcription was performed with total RNA in a 20-µl volume using the iScript Synthesis kit (Bio-Rad®, Hercules, CA, USA) according to the manufacturer’s instructions.
Real-time polymerase chain reaction (PCR) reactions were run on an Applied Biosystems 7500 RT-PCR (Applied Biosystems®, Foster City, CA, USA) using SYBR Green PCR Master Mix (Applied Biosystems®) according to the manufacturer’s instructions. H2A histone family, member Z gene (H2AFZ) used as the endogenous control for all evaluated structures.
The expression target genes was evaluated as follows: BCL2 associated X protein (BAX) and apoptosis regulator Bcl-2 (BCL2) as apoptosis markers; amphiregulin (AREG) and epiregulin (EREG) as genes related to cumulus cells expansion and resumption of meiosis; growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) as indicators of oocyte quality; facilitated glucose transporter member 1 (SLC2A1) and phosphofructokinase, platelet (PFKP) for analysis of energy metabolism in oocytes; and placenta-specific 8 (PLAC8) as indicator of embryo implantation. The coefficient of variation was less than 5% for all pairs of primers used (Table 1). Relative expression was calculated using the 2A-B/2C-D equation as described by Campos et al. (Reference Campos, Rincon, Acosta, Silveira, Pradieé, Correa, Gasperin, Pfeifer, Barros, Pegoraro and Schneider2017).
Table 1. Primer sequence used for gene expression analysis of cumulus cells, oocyte and embryos by RT-qPCR

Statistical analysis
Analyses were carried out using SAS 9.0 software (SAS, Cary, NC, EUA) and the General Linear Model approach. The polynomial regression model was used to determine the linear, quadratic or cubic effect of supplementation with 0.0, 0.05, 0.1 and 0.2 mg/ml of butafosfan on cleavage and blastocysts rates. The same analysis was performed to determine the effect on the expression of genes BAX, BCL2, AREG, EREG, GDF9, BMP15, SLC2A1, PFKP in cumulus cells and oocytes, BAX, BCL2 e PLC8 in embryos, and the BAX/BCL2 relationship in cumulus cells, oocytes and embryos. Results are shown as mean ± standard error of the mean.
Results
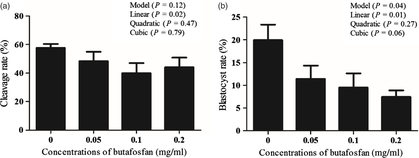
Increased butafosfan doses during IVM resulted in a reduction of cleavage and embryo development. The cleavage rate (%) was 57.67 ± 2.7, 48.38 ± 6.6, 39.92 ± 7.1 and 44.06 ± 6.8 and the blastocyst rate (%) was 19.93 ± 3.4, 11.43 ± 2.9, 9.52 ± 3.1 and 7.45 ± 1.4 in the groups supplemented with 0, 0.05, 0.1 and 0.2 mg/ml of butafosfan, respectively. A linear effect of butafosfan addition during IVM was observed for cleavage rate (P = 0.02; Fig. 1a) and blastocyst rate (P = 0.01; Fig. 1b). Nuclear maturation rate (%) of oocytes supplemented with 0, 0.05, 0.1 and 0.2 mg/ml of butafosfan was not different between groups (57.50 ± 19.2, 53.75 ± 11.7, 55.67 ± 8.1 and 53.40 ± 16.5, respectively, P > 0.05; Fig. 2).
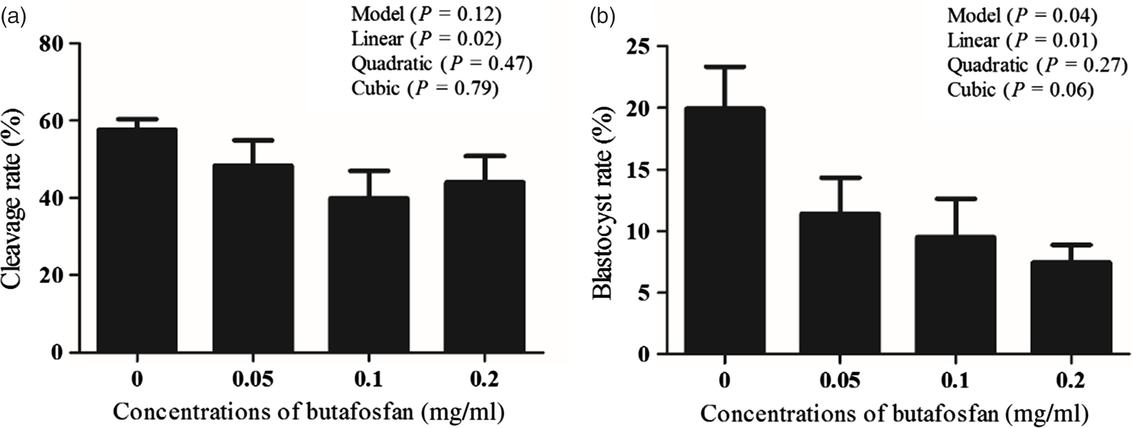
Figure 1. Cleavage rate D2 (a) and blastocyst rate on D7 (b) derived from oocytes supplemented with different doses of butafosfan during in vitro maturation.

Figure 2. Nuclear maturation rate of oocytes analyzed by Hoechst staining after in vitro maturation with different doses of butafosfan supplementation.
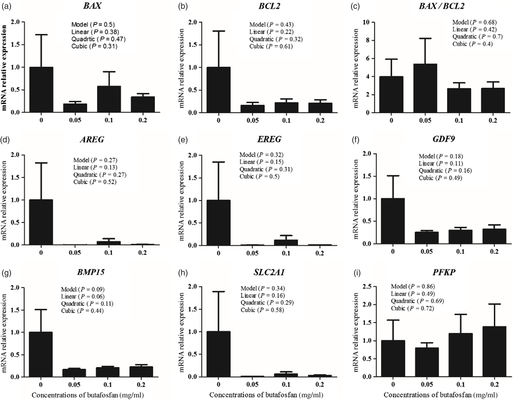
Relative expression of the genes BAX, BCL2, AREG, EREG, GDF9, BMP15, SLC2A1, PFKP and BAX/BCL2 in oocytes (Fig. 3) and cumulus cells (Fig. 4) was not different among groups (P > 0.05). In addition, no difference among groups was observed for relative expression of genes BAX, BCL2 e PLC8 and BAX/BCL2 in Day 7 embryos (P > 0.05; Fig. 5).

Figure 3. Relative expression of genes BAX (a), BCL2 (b), AREG (d), EREG (e), GDF9 (f), BMP15 (g), SLC2A1 (h), PFKP (i) and BAX/BCL2 (c), in oocytes supplemented with different doses of butafosfan during in vitro maturation. Determined by real-time PCR using H2AFZ gene as internal control.

Figure 4. Relative expression of genes BAX (a), BCL2 (b), AREG (d), EREG (e), GDF9 (f), BMP15 (g), SLC2A1 (h), PFKP (i) and BAX/BCL2 (c) in cumulus cells derived from oocytes supplemented with different doses of butafosfan during in vitro maturation. Determined by real-time PCR using H2AFZ gene as internal control.

Figure 5. Relative expression of genes BAX (a), BCL2 (b), PLAC8 (d) and BAX/BCL2 (c) in embryos derived from oocytes supplemented with different doses of butafosfan during in vitro maturation. Determined by real-time PCR using H2AFZ gene as internal control.
Discussion
In the present study, we observed that increasing doses of butafosfan in IVM linearly decreased the cleavage rate. The same effect was observed for embryo development, which was lower as the concentration of butafosfan increased, suggesting that butafosfan is detrimental for oocyte maturation. It was proposed that butafosfan may interfere with phosphorylation of molecules involved in cellular signalling (Tabeleão et al., Reference Tabeleão, Pereira, Prietsch, Feijó, Bondan, Mattei, Schmitt, Del Pino and Corrêa2017). To achieve competence, oocytes need to complete nuclear and cytoplasmic maturation (Ferreira et al., Reference Ferreira, Vireque, Adona, Meirelles, Ferriani and Navarro2009), a process dependent on several signalling pathways, such as promoting maturation factor (PMF) and regulated by different kinases and phosphatases (Belloc et al., Reference Belloc, Pique and Mendez2008).
Our results do not indicate any difference in oocyte maturation rate in groups supplemented with butafosfan during IVM, suggesting that its deleterious effects on cleavage rate and embryo development are not associated with nuclear maturation. Cytoplasmic maturation comprises the rearrangement of cytoplasmic organelles (Yamada and Isaji, Reference Yamada and Isaji2011), increase of lipid content and reduction of the Golgi complex, leading the oocyte to interrupt transcription by nucleolus condensation (Sirard and Coenen, Reference Sirard and Coenen2006; Wang and Sun, Reference Wang and Sun2007). This process gives the oocyte the ability to prevent polyspermia, to allow decondensation of the sperm in the ooplasm and to maintain embryonic development in the early stages (van den Hurk and Zhao, Reference van den Hurk and Zhao2005). Therefore, cytoplasmic maturation results in a cellular rearrangement that enables the oocyte to be fertilized (Ferreira et al., Reference Ferreira, Vireque, Adona, Meirelles, Ferriani and Navarro2009). Thereby, butafosfan may have affected cytoplasmic maturation, negatively affecting competence acquisition process. However, this study is the first using butafosfan during oocyte maturation, so doses tested here may not represent the best for beneficial potential of the butafosfan molecule in vitro.
Cumulus cells play a crucial role in oocyte maturation and competence acquisition (Tanghe et al., Reference Tanghe, Van Soom, Nauwynck, Coryn and de Kruif2002). In our study, the effect of butafosfan on the expansion of cumulus cells was also evaluated by gene expression analysis. AREG and EREG play a central role in cumulus cell expansion (Ashkenazi et al., Reference Ashkenazi, Cao, Motola, Popliker, Conti and Tsafriri2005; Park et al., Reference Park, Su, Ariga, Law, Jin and Conti2004). These genes translate into proteins responsible for mediating the effect of luteinizing hormone (LH) on cumulus cells (Ashkenazi et al., Reference Ashkenazi, Cao, Motola, Popliker, Conti and Tsafriri2005). However, no change was observed in relative expression of AREG and EREG in groups with lower cleavage rates and embryo development. Conversely, Boruszewska et al. (Reference Boruszewska, Sinderewicz, Kowalczyk-Zieba, Grycmacher and Woclawek-Potocka2015) reported an increase in relative expression of these genes in cumulus cells of oocytes maturated in medium supplemented with lysophosphatidic acid and a higher rate of embryo development. As we observed no effect on the level of transcription, it is possible that butafosfan acts post-transcriptionally or independent of these traditional markers of oocyte competence.
The effect of butafosfan in apoptosis of cumulus cells, oocytes and embryos was evaluated by relative expression analysis of BAX and BCL2 genes and the BAX/BCL2 ratio. The BAX/BCL2 ratio is associated with oocyte and embryo quality (Yang and Rajamahendran, Reference Yang and Rajamahendran2002) and higher mRNA levels of BCL2, an anti-apoptotic gene, in cumulus cells are associated with an increased embryo quality (Assou et al., Reference Assou, Haouzi, Mahmoud, Aouacheria, Guillemin, Pantesco, Reme, Dechaud, De Vos and Hamamah2008). However, when groups with higher cleavage rates and embryo development were compared with those with lower rates, no difference was observed in expression of BCL2 and BAX, a pro-apoptotic gene, or in the BAX/BCL2 relationship for cumulus cells, oocytes or embryos, suggesting that treatment with butafosfan did not increase apoptosis of these cells. In another study, IVM was supplemented with recombinant paraoxanase-1, a higher blastocyst rate was observed when compared with the control group, however no difference in the BAX/BCL2 relationship was observed in cumulus cells, oocytes or embryos (Rincon et al., Reference Rincon, Madeira, Campos, Mion, Silva, Absalon-Medina, Butler, Correa, Pegoraro and Schneider2016). Therefore, the deleterious effects of butafosfan might not be directly related to the apoptotic pathway.
Expression of genes related to oocyte quality was also evaluated by real-time PCR. Several studies have demonstrated that GDF9 and BMP15 are markers for oocyte quality (Gendelman et al., Reference Gendelman, Aroyo, Yavin and Roth2010; Hussein et al., Reference Hussein, Thompson and Gilchrist2006; Gendelman and Roth, Reference Gendelman and Roth2012). GDF9 and BMP15 regulate the crosstalk between oocytes and granulosa cells, as well as the metabolism of cumulus cells (Su et al., Reference Su, Sugiura, Wigglesworth, O’Brien, Affourtit, Pangas, Matzuk and Eppig2008). GDF9 expression is higher in oocytes with earlier cleavage than in oocytes with later cleavage (Gendelman et al., Reference Gendelman, Aroyo, Yavin and Roth2010). However, we did not observe any significant difference in expression of these genes in butafosfan treated groups. Therefore, it is possible that there are other factors regulating the expression of GDF9 and BMP15 that are not directly associated with oocyte quality.
Genes involved in glucose metabolism were also evaluated in this study. Glucose is an important energetic substrate for cumulus cells expansion, oocyte maturation and embryonic development (Rose-Hellekant et al., Reference Rose-Hellekant, Libersky-Williamson and Bavister1998). SLC2A1 is the most abundant passive glucose transporter and its expression is detected in oocytes and embryos (Augustin et al., Reference Augustin, Pocar, Navarrete-Santos, Wrenzycki, Gandolfi, Niemann and Fischer2001). During IVM, SLC2A1 promotes the passive transport of glucose to COCs. Cumulus cells are responsible for glucose metabolism and transfer of pyruvate to the oocyte through gap junctions (Cetica et al., Reference Cetica, Pintos, Dalvit and Beconi1999). In cumulus cells, glycolysis is mediated by the PFKP enzyme (Cetica et al., Reference Cetica, Pintos, Dalvit and Beconi2002). Oocytes matured in the presence of lysophosphatidic acid had a high maturation rate and reduced apoptosis, in addition to a high expression of SLC2A1 and PFKP, suggesting that the level of expression of these genes is associated with oocyte and embryonic quality (Boruszewska et al., Reference Boruszewska, Sinderewicz, Kowalczyk-Zieba, Grycmacher and Woclawek-Potocka2015). Studies have described that butafosfan increases ATP synthesis, improving the intracellular energetic status (Furll et al., Reference Furll, Deniz, Westphal, Illing and Constable2010; Rollin et al., Reference Rollin, Berghaus, Rapnicki, Godden and Overton2010; Pereira et al., Reference Pereira, Silveira, Montagner, Schneider, Schmitt, Rabassa, Pfeifer, Del Pino, Pulga and Correa2013). However, we did not observe any significant difference in SLC2A1 and PFKP expression in cumulus cells and oocytes treated with different concentrations of butafosfan during IVM. Therefore, it is likely that the deleterious effect of butafosfan is not mediated by changes in energy metabolism.
In the present study, the effect of butafosfan on the improvement of embryo–maternal signalling ability was evaluated through expression analysis of PLAC8. PLAC8 plays an important role in embryo–maternal signalling (Rekik et al., Reference Rekik, Dufort and Sirard2011). Blastocysts produced in vitro that result in pregnancy have a higher expression of PLAC8 (El-Sayed et al., Reference El-Sayed, Hoelker, Rings, Salilew, Jennen, Tholen, Sirard, Schellander and Tesfaye2006). Despite that, no difference was observed in PLAC8 mRNA levels in groups treated with butafosfan, indicating that implantation of these embryos is not compromised.
In conclusion, the supplementation of IVM with different doses of butafosfan decreases the cleavage rate and embryo development, with no difference in maturation rate and in expression of genes related to oocyte and embryo quality. Therefore, we suggest that butafosfan presents a deleterious effect to oocytes.
Ethics statement
All procedures were approved by the Ethics Committee in Animal Experimentation from Universidade Federal de Pelotas (Protocol 6936).
Conflict of interest
None of the authors of this study has any conflict of interest.
Financial support
This work was supported by FAPERGS, CNPq and CAPES.