Introduction
Seminal plasma (SP) is the liquid fraction of semen, originating from the accessory sex glands, testis and epididymis (Kumar et al., Reference Kumar, Kumar, Singh and Yadev2012). It activates and supports ejaculated spermatozoa as well as transporting them into the female reproductive tract. However, SP varies in composition between individuals and therefore may be implicated in variations in fertility (Moura et al., Reference Moura, Koc, Chapman and Killian2006). The composition of SP also varies within the ejaculate (Rodriguez-Martinez et al., Reference Rodriguez-Martinez, Kvist, Ernerudh, Sanz and Calvete2011), making interpretation of its effects difficult, e.g. the proteins in the last part of the human ejaculate cause coagulation. The method of semen collection may affect the SP: collecting semen by electroejaculation can alter the composition of SP as the contributions of the various accessory sex glands may be changed (Marco-Jiménez et al., Reference Marco-Jiménez, Vicente and Viudes-de-Castro2008). However, despite these challenges in determining the effects of SP, the possibilities of activating spermatozoa for reproductive biotechnologies by changing the SP environment are interesting (de Graaf et al., Reference De Graaf, Leahy, Marti, Evans and Maxwell2008) and therefore further study of the effects of SP are needed.
Previous studies on the effects of SP on sperm quality have used either epididymal sperm samples or ejaculated sperm samples that have been centrifuged to remove liquid SP. Both types of samples present problems when interpreting the results. Epididymal samples may be contaminated with cellular debris and blood, both of which are detrimental to sperm quality. In addition, the spermatozoa have not been activated in the same manner as they would have been during ejaculation under physiological conditions. With centrifuged ejaculated samples, even centrifugation at a high g force does not remove SP proteins that are adhering firmly to the sperm surface (Kruse et al., 2011) and may damage the spermatozoa through the release of reactive oxygen species (ROS). Therefore, adding SP to such samples may not enable its effects to be assessed. Centrifugation through a colloid, however, removes the SP proteins adhering to the sperm surface (Kruse et al., 2011), and also reduces some ROS levels (Morrell et al., 2017). Therefore, colloid centrifugation, particularly single layer centrifugation (SLC), can be used in the preparation of sperm samples to study the effects of adding SP to sperm samples under controlled conditions.
In previous experiments, the addition of small proportions of SP from bulls of high or low fertility to SP-free sperm samples before freezing was studied to determine the effects on post-thaw sperm quality (Nongbua et al., Reference Nongbua, Johannisson, Edman and Morrell2016). In the present experiment, the effects of adding 1% or 5% of homologous SP (bull's own SP) and heterologous SP (SP from a different bull) prior to freezing on post-thaw sperm quality were studied.
Materials and methods
Bovine semen collection, preparation and addition of bovine SP
The collection of the material needed for this study does not require ethical approval in Sweden at the present time. Semen was collected at a commercial semen collection station (Viking Genetics, Skara, Sweden) from bulls kept according to standard husbandry procedures. Bulls were categorized as high or low fertility according to a fertility index used routinely by the breeding company, based on the 56-day non-return rate from a minimum of 1000 artificial inseminations (Humblot et al., Reference Humblot, Decoux and Dhorne1991). Using this index a bull of average fertility will score 100; bulls of higher fertility in the cohort will have a score of >100 whereas lower fertility bulls will have a score of<100. This index was adjusted to take into account factors such as time of year of insemination, farm, age and parity of cow, and inseminator (Rodriguez-Martinez, Reference Rodriguez-Martinez2003).
Ejaculates deemed to be acceptable for freezing according to the internal quality control standards of the company were considered to be acceptable for the study. The semen was divided in two parts. The first part was used for the preparation of bovine SP by centrifuging at 1800 g for 10 min to pellet the spermatozoa. The supernatant was removed and checked by light microscopy for the presence of spermatozoa. If spermatozoa were seen, the centrifugation was repeated.
Meanwhile, the second part of the ejaculate was extended in warm (35°C) egg yolk medium (extender consisting of 20 ml egg yolk, Tris 2.422 g, glucose 1 g, citric acid 1.36 g, de-ionized water 80 ml; products were acquired from VWR, Stockholm, Sweden) to provide a sperm concentration of 50×106 spermatozoa/ml. This suspension was used for SLC with a species-specific colloid, Bovicoll (formerly Androcoll-B) (patent applied for, J. M. Morrell), following the protocol for stallion semen (Morrell et al., Reference Morrell, Johannisson, Dalin and Rodriguez-Martinez2009) with slightly modifications: the sperm sample was extended to a concentration of 50 × 106/ml, with 15 ml of sample being layered over 15 ml of Bovicoll in a 50 ml conical centrifuge tube. The preparation was centrifuged at 300 g for 20 min at room temperature. After centrifugation, the supernatant was discarded by aspiration and the sperm pellet was harvested from beneath the last 1–2 ml colloid and resuspended in cryopreservation extender (OptiXcell®; IMV, l’Aigle, France). Sperm concentration was measured using the NucleoCounter® SP-100™ (ChemoMetic AS, Allerød, Denmark) and adjusted to achieve a final concentration of 69×106 spermatozoa/ml. The suspension was divided into five aliquots of 5 ml each; homologous or heterologous bovine SP (from the same bull or from a different bull, respectively) was added at 0, 1 or 5% (v/v) of the final volume (Fig. 1). Finally, the sperm samples were cooled to 4°C and frozen by controlled rate freezing according to the company’s routine practice.
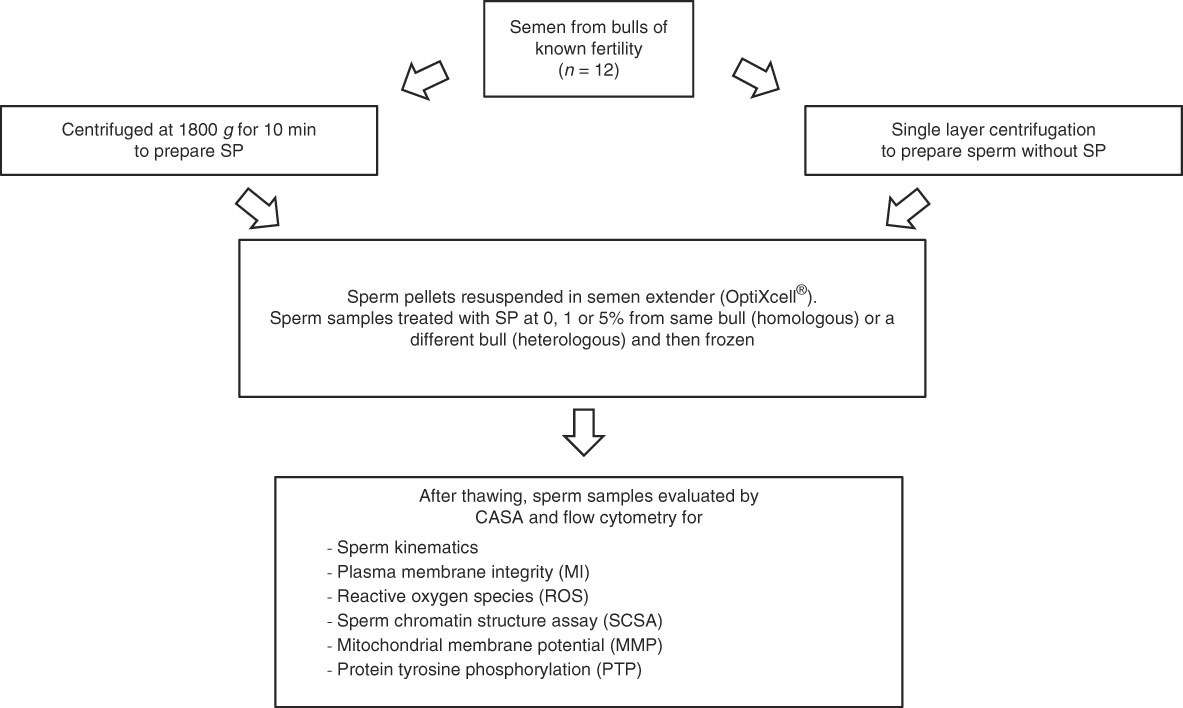
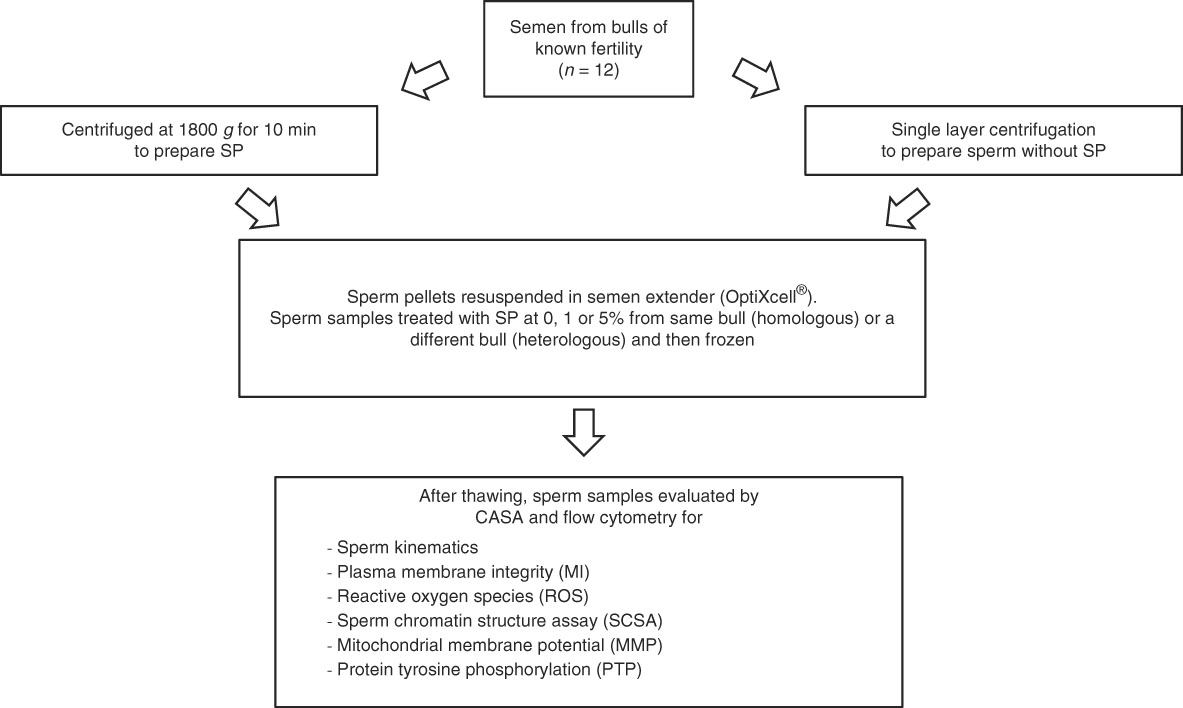
Figure 1 Experimental design.
Ejaculates were available from 12 bulls of known fertility (high fertility, n=5; low fertility, n=7) of Swedish Red or Holstein Friesian breed. Either homologous or heterologous SP was added to aliquots of SP-free sperm samples from each bull (Fig. 1). After freezing, the straws were stored in liquid nitrogen until required for analysis. One straw of each sample was thawed at 37°C for 12 s and was used for the following analyses.
Computer-assisted sperm analysis (CASA)
Motility analysis was performed using a SpermVision® analyzer (Minitube GmbH, Tiefenbach, Germany) and QualiSperm® (Biophos, AG, Switzerland). The SpermVision® was connected to a microscope (Olympus BX 51, Tokyo, Japan) equipped with a heated stage (38°C). For the samples in OptiXcell® extender, an incubation of 10 min at 37°C was carried out before the samples were analyzed. Aliquots (5 µl) of the sperm samples were pipetted onto a warm glass slide (38°C) and a coverslip was placed on top. The following parameters were evaluated: total motility (MOT,%), progressive motility (PMOT,%), velocity straight line (VSL, μm/s), velocity average path (VAP, μm/s), velocity curved line (VCL, μm/s), linearity (LIN, VSL/VCL; %), straightness (STR, VSL/VAP; %), wobble (WOB, VAP/VCL; %), amplitude of lateral head displacement (ALH, μm) and hyperactive (Hyper, %).
Sperm motility analysis with the QualiSperm® (Biophos, AG, Switzerland) was carried out using a microscope (Nikon, Eclipse E200, Tokyo, Japan) supplied with a heated stage (38°C) using a similar procedure to the SpermVision® (Minitube GmbH, Tiefenbach, Germany). The following measurements were made: total motility (TMQ, %), progressive motility (PMQ, %), velocity (μm/s), velocity class A (%, rapid progressive>50 μm/s), velocity class B velocity (%, slow progressive 50<B>15 μm/s), velocity class C (%, slow progressive<15 μm/s), velocity class D (%, non- progressive 0 μm/s).
Plasma membrane integrity (MI)
Plasma membrane integrity was evaluated by flow cytometry using SYBR14 and propidium iodide (PI) staining according to published procedures Johannisson et al. (Reference Johannisson, Morrell, Thorén, Jönsson, Dalin and Rodriguez-Martinez2009), with slight adaption for bull samples (Goodla et al., Reference Goodla, Morrell, Yusnizar, Stålhammar and Johannisson2014). Briefly, aliquots of samples were diluted with buffer B (patent applied for; J.M. Morrell and H. Rodriguez-Martinez) to a concentration of approximately 2 × 106 sperm cells/ ml (300 μl). The diluted samples were stained with 0.6 μl of 0.02 mM SYBR14, 3 μl of 2.4 mM propidium iodide (PI) (Live-Dead Sperm Viability Kit L-7011; Invitrogen, Eugene, OR, USA) and incubated at 37°C for 10 min. The stained samples were analyzed with a BD LSR flow cytometer (Becton Dickinson, San Jose, CA, USA). Excitation was induced by an argon-ion laser (488 nm). Detection of fluorescence colours was as follows: green fluorescence was evaluated via fluorescence channel (FL1) band-pass filter (530/30 nm) and red fluorescence was detected via FL3 long-pass filter (>670 nm). In total, 50,000 spermatozoa cell were evaluated and classified into three populations according to the degree of intactness of the plasma membrane: living (%) (SYBR14 positive/PI negative), dead (%) (SYBR14 negative/PI positive), and dying (%) (SYBR14 positive/PI positive).
Reactive oxygen species
The procedure of Guthrie and Welch (Reference Guthrie and Welch2006) was adapted for bull semen (Goodla et al., Reference Goodla, Morrell, Yusnizar, Stålhammar and Johannisson2014). Aliquots of samples were divided in two parts (part one and part two) and diluted to a concentration of approximately 2 × 106 sperm cells/ml with buffer B (300 μl). The samples were stained with 9 μl of 40 μM Hoechst 33258 (HO; Sigma, Stockholm, Sweden), 9 μl of 40 μM hydroethidine (HE; Invitrogen, Eugene, OR, USA), and 9 μl of 2 mM 2′,7′-dichlorodihydrofluorescein diacetate (DCFDA; Invitrogen, Eugene, OR) in final concentrations. Only the part one sample was stained with 3 μl of 20 mM menadione (Sigma, St. Louis, MO, USA) to stimulate ROS production. The use of HO permitted the simultaneous differentiation of living and dead cells; HE and DCFDA stains were used to detect superoxide anion and hydrogen peroxide (H2O2), respectively. The samples were incubated at 37°C for 30 min and analyzed with a BD LSR flow cytometer (Becton Dickinson, San Jose, CA, USA). Excitation was performed with an argon-ion laser (488 nm) and a HeCd laser (325 nm). Detection of fluorescence colours was as follows: green fluorescence was detected with a FL1 band-pass filter (530/30 nm), blue fluorescence was detected with a FL5 long-pass filter (380 nm), red fluorescence was evaluated via a FL3 long-pass filter (>670 nm). In total, 50,000 spermatozoa cell were detected and presented as percentages, with the following parameters evaluated: live superoxide-negative, live superoxide-positive, dead superoxide-positive, live H2O2-negative, live H2O2-positive, dead H2O2-negative and dead H2O2-positive.
Sperm chromatin structure (SCSA)
Sperm chromatin integrity was evaluated according to the SCSA method (Evenson et al., Reference Evenson, Larson and Jost2002) with slight modifications (Goodla et al., Reference Goodla, Morrell, Yusnizar, Stålhammar and Johannisson2014). Briefly, aliquots of sperm samples were mixed 50 µl: 50 µl with Tris–sodium chloride–EDTA (TNE) buffer (0.15 mol/l NaCl, 0.01 mol/l Tris–HCl, 1 mmol/l EDTA, pH 7.4). Immediately, the mixture was frozen in liquid nitrogen vapour and subsequently stored in a −80°C freezer. The samples were thawed on crushed ice approximately 20 min before staining. Aliquots (10 μl) of the thawed samples were extended with 90 μl of TNE buffer and subjected to partial DNA denaturation in situ via mixing with 0.2 ml of a low pH detergent solution containing 0.17% Triton X-100 (0.15 mol/l NaCl, and 0.08 mol/l HCl; pH 1.2). After waiting for 30 s the denatured sperm were stained with 0.6 ml of acridine orange (6 μg/ml in 0.1 mol/l citric acid, 0.2 mol/l Na2HPO4, 1 mmol/l EDTA, 0.15 mol/l NaCl; pH 6.0). Finally, the stained samples were analyzed within 5 min with a BD LSR flow cytometer (Becton Dickinson, San Jose, CA, USA), collecting forward scatter, side scatter, green (FL1) and red (FL3) fluorescence. In total, 10,000 cells were evaluated and presented as DNA fragmentation index (%DFI), calculated as the ratio of the percentage of cells with denatured, single-stranded DNA to total cells acquired (both with stable, double-stranded DNA, and denatured single-stranded DNA); all results were calculated using FCS Express version 2 (De Novo software, Glendale CA, USA).
Mitochondrial membrane potential
Mitochondrial membrane potential (MMP) of sperm cells was analyzed using published procedures (Cossarizza et al., 1993) using the lipophilic cationic probe 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolylcarbocyanine iodide (JC-1; Invitrogen, Eugene, OR, USA) with slight modifications (Goodla et al., Reference Goodla, Morrell, Yusnizar, Stålhammar and Johannisson2014). Briefly, aliquots of samples were diluted with buffer B to a concentration of approximately 2.5 × 106 sperm cells/ml (300 μl). Then the diluted samples were stained with 1.2 μl of 3 mM JC-1 and incubated at 37°C for 40 min. The stained samples were evaluated with a BD LSR flow cytometer (Becton Dickinson, San Jose, CA, USA). Excitation of the fluorophore stained cells was achieved by the instrument’s argon-ion laser (488 nm). Emitted fluorescence was collected using both FL1 (530/30 nm) and FL2 (585 nm) filters. The green fluorescence was evaluated in FL1 and orange in FL2. In total, 30,000 cells were analysed and presented in two classifications: sperm cells with high respiratory activity (%) (orange fluorescence) and with low respiratory activity (%) (green fluorescence) (CellQuest, version 3.3; Beckon Dickinson).
Global protein tyrosine phosphorylation (PTP)
Bull sperm PTP was evaluated using flow cytometry according to published procedures (Sidhu et al., Reference Sidhu, Mate, Gunasekera, Veal, Hetherington and Baker2004; Piehler et al., Reference Piehler, Petrunkina, Ekhlasi-Hundrieser and Töpfer-Petersen2006) with modifications (Goodla et al., Reference Goodla, Morrell, Yusnizar, Stålhammar and Johannisson2014). Briefly, aliquots of samples were diluted with buffer B to a concentration of approximately 8 × 106 sperm cells/ml (300 μl). The diluted samples were stained with 7 μl of PI (Live-Dead Sperm Viability Kit L-7011; Invitrogen, Eugene, OR, USA) and incubated at 37°C for 15 min and centrifuged at 300 g for 10 min at 20°C. The supernatant was discarded and the pellet was resuspended in 1 ml of buffer B. Then the samples was divided into two parts (parts one and two), the samples were washed in 1 ml of buffer B and centrifuged at 300 g for 10 min at 20°C. Afterwards the pellets were resuspended with 500 μl of 1% paraformaldehyde, and incubated at 4°C for 30 min. The pellets were washed by centrifugation at 1000 g for 3 min. The pellet was resuspended in 1 ml of 0.05% saponin (Sigma, St. Louis, MO, USA) in PBS, and incubated for 10 min at room temperature to permeabilize the cells. Then the samples were washed and resuspended in 500 μl PBS containing 0.1% BSA (Sigma, St. Louis, MO, USA) and 0.1% Tween 20 (MERCK-Schuchardt, Hohengrunn, Germany) to block non-specific binding sites. The samples were incubated at room temperature for 30 min and washed as above. The sample part one was stained with 100 μl of fluorescein isothiocyanate (FITC)-conjugated monoclonal anti-phosphotyrosine antibody produced in mouse clone PT-66 (Sigma, St. Louis, MO, USA) diluted 1:300 in PBS. Sample part two (control) was stained 100 μl of antibody, IgG1-FITC isotype control from murine myeloma, clone MOPC21 (Sigma, St. Louis, MO, USA) diluted 1:20 in PBS. All of the stained samples were incubated at room temperature for 20 min in the dark. The samples were washed again. Then the supernatant was removed and resuspended in 300 μl of PBS for analysis by flow cytometry. In total, 30,000 cells were measured using Cell Quest 3.3 software (Becton Dickinson). The cells were evaluated as the percentage of phosphorylated cells (%) and the mean fluorescence intensity (MFI).
Statistical analysis
The treatment means were compared for all analyses. In all response variables, residuals were investigated for normality and homoscedasticity using diagnostic plots. The comparisons between treatments were performed using mixed statistical models (Littell et al., Reference Littell, Stroup, Milliken, Wolfinger and Schabenberger2006; Olsson, Reference Olsson2011) of the SAS® (Proc Mixed, SAS® 9.3, Cary, NC, USA). The bull and bull interaction with treatment were used as random factors. The treatment group was used for the fixed part of the model to observe the response variable of the samples after adding homologous or heterologous SP. Post-hoc comparisons were adjusted for multiplicity using Tukey’s method. All values are reported as Least Squares Means±Standard Error (LSMEAN±S.E.). A P-value<0.05 was considered to be statistically significant.
Results
Effect of bovine SP on sperm kinematics
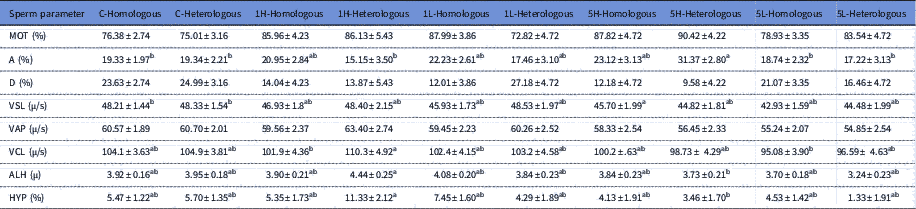
The kinematics for the treated sperm samples are shown in Table 1. The proportion of spermatozoa with velocity class A was highest for treatments 5H-Heterologous and differed from 5L-Homologous (P<0.01), 5L-Heterologous (P<0.05), 1H-Heterologous, C-Homologous (P<0.01 for each) and C-Heterologous (P<0.05) (Table 1). The VSL was significantly different in treatment 5H-Homologous compared with treatments C-Homologous and C-Heterologous (P<0.05 for both). Furthermore, treatment with 1H-Heterologous had highest VCL, ALH and HYP. The 1H-Heterologous differed from 5L-Homologous (P<0.05) in VCL, and differed from 5L-Heterologous in ALH and HYP (P<0.05 for both). Treatment 5H-Heterologous had a tendency to higher MOT and lower proportion of spermatozoa in velocity class D than control. The 5L-Homologous had a tendency to higher VAP than control
Table 1 Effect of adding homologous or heterologous bovine seminal plasma on sperm kinematics; C-Homologous (n=9), C-Heterologous (n=6), 1H-Homologous (n=5), 1H-Heterologous (n=3), 1L-Homologous (n=6), 1L-Heterologous (n=4), 5H-Homologous (n=4), 5H-Heterologous (n=5) and ), 5L-Homologous (n=8) and 5L-Heterologous (n=4)
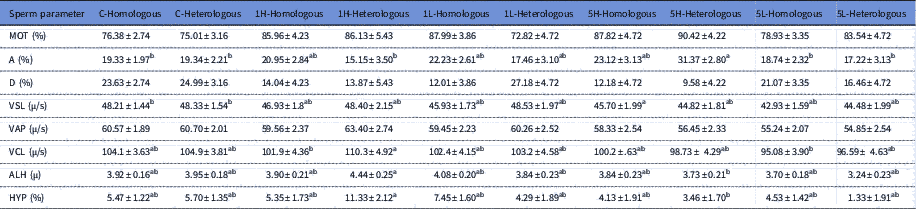
MOT (total motility;%), A (velocity class A; rapid progressive>50 μm/s), D (velocity class D; non-progressive 0 μm/s), VSL (straight line velocity), VAP (velocity average path) VCL (curvilinear velocity), ALH (amplitude of lateral head displacement), HYP (hyperactive). Different superscript letters within a row indicate statistical difference (P<0.05).
Effect of bovine SP on plasma membrane integrity (MI)
No significant differences were observed in the proportion of membrane intact spermatozoa (control 59.4±2.9%, 1H 61.9±2.5%, 1L 60.2±2.4%, 5H 59.0±2.4%, 5L 60.0±2.3%) and membrane damaged spermatozoa (control 34.8±2.2%, 1H 31.9±2.4%, 1L 32.0±2.3%, 5H 33.6±2.4%, 5L 32.6±2.2%) between treatments (P>0.05) or between homologous and heterologous SP (P>0.05) (Table 2).
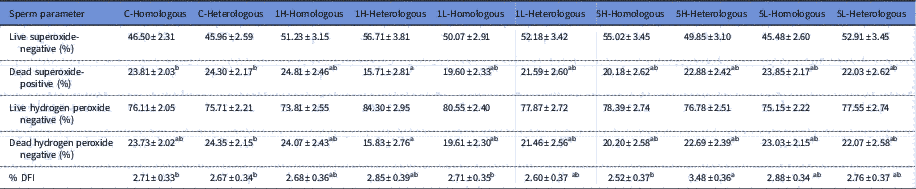
Table 2 Effect of adding homologous or heterologous bovine seminal plasma on reactive oxygen species and sperm chromatin structure assay; C-Homologous (n=9), C-Heterologous (n=6), 1H-Homologous (n=5), 1H-Heterologous (n=3), 1L-Homologous (n=6), 1L-Heterologous (n=4), 5H-Homologous (n=4), 5H-Heterologous (n=5), 5L-Homologous (n=8) and 5L-Heterologous (n=4)

% DFI (DNA fragmentation index; %). Different superscript letters within a row indicate statistical difference (P<0.05).
Effect of bovine SP on reactive oxygen species
Significant differences were observed in ROS production between groups (Table 2). Treatment 1H-Heterologous had the lowest proportion of dead, superoxide-positive spermatozoa and differed from C-Homologous (P<0.05) and C-Heterologous (P<0.05). Furthermore, treatment 1H-Heterologous had the lowest proportion of dead, H2O2-negative spermatozoa and differed from C-Heterologous (P<0.05). After adjusting by Tukey’s method, the 1H-Heterologous treatment group had a tendency to higher proportions of living superoxide-negative and living hydrogen peroxide negative spermatozoa than the other treatments.
Effect of bovine SP on sperm chromatin structure (SCSA)
There were significant differences in %DFI between treatments with homologous and heterologous SP (P<0.05) (Table 2), with treatment 5H-Heterologous having the highest %DFI compared with treatment with 5H-Homologous (P<0.05), 1L-Homologous (P<0.05), C-Homologous (P<0.05) and C-Heterologous (P<0.05).
Effect of bovine SP on mitochondrial membrane potential (MMP)
No significant differences in high MMP (control 52.3±3.0%, 1H 53.5±3.5%, 1L 52.4±3.2%, 5H 46.3±3.4%, 5L 48.6±3.0%) were seen between proportions or type of SP.
Effect of bovine SP on protein tyrosine phosphorylation (PTP)
No significant differences in the percentage of phosphorylated cells were seen among treatments (control 26.8±4.6%, 1H 32.4±5.5%, 1L 24.4±5.0%, 5H 23.5±5.2%, 5L 28.1±4.6%), regardless of whether homologous or heterologous SP was added.
Discussion
Insemination doses contain varying amounts of the bull´s own SP depending on the sperm concentration in the original ejaculate and the number of spermatozoa per straw. In the present study, the effect of adding 1% or 5% SP was studied, as previous studies had estimated the amount of SP in the insemination dose to vary between 0.8 and 12% (Hering et al., 2014; Bromfield, Reference Bromfield2016). Sperm quality was affected by the addition of SP; both the fertility of the bull from which the SP originated and whether the SP was homologous or heterologous contributed to this effect. In general, adding 5% SP tended to decrease some sperm kinematics (VCL, ALH and hyperactivity) compared with the SP-free controls and 1% SP. Sperm samples treated with SP from high fertility bulls had better sperm velocity parameters than those treated with SP from low fertility bulls. Membrane integrity, mitochondrial activity and capacitation status were not affected by adding SP, regardless of its source, whereas chromatin integrity was adversely affected by 5% heterologous SP.
These results are in partial agreement with a previous study, in which washed spermatozoa exposed to high fertility SP showed greater penetration of zona-free bovine oocytes than spermatozoa mixed with low fertility SP (Henault and Killian, 1996). In another study, SP from bulls of known fertility was shown to increase the motility of epididymal sperm samples but did not affect their membrane integrity or MMP (Holden et al., Reference Holden, Fernandez-Fuertes, Murphy, Lonergan and Fair2017), although the functionality of the treated spermatozoa was not affected in an IVF system. Using electroejaculates from Nellore bulls, Campanholi et al. (Reference Campanholi, Monteiro, Ribeiro Dias, Mercadante, de Paz, Dell’Aqua Junior, Papa, Dell’Aqua, Vantini and Garcia2017) showed that sperm velocity was improved if SP was removed from the samples prior to cryopreservation using either centrifugation or a filtration technique, and lower oxidative stress was observed. However, plasma membrane integrity was reduced by SP removal in their study, possibly as a consequence of the centrifugation or filtration technique. Blastocyst production in IVF was adversely affected by centrifugation but not by filtration. However, the studies of Holden et al. (Reference Holden, Fernandez-Fuertes, Murphy, Lonergan and Fair2017) and Campanholi et al. (Reference Campanholi, Monteiro, Ribeiro Dias, Mercadante, de Paz, Dell’Aqua Junior, Papa, Dell’Aqua, Vantini and Garcia2017) did not compare the effects of heterologous and homologous SP on sperm quality, nor did they study the effect of SP-treated samples in artificial insemination. Fertility in IVF differs from in vivo fertility, being less dependent on spermatozoa being able to navigate their way through the female reproductive tract while still retaining functionality (Thys et al., 2009). Therefore, the effects of adding SP on the functionality of bull spermatozoa in AI have still to be determined.
A previous study in stallions has shown differences in effect between homologous and heterologous SP (Morrell & Johannisson, Reference Morrell and Johannisson2014). In the present study, adding 5% heterologous bovine SP from high fertility bulls had a positive effect on spermatozoa velocity class A, ALH and HYP compared with 1% heterologous bovine SP, which is in agreement with results reported for stallion spermatozoa (Morrell & Johannisson, Reference Morrell and Johannisson2014). Samples treated with 1% heterologous bovine SP from high fertility bulls showed reduced proportions of dead, superoxide-positive and dead, H2O2-negative spermatozoa compared with samples without SP. Furthermore, there was a tendency to higher proportions of live, superoxide-negative spermatozoa live, H2O2-negative spermatozoa than in the other samples. Hydrogen peroxide can be associated with a decrease in the proportion of capacitated spermatozoa (O’Flaherty et al., Reference O’Flaherty, Beconi and Beorlegui1997), and an excess of ROS in general are detrimental to sperm survival (Guthrie and Welch, Reference Guthrie and Welch2006).
Nonetheless, our study determined that treatment with 5% heterologous SP from high fertility bulls had a negative effect on %DFI compared with treatment with 5% homologous SP from high fertility bulls or control. This result is similar to the study of the effects of heterologous stallion SP, in which the SP of most individuals caused an increase in %DFI in the spermatozoa of other stallions, with one exception (Morrell and Johannisson, Reference Morrell and Johannisson2014). Our result are also in partial agreement with those of Henault and Killian (1996) who showed that spermatozoa from a low fertility bull combined with high fertility SP had high penetrating ability compared with adding low fertility SP. The spermatozoa from a low fertility bull treated with his own SP had a greater ability to penetrate oocytes than did spermatozoa from a bull of high fertility combined with low fertility SP (Henault and Killian, 1996). In contrast, our results suggested that the presence of 5% heterologous bovine SP had a negative effect on sperm motility and chromatin integrity (Nongbua et al., Reference Nongbua, Johannisson, Edman and Morrell2016). However, the fertility of the treated samples was not examined in the present study.
In a previous study, 5% SP was found to reduce MMP (T. Nongbua, unpublished data), although no difference in MMP was seen in the present study. However, this apparent difference may indicate an interaction between SP and extender, as OptiXcell was used in the present study whereas Andromed was the extender used in the previous study.
Further studies are needed to investigate other factors involved e.g. breed of bull, SP concentrations, homologous or heterologous spermatozoa; the combination of SP and different extenders should be investigated, and also the fertility of manipulated samples. In future, it might be possible to optimize fertility in sperm doses for artificial insemination by adding SP to sperm samples that have had their own SP removed by colloid centrifugation. Moreover, if the activating substances could be identified and synthesized, it might be possible to obtain the stimulating effect without adding SP from other animals.
In conclusion, sperm velocity was increased by the addition of 5% bovine SP, with heterologous SP having the greatest effect. However, 5% heterologous SP from high fertility bulls had a deleterious effect on chromatin integrity. Other parameters of sperm quality were not influenced by the addition of bovine SP. Therefore, adding SP to sperm samples with the intention of improving fertility should be approached with caution but further studies of SP composition between low and high fertility bulls, including fertility trials, are needed to identify the factors involved.
Acknowledgements
We are grateful to the staff in the barn and laboratory at Viking Genetics, Skara, for supplying the ejaculates used in this study and for freezing all the samples. We would also like to thank Professor Ulf Olsson for his suggestions and comments on the statistical analyses.
Financial support
TN is funded by Mahasarakham University, Thailand. JMM and AJ are funded by the Swedish Research Council for the environment, agricultural sciences and spatial planning (FORMAS), project number 221-2010-1241.
Statement of interest
The authors declare that they have no competing interests. JMM is the inventor and patent holder of the colloid and buffer B used in the experiment. However, as the colloid was not the subject of the study, it is not considered to be a potential conflict of interest.
Ethical standards
The collection of the material needed for this study does not currently require ethical approval.